There are extraordinary examples of evolutionary conservation in biology, one of which is revealed in aging biology. A robust case in point are three interrelated endocrine systems that have been linked to longevity: the growth hormone (GH), insulin (INS), and insulin-like growth factor (IGF) pathways. As far down the evolutionary ladder as yeast, there is strong evidence that a carbohydrate regulatory system exists and that if perturbed, life-span extension is observed (1). As one moves up the rungs of the ladder to nematodes and fruit flies, both insulin and IGF become important in the maintenance of metabolism (Figure 1). Like yeast, when levels or activity of ligand, receptor or cognate signaling factors in these pathways are disrupted (ie, nematode age-1, daf-2; Drosophila InR, chico), an extension of life span is observed (2–6). In the mammalian system, the endocrine system becomes more complex with the addition of GH, a hormone that controls circulating IGF-1 levels and thus has somatic actions yet also exhibits key metabolic functions that are independent of IGF-1 (7–9). Disturbing the GH pathway by severely reducing plasma levels or by receptor disruption significantly extends health span and life span in mice (10–13). Elevating these findings to the more advanced mammals, humans, as with many biological tenets has added complexity for a variety of reasons.
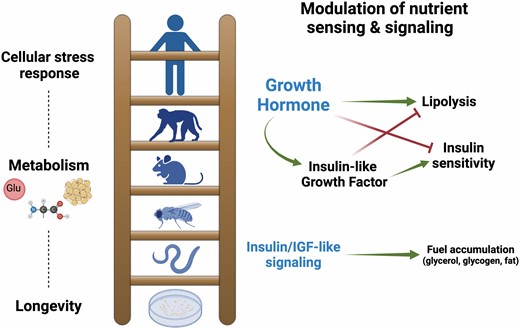
Figure 1.
Growth hormone (GH) is a multifunctional hormone in mammals that drives growth and metabolism. Reduced GH signaling results in enhanced cellular stress resistance and altered metabolism promoting delays in aging processes, lowering age-related disease incidence and progression, and extending life span. Disrupted insulin and insulin-like growth factor (IGF)-like receptor signaling in invertebrates and yeast promotes life-span extension via altered metabolism and cellular defense. Created with BioRender.com.
What is troublesome is that IGF-1 is very often cited as the key determinant of longevity in mammals. This is most likely due to the large body of evidence supporting the role of insulin and IGF-1 in longevity of yeast, worms, flies, and their presence in mammals. There is no question that IGF-1 is a major player in life-span determination in invertebrates. However, in mice, the data linking growth hormone with health-span and life-span regulation is far more robust yet often overlooked even though it regulates circulating IGF-1 production and secretion. The actions of these two closely linked hormones are not identical and sometimes opposite of one another.
Growth hormone is a pituitary-derived hormone that has both somatic and metabolic functions. The somatic actions of GH are mediated in part via the stimulation of liver IGF-1 secretion and its subsequent actions on bone, cartilage, and muscle. Somatic growth is driven by components of this pathway; therefore, body size differences are obvious in many GH/IGF-1 mutant mice as well as dogs and horses (14–18) and humans (19,20). Individual contributions of each hormone to body growth have been determined using knockout mice (21). This study estimated that GH accounted for 14%, IGF-1 contributed 35%, and the overlapping actions of both contributed to 34% of total postnatal growth in mice. Thus, in mammals, an intact GH/IGF-1 axis is required for normal growth (22). Along with growth, the proliferative drive (neoplastic growth, cancer) stimulated by growth-related hormones is also a consideration with little relevance in invertebrates. High levels of GH and IGF-1 are associated with tumor promotion and accelerated aging (23–28).
The metabolic activity of GH includes but is not limited to actions on glucose regulation, lipolysis, and oxidative metabolism. The actions of GH on glucose regulation and insulin signaling are well known. This hormone is considered a diabetogenic factor in that it counteracts the actions of insulin and promotes insulin resistance. GH elevates plasma glucose concentrations by stimulating gluconeogenesis and glycogenolysis and inhibiting glucose uptake at the tissue level. Humans and transgenic mice with elevated plasma GH levels exhibit hyperinsulinemia, hyperglycemia, and/or insulin resistance (29,30). Greater than 50% of humans with supraphysiological GH levels become diabetic and develop micro- and macrovascular complications associated with hyperglycemia. Whereas GH-deficient and GH-resistant animals exhibit low glucose, low blood insulin, and increased insulin sensitivity (31–34). In contrast to GH, IGF-1 has insulin-like effects and directly stimulates glucose uptake in skeletal muscle (35,36). Hepatocytes (liver) and adipocytes represent additional tissues of significance in glucose homeostasis and insulin signaling that are affected by GH directly, whereas these tissues lack IGF-1 receptors. Importantly, many of the actions of IGF-1 on glucose regulation are indirectly mediated via GH suppression indicating the overall contribution of GH on this key aspect of health and longevity in mammals.
Growth hormone has profound effects on lipid metabolism by promoting lipolysis and thus reducing fat mass; and the impact of GH on adiposity is distinct from that of IGF-1 (37,38). Differential effects of GH and IGF-1 are also observed on lipid parameters (39). High total serum cholesterol and triglyceride levels are observed in GH transgenic mice, mice treated with GH, and in humans with acromegaly, whereas IGF-1 treatment does not affect lipid levels (39–42). GH also increases apolipoprotein E, whereas IGF-1 does not (39). Thus, GH appears to directly affect aspects of lipid metabolism that affect health while IGF-1 lacks a direct action.
The role of GH on aspects of oxidative metabolism and stress resistance have been previously reviewed and indicate direct effects that would intuitively counter health and life-span extension (43). Evidence for the role of GH and GH receptor deficiency on immune metabolism and function are also emerging indicating GH signaling in the control of inflammation in aging (44,45).
A few key papers have examined the specific role of reduced IGF-1 signaling on life span in mice. An early report described studies in IGF-1R heterozygous mice indicating a 26% increase in life span (46). However, the wild-type mice in these studies only lived 18 months and these authors later reported that genetic background specifically affected the results as the life extension was very limited on a C57Bl/6 background (11% in females, no extension or shorter-lived in males; (47)). An additional study supported this finding demonstrating that male IGF-1 receptor heterozygous mice do not live longer than wild-type mice (nor do they exhibit differences in end-of-life pathology), and the extension of longevity in females was very modest (less than 5%; (48)). These investigators concluded that a reduction in circulating IGF-1 levels in mice plays little if any role in delayed aging and longevity.
Germane to this discussion, GH action is responsible for 70%–90% of circulating IGF-1 (49,50). Hepatic IGF-1 receptor gene deletion resulted in mice of normal body weight, length, and development. However, liver-specific GH receptor gene deletion produced mice with decreased body size, low IGF-1, impaired glucose metabolism, and hepatic steatosis (51). A reduction in IGF-1 signaling does not alter life span at nearly the levels observed in reduced GH signaling mutants (5% vs 40%–70%, respectively). In complete agreement, a metanalysis including 42 survival studies examined reduced somatotropic signaling (GH, IGF-1, insulin receptor substrate [IRS]) and found that mice with mutations that alter GH signaling exhibited greater relative reductions in mortality compared to those with mutations that affected either IGF-1 or IRS (52). This report concluded that reductions in GH signaling “robustly” increase median life span, exhibit low heterogeneity in the life-span response (5.6% vs IGF-1 34.1% and IRS 47.2%), and are not sex- or control strain-dependent (52). In contrast, reductions in IGF-1 signaling preferentially extend life span in females and short-lived strains of mice. Moreover, life span is not increased in transgenic mice expressing a GH antagonist where levels of circulating IGF-1 are decreased, and insulin levels and sensitivity are not altered (53). Overall, this evidence supports our work and others that strongly propose that reduced GH rather than the secondary reduction of IGF-1 provides beneficial effects on aging and is the key to longevity in mammalian systems perhaps through stress resistance, reduced inflammation, and insulin sensitivity.
In humans, these signaling pathways are relevant to biological aging with life-span implications that reveal more intricate relationships. Briefly, it is clear that genes within the somatotropic axis and downstream targets of these pathways influence aging and longevity (54–63). For example, studies of centenarians show that enhanced insulin sensitivity is strongly correlated with longevity and that exceptional longevity is associated with reduced IGF-1 (61,64–67). There are also differences in long-lived families indicating that less GH is secreted and is under tighter regulation (68). However, while neither lifelong GH deficiency nor GH resistance consistently extends human life span, they do result in major protection from diabetes, cancer, atherosclerosis, and other age-related diseases (69–74).
We also know that in the case of GH excess in both humans (acromegaly) and mice (transgene expression), life span is severely reduced (75–81). Edema, arthralgia, symptoms of carpal tunnel syndrome, and insulin resistance are among the detriments experienced by individuals administered GH (82). In GH transgenic mice, signs of premature aging are abundant and early death is related to kidney dysfunction and tumor development and progression (83–85). In contrast, IGF-1 transgenic mice do not experience such severe pathological changes as GH transgenic mice (86). Perhaps the natural age-related decline in plasma GH levels (14% per decade during adult human life) and the concomitant decrease in IGF-I that occurs in mammals is a protective mechanism to decrease metabolic activity and cellular division and increase autophagy (87,88).
Although IGF-1 is likely the major determinant of life span in invertebrates, it is GH that affects multiple physiological processes that significantly affect longevity in mammals. Keys that factor into the influence of growth hormone on longevity may be the major role it plays metabolically in vertebrate organisms stemming from the wide expression of GH receptors centrally and peripherally and its regulation of circulating IGF-1 levels among others. In addition, growth hormone’s influence on aging trajectories and longevity is not limited to early- or late-life actions (89,90).
The example provided is indicative of the need to read the literature, know the history, understand the physiology, and cite supportive work. Aging is a very complex field and we have so much more to learn! Let’s strive to discover more about these fascinating hormones and their role in aging biology.
What does it mean to be a Fellow of the Gerontological Society of America? To me, fellowship status in an association is the recognition by your scientific colleagues, your community of peers, that your contributions to the field have been impactful. Contributions not based solely on one’s research productivity but on your service, untold efforts, and time, that aimed to benefit the larger community composed of colleagues, trainees, as well as the public. That said, I believe that one’s training and experiences should be shared to connect and educate various groups, develop, and foster science communication, elevate our understanding of aging through research, all of which will improve/benefit our relationships between scientists, policymakers, and the lay public and reveal how important studying aging biology and gerontology is for all. Thus, I am appreciative of fellowship status in the Gerontological Society of America. My only issue with fellowship—is the word “fellow”—it is an antiquated, exclusive term.
Funding
This work was supported by the National Institutes of Health (AG067734 to H.M.B.-B.); and UND.
Conflict of Interest
None declared.
Author Contributions
H.M.B.-B. is the sole author of the manuscript. H.M.B.-B. drafted the text and created the accompanying figure.
References
1.Fabrizio
P
, Pozza
F
, Pletcher
SD
, Gendron
CM
, Longo
VD
.
Regulation of longevity and stress resistance by Sch9 in yeast
.
Science.
2001
;
292
:
288
–
290
. doi:
10.1126/science.1059497 2.Friedman
DB
, Johnson
TE
.
A mutation in the age-1 gene in Caenorhabiditis elegans lengthens life and reduces hermaphrodite fertility
.
Genetics.
1988
;
118
:
75
–
86
. doi:
10.1093/genetics/118.1.75 3.Kenyon
C
, Chang
J
, Gensch
E
, Rudner
A
, Tabtiang
R
.
A C. elegans mutant that lives twice as long as wild type
.
Nature.
1993
;
366
:
461
–
464
. doi:
10.1038/366461a0 4.Kimura
KD
, Tissenbaum
HA
, Liu
Y
, Ruvkun
G
.
daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans
.
Science.
1997
;
277
:
942
–
946
. doi:
10.1126/science.277.5328.942 5.Tatar
M
, Kopelman
A
, Epstein
D
, Tu
MP
, Yin
CM
, Garofalo
RS
.
A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function
.
Science.
2001
;
292
:
107
–
110
. doi:
10.1126/science.1057987 6.Clancy
DJ
, Gems
D
, Harshman
LG
, et al.
Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein
.
Science.
2001
;
292
:
104
–
106
. doi:
10.1126/science.1057991 7.Melmed
S
, Polonsky
KS
, Larsen
PR
, Kronenberg
H.
Williams Textbook of Endocrinology
.
Philadelphia, PA
:
Elsevier
;
2016
.
8.Bartke
A
, Sun
LY
, Longo
V
.
Somatotropic signaling: trade-offs between growth, reproductive development, and longevity
.
Physiol Rev.
2013
;
93
(
2
):
571
–
598
. doi:
10.1152/physrev.00006.2012 9.Veldhuis
JD
.
Aging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and women
.
Ageing Res Rev.
2008
;
7
:
189
–
208
. doi:
10.1016/j.arr.2007.12.005 10.Brown-Borg
HM
, Borg
KE
, Meliska
CJ
, Bartke
A
.
Dwarf mice and the ageing process
.
Nature
1996
;
384
:
33
. doi:
10.1038/384033a0 11.Flurkey
K
, Papaconstantinou
J
, Miller
RA
, Harrison
DE
.
Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production
.
Proc Natl Acad Sci USA.
2001
;
98
:
6736
–
6741
. doi:
10.1073/pnas.111158898 12.Coschigano
KT
, Clemmons
D
, Bellush
LL
, Kopchick
JJ
.
Assessment of growth parameters and life span of GHR/BP gene-disrupted mice
.
Endocrinology.
2000
;
141
:
2608
–
2613
. doi:
10.1210/endo.141.7.7586 13.Sun
LY
, Spong
A
, Swindell
WR
, et al.
Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice
.
Elife.
2013
;
2
:
e01098
. doi:
10.7554/eLife.01098 14.Bartke
A
.
Histology of the anterior hypophysis, thyroid and gonads of two types of dwarf mice
.
Anat Rec.
1964
;
149
:
225
–
235
. doi:
10.1002/ar.1091490206 15.Miller
RA
, Harper
JM
, Galecki
A
, Burke
DT
.
Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice
.
Aging Cell.
2002
;
1
:
22
–
29
. doi:
10.1046/j.1474-9728.2002.00006.x 16.Rollo
CD
, Carlson
J
, Sawada
M
.
Accelerated aging of giant transgenic mice is associated with elevated free radical processes
.
Can J Zool.
1996
;
74
:
606
–
620
. doi:
10.1139/z96-070 17.Patronek
GJ
, Waters
DJ
, Glickman
LT
.
Comparative longevity of pet dogs and humans: implications for gerontology research
.
J. Gerontol.
1997
;
52A
:
B171
–
B178
. doi:
10.1093/gerona/52a.3.b171 18.Brosnahan
MM
, Paradis
MR
.
Demographic and clinical characteristics of geriatric horses: 467 cases (1989–1999)
.
J Am Vet Med Assoc.
2003
;
223
(
1
):
93
–
98
. doi:
10.2460/javma.2003.223.93 19.He
Q
, Morris
BJ
, Grove
JS
, et al.
Shorter men live longer: association of height with longevity and FOXO3 genotype in American men of Japanese ancestry
.
PLoS One.
2014
;
9
(
5
):
e94385
. doi:
10.1371/journal.pone.0094385 20.Samaras
TT
, Bartke
A
, Rollo
CD.
Human Body Size and the Laws of Scaling: Physiological, Performance, Growth, Longevity, and Ecological Ramifications
.
New York
:
Nova Science Publishers
;
2007
.
21.Lupu
F
, Terwilliger
JD
, Lee
K
, Segre
GV
, Efstratiadis
A
.
roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth
.
Dev Biol.
2001
;
229
(
1
):
141
–
162
. doi:
10.1006/dbio.2000.9975 22.Wang
J
, Zhou
J
, Cheng
CM
, Kopchick
JJ
, Bondy
CA
.
Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth
.
J Endocrinol.
2004
;
180
:
247
–
255
. doi:
10.1677/joe.0.1800247 23.Bartke
A
.
Can growth hormone (GH) accelerate aging? Evidence from GH transgenic mice
.
Neuroendocrinology.
2003
;
78
:
210
–
216
. doi:
10.1159/000073704 24.Steuerman
R
, Shevah
O
, Laron
Z
.
Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies
.
Eur J Endocrinol.
2011
;
164
:
485
–
489
. doi:
10.1530/EJE-10-0859 25.Dal
J
, Leisner
MZ
, Hermansen
K
, et al.
Cancer incidence in patients with acromegaly: acohort study and meta-analysis of the literature
.
J Clin Endocrinol Metab.
2018
;
103
(
6
):
2182
–
2188
. doi:
10.1210/jc.2017-02457 26.Burroughs
KD
, Dunn
SE
, Barrett
JC
, Taylor
JA
.
Insulin-like growth factor-I: a key regulator of human cancer risk?
J Natl Cancer Inst.
1999
;
91
(
7
):
579
–
581
. doi:
10.1093/jnci/91.7.579 27.Yu
H
, Rohan
T
.
Role of the insulin-like growth factor family in cancer development and progression
.
J Natl Cancer Inst.
2000
;
92
(
18
):
1472
–
1489
. doi:
10.1093/jnci/92.18.1472 28.Swerdlow
AJ
, Cooke
R
, Beckers
D
, et al.
Cancer risks in patients treated with growth hormone in childhood: the SAGhE European Cohort Study
.
J Clin Endocrinol Metab.
2017
;
102
:
1661
–
1672
. doi:
10.1210/jc.2016-2046 29.Balbis
A
, Bartke
A
.
Turyn D. Overexpression of bovine growth hormone in transgenic mice is associated with changes in hepatic insulin receptors and in their kinase activity
.
Life Sci.
1996
;
59
:
1362
–
1371
. doi:
10.1016/0024-3205(96)00462-6 30.Dominici
FP
, Balbis
A
, Bartke
A
, Turyn
D
.
Role of hyperinsulinemia on hepatic insulin binding and insulin receptor autophosphorylation in the presence of high growth hormone (GH) levels in transgenic mice expressing GH gene
.
J Endocrinol.
1998
;
159
:
1
–
25
. doi:
10.1677/joe.0.1590015 31.Borg
KE
, Brown-Borg
HM
, Bartke
A
.
Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse
.
Proc. Soc. Exp. Bio. Med.
1995
;
210
:
126
–
133
. doi:
10.3181/00379727-210-43931 32.Dominici
FP
, Arostegui Diaz
G
, Bartke
A
, Kopchick
JJ
, Turyn
D
.
Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice
.
J Endocrinol.
2000
;
166
:
579
–
590
. doi:
10.1677/joe.0.1660579 33.Dominici
FP
, Argentino
DP
, Bartke
A
, Turyn
D
.
The dwarf mutation decreases high dose insulin responses in skeletal muscle, the opposite of effects in liver
.
Mech Ageing Dev.
2003
;
124
:
819
–
827
. doi:
10.1016/s0047-6374(03)00136-2 34.Hauck
SJ
, Bartke
A
.
Free radical defenses in the liver and kidney of human growth hormone transgenic mice: possible mechanisms of early mortality
.
J Gerontol A Biol Sci Med Sci.
2001
;
56
:
B153
–
B162
. doi:
10.1093/gerona/56.4.b153 35.Haluzik
M
, Yakar
S
, Gavrilova
O
, Setser
J
, Boisclair
Y
, LeRoith
D
.
Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance
.
Diabetes
.
2003
;
52
(
10
):
2483
–
2489
. doi:
10.2337/diabetes.52.10.2483 36.Vijayakumar
A
, Yakar
S
, Leroith
D
.
The intricate role of growth hormone in metabolism
.
Front Endocrinol (Lausanne).
2011
;
2
:
32
. doi:
10.3389/fendo.2011.00032 37.Berryman
DE
, List
EO
, Sackmann-Sala
L
, Lubbers
E
, Munn
R
, Kopchick
JJ
.
Growth hormone and adipose tissue: beyond the adipocyte
.
Growth Horm IGF Res.
2011
;
21
(
3
):
113
–
123
. doi:
10.1016/j.ghir.2011.03.002 38.Laron
Z
, Ginsberg
S
, Lilos
P
, Arbiv
M
, Vaisman
N
.
Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity)
.
Clin Endocrinol (Oxf).
2006
;
65
(
1
):
114
–
117
. doi:
10.1111/j.1365-2265.2006.02558.x 39.Ding
J
, Berryman
DE
, Kopchick
JJ
.
Plasma proteomic profiles of bovine growth hormone transgenic mice as they age
.
Transgenic Res.
2011
;
20
(
6
):
1305
–
1320
. doi:
10.1007/s11248-011-9499-5 40.Olsson
B
, Bohlooly-Y
M
, Fitzgerald
SM
, et al.
Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet
.
Endocrinology.
2005
;
146
(
2
):
920
–
930
. doi:
10.1210/en.2004-1232 41.Palmer
AJ
, Chung
MY
, List
EO
, et al.
Age-related changes in body composition of bovine growth hormone transgenic mice
.
Endocrinology.
2009
;
150
(
3
):
1353
–
1360
. doi:
10.1210/en.2008-1199 42.Colao
A
, di Somma
C
, Pivonello
R
, et al.
The cardiovascular risk of adult GH deficiency (GHD) improved after GH replacement and worsened in untreated GHD: a 12-month prospective study
.
J Clin Endocrinol Metab.
2002
;
87
(
3
):
1088
–
1093
. doi:
10.1210/jcem.87.3.8336 43.Brown-Borg
HM
.
Hormonal control of aging in rodents: the somatotropic axis
.
Mol Cell Endocrinol.
2009
;
299
:
64
–
71
. doi:
10.1016/j.mce.2008.07.001 44.Qin
YJ
, Chan
SO
, Chong
KK
, et al.
Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation
.
Proc Natl Acad Sci USA.
2014
;
111
:
18303
–
18308
. doi:
10.1073/pnas.1421815112 45.Spadaro
O
, Goldberg
EL
, Camell
CD
, et al.
Growth hormone receptor deficiency protects against age- related NLRP3 inflammasome activation and immune senescence
.
Cell Rep.
2016
;
14
(
7
):
1571
–
1580
. doi:
10.1016/j.celrep.2016.01.044 46.Holzenberger
M
, Dupont
J
, Ducos
B
, Leneuve
P
, Geloen
A
, Even
PC
.
IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice
.
Nature.
2003
;
421
:
182
–
187
. doi:
10.1038/nature01298 47.Xu
J
, Gontier
G
, Chaker
Z
, Lacube
P
, Dupont
J
, Holzenberger
M
.
Longevity effect of IGF 1R(_/_) mutation depends on genetic background-specific receptor activation
.
Aging Cell.
2014
;
13
:
19
–
28
. doi:
10.1111/acel.12145 48.Bokov
AF
, Garg
N
, Ikeno
Y
, et al.
Does reduced IGF-1R signaling in Igf1r_/_ mice alter aging?
PLoS One.
2011
;
6
:
e26891
. doi:
10.1371/journal.pone.0026891 49.Yakar
S
, Liu
JL
, Stannard
B
, et al.
Normal growth and development in the absence of hepatic insulin-like growth factor I
.
Proc. Natl. Acad. Sci.
1999
;
96
:
7324
–
7329
. doi:
10.1073/pnas.96.13.7324 50.Yakar
S
, Setser
J
, Zhao
H
, et al.
Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice
.
J Clin Invest.
2004
;
113
(
1
):
96
–
105
. doi:
10.1172/JCI17763 51.List
EO
, Berryman
DE
, Funk
K
, et al.
Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles
.
Endocrinology.
2014
;
155
(
5
):
1793
–
1805
. doi:
10.1210/en.2013-2086 52.Garratt
M
, Nakagawa
S
, Simons
MJP
.
Life-span extension with reduced somatotrophic signaling: moderation of aging effect by signal type, sex, and experimental cohort
.
J Gerontol A Biol Sci Med Sci.
2017
;
72
(
12
):
1620
–
1626
. doi:
10.1093/gerona/glx010 53.Coschigano
KT
, Holland
AN
, Riders
ME
, List
EO
, Flyvbjerg
A
, Kopchick
JJ
.
Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span
.
Endocrinology.
2003
;
144
(
9
):
3799
–
3810
. doi:
10.1210/en.2003-0374 54.van Heemst
D
, Beekman
M
, Mooijaart
SP
, et al.
Reduced insulin/IGF-1 signaling and human longevity
.
Aging Cell.
2005
;
4
(
2
):
79
–
85
. doi:
10.1111/j.1474-9728.2005.00148.x 55.Suh
Y
, Atzmon
G
, Cho
MO
, et al.
Functionally significant insulin-like growth factor I receptor mutations in centenarians
.
Proc Natl Acad Sci USA.
2008
;
105
:
3438
–
3442
. doi:
10.1073/pnas.0705467105 56.Bonafè
M
, Barbieri
M
, Marchegiani
F
, et al.
Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control
.
J Clin Endocrinol Metab.
2003
;
88
:
3299
–
3304
. doi:
10.1210/jc.2002-021810 57.Bonafè
M
, Olivieri
F
.
Genetic polymorphism in long-lived people: cues for the presence of an insulin/IGF-pathway-dependent network affecting human longevity
.
Mol Cell Endocrinol.
2009
;
299
:
118
–
123
. doi:
10.1016/j.mce.2008.10.038 58.Willcox
BJ
, Donlon
TA
, He
Q
, et al.
FOXO3A genotype is strongly associated with human longevity
.
Proc Natl Acad Sci USA.
2008
;
105
(
37
):
13987
–
13992
. doi:
10.1073/pnas.0801030105 59.Tazearslan
C
, Huang
J
, Barzilai
N
, Suh
Y
.
Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles
.
Aging Cell.
2011
;
10
:
551
–
554
. doi:
10.1111/j.1474-9726.2011.00697.x 60.Junnila
RK
, List
EO
, Berryman
DE
, Murrey
JW
, Kopchick
JJ
.
The GH/IGF-1 axis in ageing and longevity
.
Nat Rev Endocrinol.
2013
;
9
(
6
):
366
–
376
. doi:
10.1038/nrendo.2013.67 61.Milman
S
, Atzmon
G
, Huffman
DM
, et al.
Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity
.
Aging Cell.
2014
;
13
:
769
–
771
. doi:
10.1111/acel.12213 62.van der Spoel
E
, Rozing
MP
, Houwing-Duistermaat
JJ
, et al.
Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden Longevity Study
.
Aging (Albany NY).
2015
;
7
(
11
):
956
–
963
. doi:
10.18632/aging.100841 63.Ben-Avraham
D
, Govindaraju
DR
, Budagov
T
, et al.
The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature
.
Sci Adv.
2017
;
3
(
6
):
e1602025
. doi:
10.1126/sciadv.1602025 64.Paolisso
G
, Gambardella
A
, Ammendola
S
, et al.
Glucose tolerance and insulin action in healthy centenarians
.
Am J Physiol.
1996
;
270
(
5 Pt 1
):
E890
–
E894
. doi:
10.1152/ajpendo.1996.270.5.E890 65.Paolisso
G
, Barbieri
M
, Rizzo
MR
, et al.
Low insulin resistance and preserved beta-cell function contribute to human longevity but are not associated with TH-INS genes
.
Exp Gerontol.
2001
;
37
(
1
):
149
–
156
. doi:
10.1016/s0531-5565(01)00148-6 66.Kojima
T
, Kamei
H
, Aizu
T
, et al.
Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways
.
Exp Gerontol.
2004
;
39
(
11–12
):
1595
–
1598
. doi:
10.1016/j.exger.2004.05.007 67.Vitale
G
, Brugts
MP
, Ogliari
G
, et al.
Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians’ offspring
.
Aging (Albany NY).
2012
;
4
(
9
):
580
–
589
. doi:
10.18632/aging.100484 68.van der Spoel
E
, Jansen
SW
, Akintola
AA
, et al.
Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity
.
Aging Cell.
2016
;
15
(
6
):
1126
–
1131
. doi:
10.1111/acel.12519 69.Aguiar-Oliveira
MH
, Oliveira
FT
, Pereira
RM
, et al.
Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene
.
J Clin Endocrinol Metab.
2010
;
95
:
714
–
721
. doi:
10.1210/jc.2009-1879 70.Krzisnik
C
, Kolacio
Z
, Battelino
T
, Brown
M
, Parks
JS
, Laron
Z
.
The “Little People” of the island of Krk-revisited. Etiology of hypopituitarism revealed
.
J Endocr Genet.
1999
;
1
:
9
–
19
.
71.Krzisnik
C
, Grguri´c
S
, Cvijovi´c
K
, Laron
Z
.
Longevity of the hypopituitary patients from the island Krk: a follow-up study
.
Pediatr Endocrinol Rev.
2010
;
7
:
357
–
362
.
72.Guevara-Aguirre
J
, Balasubramanian
P
, Guevara-Aguirre
M
, et al.
Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans
.
Sci Transl Med.
2011
;
3
:
70ra13
. doi:
10.1126/scitranslmed.3001845 73.Laron
Z
.
Lessons from 50 years of study of Laron Syndrome
.
Endocr Pract.
2015
;
21
(
12
):
1395
–
1402
. doi:
10.4158/EP15939.RA 74.Nashiro
K
, Guevara-Aguirre
J
, Braskie
MN
, et al.
Brain structure and function associated with younger adults in growth hormone receptor-deficient humans
.
J Neurosci.
2017
;
37
:
1696
–
1707
. doi:
10.1523/JNEUROSCI.1929-16.2016 75.Bengtsson
BA
, Edén
S
, Ernest
I
, Odén
A
, Sjögren
B
.
Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984
.
Acta Med Scand.
1988
;
223
(
4
):
327
–
335
. doi:
10.1111/j.0954-6820.1988.tb15881.x 76.Orme
SM
, McNally
RJ
, Cartwright
RA
, Belchetz
PE
.
Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group
.
J Clin Endocrinol Metab.
1998
;
83
:
2730
–
2734
. doi:
10.1210/jcem.83.8.5007 77.Wolf
E
, Kahnt
E
, Ehrlein
J
, Hermanns
W
, Brem
G
, Wanke
R
.
Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models
.
Mech Ageing Dev.
1993
;
68
(
1–3
):
71
–
87
. doi:
10.1016/0047-6374(93)90141-d 78.Cecim
M
, Bartke
A
, Yun
YS
, Wagner
T
.
Expression of human, but not bovine growth hormone genes promotes development of mammary tumors in transgenic mice
.
Transgenics.
1994
;
1
:
431
–
437
.
80.Dekkers
OM
, Biermasz
NR
, Pereira
AM
, Romijn
JA
, Vandenbroucke
JP
.
Mortality in acromegaly: a metaanalysis
.
J Clin Endocrinol Metab.
2008
;
93
(
6
):
1
–
67
. doi:
10.1210/jc.2007-1191 81.Ritvonen
E
, Löyttyniemi
E
, Jaatinen
P
, et al.
Mortality in acromegaly: a 20-year follow-up study
.
Endocr Relat Cancer.
2016
;
23
(
6
):
469
–
480
. doi:
10.1530/ERC-16-0106 82.Blackman
MR
, Sorkin
JD
, et al.
Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial
.
JAMA.
2002
;
288
:
2282
–
2292
. doi:
10.1001/jama.288.18.2282 83.Wanke
R
, Wolf
E
, Hermanns
W
, Folger
S
, Buchmuller
T
, Brem
G
.
The GH-transgenic mouse as an experimental model for growth research: clinical and pathological studies
.
Horm Res.
1992
;
3
:
74
–
87
. doi:
10.1159/000182406 84.Steger
RW
, Bartke
A
, Cecim
M
.
Premature aging in transgenic mice expressing growth hormone genes
.
J. Repro. Fertil. Suppl.
1993
;
46
:
61
–
75
.
85.Yang
XF
, Beamer
W
, Huyn
HT
, Pollak
M
.
Reduced growth of human breast cancer xenografts in host homozygous for the “lit” mutation
.
Cancer Res.
1996
;
56
:
1509
–
1511
.
86.Doi
T
, Striker
LJ
, Quaife
C
, et al.
Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulin like growth factor-1
.
Am J Pathol.
1988
;
131
:
398
–
403
.
87.Iranmanesh
A
, Lizarralde
G
, Veldhuis
JD
.
Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men
.
J Clin Endocrinol Metab.
1991
;
73
(
5
):
1081
–
1088
. doi:
10.1210/jcem-73-5-1081 88.Zhang
WB
, Ye
K
, Barzilai
N
, Milman
S
.
The antagonistic pleiotropy of insulin-like growth factor 1
.
Aging Cell.
2021
;
20
(
9
):
e13443
. doi:
10.1111/acel.13443 90.Junnila
RK
, Duran-Ortiz
S
, Suer
O
, et al.
Disruption of the GH receptor gene in adult mice increases maximal longevity in females
.
Endocrinology.
2016
;
157
(
12
):
4502
–
4513
. doi:
10.1210/en.2016-1649
© The Author(s) 2022. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail:
[email protected].
PDF




