-
PDF
- Split View
-
Views
-
Cite
Cite
Millissia Ben Maamar, Eric E Nilsson, Michael K Skinner, Epigenetic transgenerational inheritance, gametogenesis and germline development†, Biology of Reproduction, Volume 105, Issue 3, September 2021, Pages 570–592, https://doi.org/10.1093/biolre/ioab085
Close - Share Icon Share
Abstract
One of the most important developing cell types in any biological system is the gamete (sperm and egg). The transmission of phenotypes and optimally adapted physiology to subsequent generations is in large part controlled by gametogenesis. In contrast to genetics, the environment actively regulates epigenetics to impact the physiology and phenotype of cellular and biological systems. The integration of epigenetics and genetics is critical for all developmental biology systems at the cellular and organism level. The current review is focused on the role of epigenetics during gametogenesis for both the spermatogenesis system in the male and oogenesis system in the female. The developmental stages from the initial primordial germ cell through gametogenesis to the mature sperm and egg are presented. How environmental factors can influence the epigenetics of gametogenesis to impact the epigenetic transgenerational inheritance of phenotypic and physiological change in subsequent generations is reviewed.
Introduction
The germ line is an enduring link between all generations of a species. After the fertilization of the oocyte by the sperm, a totipotent zygote will give rise to all cell lineages of the organism, including the germ line itself. The primordial germ cells (PGCs) are the precursor pluripotent stem cells for the sperm and egg. They are established during the perigastrulation epiblast stage of the mammalian embryo. The PGCs specification is regulated by a unique and complex gene network induced by signals from extra-embryonic tissues [1]. In the gonads, these PGCs will differentiate into the male prospermatogonia or female oogonia in response to Sertoli or granulosa cell signaling. The prospermatogonia continue in the gametogenesis process and undergo spermatogenesis to develop into the mature sperm. The oogonia continue into the gametogenesis process and undergo oogenesis to develop into the mature oocyte. Therefore, gametogenesis can be seen as a crucial first step for the perpetuation of the mammalian life cycle [2].
The crucial aspect of gametogenesis is the production of genetically and epigenetically competent gametes, which are necessary for normal fertilization and the organism’s development. Epigenetics is defined as the factors and processes around DNA that require genome activity independent of DNA sequence, and are mitotically stable. The components include DNA methylation, histone modifications, and non-coding RNA chromatin structure that regulate gene activity independent of DNA sequence [3]. Maternal and paternal gametes display genomic imprinted differences due to DNA methylation and other epigenetic modifications established in the germ line during gametogenesis, but this is only a small component of the epigenome and its regulation of biology.
Interestingly, previous studies have shown that epigenetic modifications can occur during gametogenesis under the influence of environmental factors (stress, diet, pollutants, etc.), which can lead to phenotypical defects in the individuals exposed and in the subsequent generations through the germline. This non-genetic form of inheritance is termed epigenetic transgenerational inheritance and is mediated through epigenetic alterations (i.e. epimutations) in the sperm or egg.
The current review presents the molecular basis of germ cell development (i.e. gametogenesis), and also how the environment can induce stable epimutations and modified phenotypes through the transgenerational inheritance phenomenon.
Mammalian gametogenesis and primordial germ cell development
Primordial germ cell specification
The totipotent stem cells derived from the zygote are the product of fertilization of the oocyte by the sperm, which gives rise to all cell lineages of an organism, and the germline itself. Thus, the specification of primordial germ cells (PGCs) is a pivotal first step for the acquisition of the germline pluripotent cell and the continuation of the mammalian life cycle [1]. In metazoans, two different processes form the germline in the male and female, giving rise to sperm and oocytes. Caenorhabditis elegans and Drosophila melanogaster have been used to describe the mechanism for PGC specification in invertebrates, and Zebrafish and Xenopus in non-mammalian vertebrates [4–7]. At the onset of development, preformation of germ plasm segregates the germ and the soma. The germ plasm is comprised of RNA, proteins, and organelles that are grouped in a specific location within the oocytes, then allocated to a few cells in the germline of the developing embryo [8]. In this instance, the germline is always differentiated from somatic cells across generations. Alternatively, in mammals, the germline is induced within a population of pluripotent cells. The ectopic expression of germline genes in the soma can be tumorigenic [9], however, specification failure in the germline is a reproductive dead end. This specification process requires a precise orchestration to ensure a timely restriction from the soma. Somatic cells not allocated to the germline will undergo differentiation and perish, whereas the germline has the ability of establishing a new organism in the next generation [10]. The primordial germ cells are specified during early embryonic development. Bone morphogenic protein (BMP) signaling is indispensable for PGC specification, and targeted disruption of Bmp2, Bmp4, Bmp8b, or BMP signaling transducers Smad1, Smad4, Smad5 or Alk2, all demonstrate loss or reduced numbers of PGC [11–16].
The first phase of gametogenesis happens in early embryogenesis, during the formation and migration of PGCs into the gonadal ridge [17, 18]. Mouse models have been primarily used to study the mammalian germ cell development. In the early post-implantation embryo epiblast, PGCs specification is initiated. At mouse embryonic day E6.25 in some pluripotent epiblast cells, bone morphogenetic protein (BMP) and WNT signals from extra-embryonic tissues to induce the expression of a key regulator of PGC fate: the PR domain zinc-finger protein 1 (PRDM1, also known as BLIMP1) [2, 19]. Two other factors are upregulated next, PRDM14 and the transcription factor AP2γ (encoded by Tfap2c) [20, 21]. The germ cell fate is then induced by the transcription factor network formed by PRDM1, PRDM14, and AP2γ [22–24]. This tripartite network suppresses somatic gene expression such as Hoxa1, Hoxb1, Lim1, Evx1, Fgf8, and Snail genes, while initiating the germ cell transcriptional program but also setting off a genome-wide epigenetic reprogramming [13, 20, 22, 25–28]. Interestingly, Blimp1 and Prdm14 have distinct binding patterns relative to promoters, whereas Blimp1 is important for the repression of almost all genes usually downregulated in PGCs, as well as for the restoration of pluripotency and epigenetic reprogramming (Figure 1) [29]. The restoration of pluripotency and epigenetic reprogramming are regulated by Prdm14, independently from Blimp1, that defines a novel genetic pathway with strict specificity to the germ cell lineage [30]. In mice, the knockout (KO) of Blimp1, Prdm14, or Ap2γ result in PGCs specification defects highlighting the fact that these three factors are dominant coordinators of the transcriptional program for the establishment of the germ cell fate. In addition, the concomitant overexpression of these three factors in cells in vitro induces mouse germ cell formation in the absence of cytokines [22] shows again the importance of these three transcription factors. After embryonic day 7 (E7) in the mouse, the PGCs are then specified and express PGC-characteristic markers, such as stage-specific embryonic antigen 1 (SSEA1) or developmental pluripotency associated 3 (DPPA3 or STELLA) [31–34]. The expression of several pluripotent genes is also maintained in the PGCs such as Nanog, Oct4 and SRY (sex-determining region-Y) [35–38]. Sybirna and collaborators recently revealed a crucial role for PRDM14 in human germ cell fate, where a loss of function affects the efficiency of specification and results in an aberrant hPGCLC transcriptome. Moreover, their study showed that PRDM14 targets are not conserved between mouse and human, which highlights the evolutionary divergence in the molecular network for PGC specification [39].
![Human germline development. Just after fertilization, a zygote is formed. At week 1, the blastocyst develops and contains pluripotent epiblast cells, which will give rise to all lineages in the embryo, including the germ line. At week 2, the blastocyst implants into the uterine wall. The human primordial germ cells (hPGCs) are probably specified around the time of gastrulation around week 3. At week 4, the hPGCs are localized near the yolk sac wall close to the allantois. After that stage, the hPGCs migrate through the hindgut to the developing genital ridges. At this developmental stage, the migrating hPGCs go through a genome-wide epigenetic reprogramming, including global DNA demethylation, to erase imprints and other somatic epigenetic marks. During the fetus development and adult life, the germ line will undergo meiosis and gametogenesis to differentiate into sperm and eggs. At the same time, the genome is remethylated and acquires appropriate epigenetic signatures for the generation of a totipotent zygote upon fertilization (modified from [1]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolreprod/105/3/10.1093_biolre_ioab085/2/m_ioab085f1.jpeg?Expires=1750871241&Signature=hGSuh1gwDC6fGq2VHoYAQR7tcM4adCy4REwdMy-y-4U43irB~IFf0zmLSJmGKr4ljoS5jccHb~y8YKwVLGpOLh4kD3xJI8bPqjdxXW1NN-jEP8K1pw7YGr-sdjK~IXTh5BbAw-DuM1xk2D7UcCr4bIu1CoilZZXCDhquBXFza27vRcMWp2uhFWobQgDDd672Y4H2-RF98AbhbQep071UzqU6IVJomSMWcpId984vD1Dv0iHXhMzOjUcpgk9rQH1Gk3NudDoBzYWWEdGVrIxo4PgE~Lye~U3SLsTEuL84BY4NIDBCTYmxMFH0XNP2E2JWUx0YAYIPCYg4Lkr3U-VUeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Human germline development. Just after fertilization, a zygote is formed. At week 1, the blastocyst develops and contains pluripotent epiblast cells, which will give rise to all lineages in the embryo, including the germ line. At week 2, the blastocyst implants into the uterine wall. The human primordial germ cells (hPGCs) are probably specified around the time of gastrulation around week 3. At week 4, the hPGCs are localized near the yolk sac wall close to the allantois. After that stage, the hPGCs migrate through the hindgut to the developing genital ridges. At this developmental stage, the migrating hPGCs go through a genome-wide epigenetic reprogramming, including global DNA demethylation, to erase imprints and other somatic epigenetic marks. During the fetus development and adult life, the germ line will undergo meiosis and gametogenesis to differentiate into sperm and eggs. At the same time, the genome is remethylated and acquires appropriate epigenetic signatures for the generation of a totipotent zygote upon fertilization (modified from [1]).
Although, most studies have been conducted in mice, recent findings in non-rodent mammals have highlighted the similarities and differences between species. In humans, the PGC formation occurs in the third week of gestation. In vitro studies of human PGCs have shown that these cells originate from mesodermal precursor cells, and BMP and WNT signaling pathways are also essential for specification [1, 40, 41]. In contrast to mice, human PGCs lack Sox2 expression. Therefore, the species differences between human and mice PGC transcriptional network may be explained by the differences in either pluripotency circuitry or embryonic origin [40].
Primordial germ cell migration
Just after their specification, between E7.5 and E10.5, while proliferating the PGCs migrate through the hindgut and genital ridge then into the developing gonads to differentiate into gametes [27, 42, 43]. Two germ cell–soma signaling pathways cKIT-STEEL and SDF-CXCR4 are required for the normal migration of PGCs. In mice, germ cells express c-KIT, whereas STEEL is expressed in the somatic cells lining the route to the gonad. The interaction between c-KIT and STEEL is fundamental for PGC proliferation, survival, and migration from the primitive streak to the genital ridge [44–48]. Sterility because of a lack of spermatogonial stem cells and thus differentiated germ cells has been observed in homozygous cKit and Steel mutant mice [46, 49–51]. The chemoattractant stromal cell-derived factor 1 (SDF-1) expressed at the genital ridges in the surrounding mesenchyme also facilitates the directional PGC migration. SDF-1 is detected by its receptor C-X-C chemokine receptor type 4 (CXCR4) expressed on the surface of PGCs [47]. A knockdown of the activity of CXCR4b and of the SDF-1a ligand has been shown to result in severe PGC migration defects such as very few PGCs reaching the genital ridge [52]. Alternatively, the migration of PGCs can be redirected toward sites of ectopically expressed SDF-1a [52–54]. This ectopic expression of SDF-1 can account for the development of some extra-gonadal cell tumors in humans [27].
During their migration, PGCs continue to proliferate and reach 500 cells in each fetal gonad at E10.5 in the mouse [55]. At this stage, the PGC differentiate into oogonia in females or gonocytes (i.e. prospermatogonia) in males. To form germline cysts, between E10.5 and E14.5, the fetal germ cells undergo five additional mitotic divisions with incomplete cytokinesis [56]. These cysts cluster together and will form germ cell nests in both female and male fetal gonads [55–57]. These germ cell nests will then resolve and generate the primary oocytes or prospermatogonia in the differentiated female and male gonads, respectively.
Primordial germ cells and epigenetics
DNA methylation
The first DNA methylation erasure period is incomplete and happens in the early embryo, leaving paternally and maternally imprinted genes intact and in all somatic lineages subsequently derived. The second DNA methylation erasure period is more comprehensive and occurs during the germline specification. The function of the DNA methylation erasure is to generate in the case of the embryo a totipotent stem cell population, and for PGCs a pluripotent stem cell population. However, despite these two epigenetic methylation erasure processes, epigenetic information can be passed down to the offspring, similar to imprinted genes, even though the mechanisms behind this process remain to be elucidated (Figure 2).
![Epigenetic reprogramming (DNA methylation erasure) during primordial germ cell development at gonadal sex determination and following fertilization in the early embryo (modified from [283]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolreprod/105/3/10.1093_biolre_ioab085/2/m_ioab085f2.jpeg?Expires=1750871241&Signature=LvsxGO6oaPkJ44c2kQ9er0eeOuCjDc5efKnfsZSVP8N5gZi9PQGMi7JgXRyzzZJ1AI2qdkHrwjqzD-BjcC-q7nfITHYVlOrK9Kbg6z0EBjdi8s03pEqT4Fszbl5xfIHb-ieTWoDbxMfBFRCGzkLAO4RYK6jBgPYNxIZBZsA-g4hX2EWC9N6v8P7ZdomVCV1GtDEK4IV5BuJGKm~KpZUkCZqzJ~hpdiIV9K65Y7TRZdpgBN02LaL-IV27vrgexeoMYvinSV6IhvXt5E23RkVycWMvKNzPInko-k5xu4oHV6dVhNPRpDKNTNC4r6hdTzcTQMmvmy-2qKrJl3F07C3tFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Epigenetic reprogramming (DNA methylation erasure) during primordial germ cell development at gonadal sex determination and following fertilization in the early embryo (modified from [283]).
Upon specification, during the rapid proliferation of PGCs, the first DNA methylation erasure happens and consists of a passive DNA methylation between E6.5 and E10.5 in the mouse, which results from repressing de novo DNA methyltransferases DNMT3a/b [58–62]. Still, the maintenance of DNA methyltransferase 1 (DNMT1) prevents the dilution of DNA methylation modifications on parentally imprinted regions and meiotic gene promoters [63]. Moreover, a loss of Dnmt1 in PGCs results in a premature meiotic entry in females and precocious differentiation in prospermatogonia in males, and causes infertility [63].
Further demethylation erasure takes place between E10.5 and E12.5 in the mouse, while the PGCs migrate to the genital ridge and begin sex determination. At this period, DNA methylation is at its lowest level due to enzymatic activity from TET1 and TET2 removing DNA methylation [64–66]. During this DNA demethylation erasure window, the maternally and paternally imprinted loci and resistant promoter regions are erased [65, 67]. Interestingly, several studies have demonstrated that a loss of TET1 and 2 in the germ cells does not impact infertility, and few loci showed altered epigenetic states [65, 68–70]. Despite the near complete DNA demethylation erasure process, some genome loci remain methylated in human and mouse PGCs and are referred as ‘escapees’ [58, 64, 71, 72]. In both species, these escapees have been shown to be associated with retrotransposable elements [58, 64, 71–73], subtelomeric regions [71] and pericentrometric satellite repeats [72] also display these escapees.
The PGCs extensive epigenetic reprogramming includes a genome-wide loss of approximately 90% of 5-methylcytosine (5mC) [58, 64, 65, 68, 71, 74–77]. Even though the underlying molecular mechanisms of this process have until recently remained unclear, a set of germline reprogramming responsive (GRR) genes is crucial for the correct progression of PGC development and gametogenesis. These genes show unique promoter sequence characteristics, with high levels of both 5mC and 5-hydroxymethylcytosine (5hmC). These genes are targets of TET1, the ten eleven translocation (TET) enzymes, which oxidize 5mCs and promote locus-specific reversal of DNA methylation [78]. The PRC1 is the canonical polycomb repressive complex PRC1, which promotes compact local chromatin structures and longer-range chromatin interactions [79]. The loss of DNA methylation combined with PRC1 repression is uniquely required for GRR gene activation. In this epigenetically poised state, TET1 is required to potentiate a full and efficient activation. TET1 seems to be especially important in female PGCs [68], since they start meiotic prophase soon after completion of epigenetic reprogramming, thus requiring the timely expression of these genes. A slight hypermethylation at GRR gene promoters in the mouse E14.5 Tet1−/− PGCs, Hill and collaborators also demonstrate that TET1 stimulates transcription of GRR genes via a DNA demethylation-independent mechanism [80, 81]. In addition, TET1 may also enhance transcription through regulation of the levels of 5mC and 5hmC at non-promoter cis-active elements, such as enhancers [79]. TET1 might also have a critical role in the subsequent removal of aberrant residual and/or de novo DNA methylation [79]. This suggests that global reprogramming events require efficient protection from de novo DNA methylation to stabilize the newly acquired epigenetic state after the removal of 5mC. PGC epigenetic reprogramming entails complex erasure of epigenetic information and suggests that to enable gametogenesis, a timely and efficient activation of GRR genes is required [79].
Usually, a loss of DNA methylation in somatic cells induces an ectopic expression of retrotransposons, an anarchic proliferation, and apoptosis [82]. However, PGCs develop normally despite this hypomethylated state. The hypothesis is that the chromatin reorganization could enhance the genome stability and ensure proper chromosome alignment and segregation during mitosis, as well as global transcriptional quiescence during this developmental period [25, 58, 64, 71, 74, 83]. Studies in different models have shown that this process seem to be conserved in multiple species. Experiments with human PGCs have found that these DNA demethylation events follow the same patterns as the ones found in the rodents [72, 73, 84].
Histone marks
In a murine model, before the DNA methylation erasure, the pre-migratory PGCs start a process of reprogramming that erases epigenetic marks. One of the central epigenetic changes in pre-migratory and early migratory mouse PGCs is the loss of H3K9me2. This event is followed by an accumulation of H3K27me3 signal [74, 83, 85]. Eguizabal and collaborators showed in early human gonadal PGCs similar chromatin changes in the human early developing germ line [86]. An erasure of genomic imprints and dynamic changes in chromatin modifications are observed in the mouse PGCs after their entry in the genital ridge [74]. After week 9 of gestation, and similarly to porcine PGCs, human PGCs lose H3K27me3 [86].
Spermatogenesis & spermiogenesis
Male fertility relies on the production by the testis of large numbers of normal spermatozoa. This process is known as spermatogenesis, which can be divided in three major steps: (i) mitosis with the multiplication of the spermatogonia, (ii) meiosis to reduce the number of chromosomes from diploid to haploid cells, which starts with type B spermatogonia into the prophase of the first meiotic division. The cells are then called primary spermatocytes that then divide to form secondary spermatocytes, which will divide and undergo meiosis to form the round spermatids, (iii) and finally, the spermiogenesis, which refers to the successful maturation of round spermatids into spermatozoa [87]. All of these steps are central in the spermatogenic process, and any defect during the spermatogenesis can result in the reduction or absence of sperm production, or production of abnormal sperm (Figure 3).
![Gametogenesis and spermatogenic germ cell stages (modified from [162]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolreprod/105/3/10.1093_biolre_ioab085/2/m_ioab085f3.jpeg?Expires=1750871241&Signature=ku77fxK3C9ZQGadFQM47ccpGTaz1JSNstJlFCO6fV851~PCF4mz8itImPJ4vTpzqjpCDdpwVL1UUlNSk1gbzQPVDvRD2zZThIQ1Y6hkZW8ikOc2T5FJ2ENUWv8hj43iHrqemBQsazrb8wkyoDf-yfBjAIWgNdKGNzPwB2mV5yCWIT5jhtqJS9bDjFvl8cBPgH1r~GtYSBIMb~qVPtcfCTDHSABssG89ca13kC3kLdEwjWNuIB48QzSW7lvW5rMsy6VSFsOLyfBh65Q6fhxpwdWH8Bzm9ws~p~EMo7-d1TWT2HKYvdn7bS56~GJzO~AobqTAti-pN4n-Gc5dRpx38aA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Gametogenesis and spermatogenic germ cell stages (modified from [162]).
Endocrine and paracrine regulation
FSH
Many studies in the rat have defined the stages at which testosterone and follicle stimulating hormone (FSH) act during spermatogenesis. The general consensus, until the mid-1990s, was that FSH was paramount for the initiation of spermatogenesis, but its role in the adult was to maintain a normal quantitative germ cell production [88, 89]. However, the study of transgenic mice lacking FSH or its receptor (FSHR) showed that the males were fertile but with a reduced germ cell number [90–94]. Closer observations of these KO mice revealed a reduction in spermatogonia, spermatocytes, and spermatids numbers, which suggests that FSH increases the number of spermatogonia and facilitates their entry into meiosis [90–94]. However, studies on hpg. SCARKO (hypogonadal mice lacking gonadotrophins and intratesticular androgen crossed with mice lacking androgen receptors specifically on the Sertoli cells) or hpg. ARKO mice (hypogonadal mice lacking gonadotrophins and intratesticular androgen crossed with mice lacking androgen receptors ubiquitously) have shown that FSH does not induce round spermatid formation [95–98].
While a lack of FSH action has been shown to impact spermatogenesis, it is difficult to determine the role of this hormone on maintaining this process. This is explained by the fact that FSH or its receptor are missing from the start of reproductive development. During normal adult spermatogenesis, apoptosis is a sporadic event, occurring mainly among spermatogonia. Lack of FSH, before sexual maturity and during the first wave of spermatogenesis, which is accompanied by an outburst of focal apoptosis among germ cells, increases the level of cells dying which may impact the adult spermatogenesis [99]. Other studies in rats have suggested that FSH-treatment acts to increase spermatogonia and spermatocyte numbers but displays a limited or incomplete effect on spermatogenesis [100, 101]. While these findings mostly agree with the data with the FSHRKO mice, there are significant differences. In these two studies, it was proposed that FSH could promote the completion of meiosis in rats, which was not observed in the transgenic mice models [101, 102]. The mechanism of action of FSH remains unclear even though FSH can act indirectly through Sertoli cells to increase spermatogonial differentiation/proliferation, but also modify rates of germ cell apoptosis [103–107]. A further role for FSH in the testis might be maintenance of Sertoli cell water balance as an accumulation of fluid was observed in FSHRKO mice cells [108]. As a result, this can alter cell morphology and interactions between germ cells and Sertoli cells, thus could reduce normal spermatogenic efficiency.
Androgen
Androgen plays an essential role in development and maintenance of spermatogenesis, which has been emphasized by a study demonstrating that the precocious expression of androgen receptors (ARs) in Sertoli cells leads to premature spermatogenic cell development [109]. The role of androgens has been clearly demonstrated in any animal model in which androgen levels are reduced, such as through hypophysectomy, GnRH-treatment (agonist or antagonist), ethanedimethane sulfonate treatment (EDS) (which ablates Leydig cells), or in gonadotrophin-deficient mice. In all of these cases, significant loss of pachytene spermatocytes and round spermatids, especially at stages VII and VIII of spermatogenesis, can be reversed by a treatment with testosterone [98, 110–117]. Moreover, in mice lacking functional androgen receptors (tfm or ARKO), there is a significant loss of spermatocytes which are also unable to complete meiosis and form round spermatids [118–121]. Androgen maintains indirectly through the Sertoli cells meiosis, which appears to ensure the survival of pachytene spermatocytes and enable diplotene spermatocytes to enter meiotic division [121]. However, the role of androgens in spermiogenesis and spermiation remains unclear in regard to its role on the germ cell niche. Most testis cell types express androgen receptors, except the germ cells. Several studies have concluded that androgen action in the testis is only mediated through somatic cell populations [107, 122, 123].
Estrogen
The physiological role of estrogens in the adult testis has yet to be completely understood. However, some studies suggest that estrogen action is required in the neonate to enable a normal spermatogenesis in adulthood. Estrogen has multiple indirect effects through endocrine regulation and through other tissues on the testis, which makes the study of its action even more difficult. In the adult hpg mouse, estrogens stimulate spermatogenesis by actually stimulating the FSH release from the pituitary [124, 125]. Interestingly, exogenous estrogens inhibit spermatogenesis in normal adult animals by inhibiting LH secretion and intratesticular testosterone levels [126]. In some species, the aromatase activity present in the testis can convert androgens to estrogens, such as the horse testes, which produce large concentrations of estrogens [127, 128]. Several cell types in the testis, including the germ cells, express nuclear estrogen receptors (ERα and ERβ), and the membrane cell receptor GPR30 [129, 130]. In ArKO mice (lacking aromatase), the males are initially fertile, but spermatogenesis degenerates and an arrest is observed in the early stages of spermiogenesis and multinucleated cells in the tubular lumen appear [131]. Moreover, during neonatal period, estrogen-dependent ERα signaling is required for a normal adult spermatogenesis and fertility [132].
Spermiogenesis seems to be clearly affected by estrogens. In fact, after irradiation damage to the testis, estrogens are able to stimulate spermatogonial differentiation [133, 134], which is not correlated to intratesticular testosterone suppression [135]. Numerous studies have shown that estrogens are involved in the early development of spermatogenesis and are able to affect spermatogenesis. However, their role in the normal adult spermatogenesis still needs to be determined.
Activin
The majority of testicular cell types produce activins and activin-related proteins [136]. Even though they can act as hormones, they also behave as growth factors in regard to spermatogenesis. Sertoli cells and germ cells express activin receptors [136]. Culture of stem spermatogonia cells, spermatogonia and spermatocytes has shown that these cells are sensitive to activin [137–139]. Follistatin (FST) and follistatin-like 3 (FSTL3) are two activin-binding proteins that can act as antagonists to activin activity. However, overexpression of FST does not reduce local activin levels but causes infertility without clear effects on FSH levels [140]. In KO mice for FSLT3, an increase in germ cell numbers was observed which was correlated to the increase in Sertoli cell numbers [141]. These different findings suggest that activins have probably a regulatory role in maintaining spermatogenesis and ensuring normal Sertoli cell development and activity.
Spermatogonial formation and renewal
In the mammalian testis, once the primordial germ cells migrate there during fetal development, they associate themselves with the mesenchymal cells, which will later give rise to the Sertoli cells. At this point, the sex cords are formed. The primordial germ cells then differentiate into prospermatogonia and remain centrally positioned in the cords surrounded by immature Sertoli cells. After a period of proliferation, postnatal in the rodent and prenatal in the human, the prospermatogonia migrate to the basement membrane of the sex cords to divide and form type A spermatogonia (Figure 3). Depending on the species, differences have been reported. In the human, A pale, A dark, and B spermatogonia types have been identified [142]. In the rodent testis, multiple type A spermatogonia, intermediate and type B spermatogonia have been reported and their appearance in the testis is temporally controlled. In the rat, around postnatal day 4 or 5, spermatogonial proliferation begins with type B spermatogonia identified at P6. After a series of mitotic divisions, whose mechanisms are only starting to be understood, during puberty the type B spermatogonia develop the capacity to develop into the preleptotene stage of the meiotic process.
In the testis, the migration of primordial germ cells depends on their surface expression of c-kit protein, which is the receptor for stem cell factor (SCF), produced by the immature Sertoli cells. Mutations of the c-kit receptor will result in failure of spermatogenesis due to the absence of germ cells from the testis [143]. An upregulation of SCF mRNA on E5 has been and is concurrent with the beginning of spermatogonial division [144], which indicates an interaction between c-kit and SCF to mediate and modulate spermatogonial proliferation. Other essential growth factors in the fetus testis include transferring growth factor alpha (TGFα) [145] and neurotrophic factors [146].
SCF and c-kit have also been shown to regulate the adult testis survival of spermatogonia and spermatocytes [147]. Rat tubules cultures with FSH have been shown to influence germ cell apoptosis, affecting both mitotic and meiotic cell populations [106, 148]. GDNF and CSF1 signaling are important for spermatogonial stem cell renewal in the stem cell niche [149, 150]. Survival and developmental progression of spermatogonia depend upon expression of several genes, including the transcription factor ID4 [151] and the RNA binding protein NANOS2 [152]. Different sub-populations of spermatogonial stem cells express different genes, depending on whether that population is undergoing self-renewal, differentiation and progression, or replenishment of earlier stem cell stages [153, 154].
Spermatogenic stage germ cell development and epigenetics
Many studies have shown that the dynamics of epigenetic modifications and their regulatory networks are essential for normal spermatogenesis. Any perturbations of these epigenetic modifications is likely to cause degrees of infertility and these perturbations could result in phenotypic defects in subsequent generations [3, 155–157]. Two studies have shown that a high fat or low protein in male mice can alter the metabolic gene expression in the offspring mediated by small noncoding RNAs (sncRNAs) derived from transition ncRNAs [158, 159]. Abnormal DNA methylation is associated with altered histone modifications, dysregulation of ncRNA, abnormal protamination, and all of these contribute to male infertility. In the prospermatogonia, prepachytene piRNAs are necessary for silencing mobile elements through guiding the de novo DNA methylations of transposable elements in order to guarantee genome stability [160]. In late spermatocytes and round spermatids, the pachytene piRNAs could silence the retrotransposon sequences through degrading the 3′UTR of retrotransposon mRNAs or recruiting the DNMT3L to the retrotransposon locus [161]. Most studies on the different spermatogenesis stages have focused on one type of cells. In the field of infertility, histone modifications are mostly studied in the mature sperm. Our lab investigated the developmental alterations in DNA methylation during gametogenesis from PGCs to sperm. Rat fetal PGCs, prospermatogonia, spermatogonia, meiotic pachytene spermatocytes, haploid round spermatids, caput spermatozoa and mature cauda sperm were isolated and purified. Differential DNA methylation regions between each developmental stage involved were compared. The study identified a dynamic cascade of epigenetic changes during development, the most dramatic happening during the early developmental stages, which suggests complex alterations to regulate genome biology and gene expression during gametogenesis [162].
Epididymal maturation and epigenetics
Although spermatogenesis is complete with the formation of the spermatozoa following spermatogenesis, additional maturation of the sperm occurs in the epididymis [163–166]. The spermatozoa released into the seminiferous tubules collect in the rete testes and pass through the efferent ducts into the head of the epididymis called the caput epididymis. The spermatozoa in the caput epididymis go through a further maturation as it passes through the caput to the corpus epididymis and finally, to the cauda epididymis. The caput epididymis spermatozoa do not have the capacity to have motility [167, 168]. During the transit through the epididymis, the epididymal epithelial cells produce proteins that are acquired and modify the maturation of the sperm to then in the cauda epididymis gain the capacity to become motile following ejaculation from the vas deferens where sperm are collected following epididymal maturation and stored. Therefore, the caput spermatozoa undergo a final stage of maturation during epididymal transit to the cauda epididymis to mature and gain the capacity to develop motility. The cauda epididymal sperm are then stored in the vas deferens. The molecular level maturation remains to be fully elucidated, but some aspects of epididymal maturation are known [169, 170].
Epigenetic alterations during epididymal maturation of the sperm largely remain to be elucidated [171]. Although the sperm nuclei is transcriptionally silent due to the compaction of DNA with protamines in testicular spermatogenesis, protein and epididymal components like ncRNA can be passed to the sperm and localized in the head of the sperm in the acrosome vesicle [171]. The localization of epigenetic components like ncRNA in the sperm nuclei remains to be established, but has been speculated in previous literature [172, 173]. Therefore, the role of epididymal ncRNA for sperm epididymal maturation requires further research, but is potentially an important epigenetic aspect of sperm maturation to consider [174].
Recent studies have investigated the epigenetic alterations between the caput epididymal spermatozoa and the mature cauda epididymal sperm. Environmentally induced (DDT and vinclozolin) epigenetic alterations in sperm have been shown to alter differential DNA methylation regions (DMRs) between the caput and cauda epididymal stage sperm [175, 176]. In addition, analysis of histone retention sites in the caput spermatozoa versus cauda sperm have shown differential histone retention regions (DHRs) [177]. Therefore, during epididymal maturation of sperm, there are DNA methylation and histone retention alterations that occur and are a further epigenetic developmental aspect of gamete development.
Oogenesis
Oogenesis involves the production of female gametes called eggs, and begins with the differentiation of primordial germ cells into oogonia at the period of sex determination [178]. In mammals, oogonia proliferate mitotically during fetal life to form a pool of primary oocytes that arrest in the prophase stage of the first meiotic division and stay in this state of meiotic arrest until the female reaches adulthood [179]. In the developing embryo, the nests of arrested oogonia are surrounded by somatic pre-granulosa cells [180]. Interaction and communication between oogonia and the surrounding somatic cells are vital for normal follicle and oocyte development [181, 182]. The oogonia nests subsequently break down, many oogonia undergo programmed cell death, and the pre-granulosa cells migrate to surround each remaining arrested oocyte in a process termed primordial follicle assembly [183, 184] (Figure 4). A primordial follicle is composed of a single oocyte surrounded by a single layer of flattened pre-granulosa cells. Oocytes are maintained in primordial follicles until sexual maturity, at which point follicles begin to be recruited out of the pool of primordial follicles and undergo primordial to primary follicle transition [185, 186].
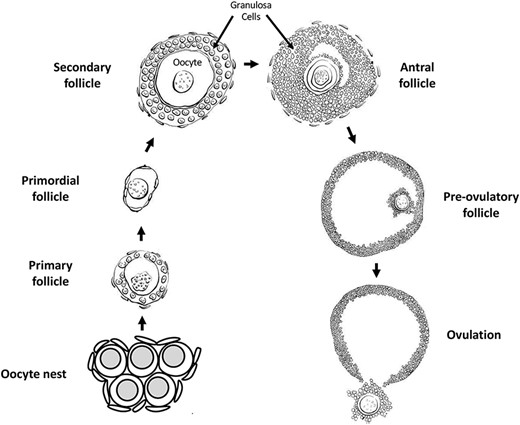
Once a primordial follicle undergoes transition and begins developing, the surrounding flattened pre-granulosa cells become cuboidal and begin proliferating, themselves surrounded by the ovarian stromal cells destined to become theca cells. The follicle is now termed a primary follicle [180]. Subsequent growth and development of the follicle into secondary and pre-antral follicle stages involves continued proliferation of the granulosa cells to form multiple layers, and the initial development of a theca cell layer around the granulosa cells. Follicles with multiple layers of granulosa cells gain sensitivity to follicle stimulating hormone (FSH) secreted by the pituitary, and thus are regulated to grow and develop in cyclic waves in coordination with the estrous cycle [187]. As the granulosa and theca cell layers proliferate a fluid-filled space or antrum forms in the follicle, eventually dividing the granulosa cells into cumulus granulosa surrounding the oocyte and mural granulosa around the inside periphery of the follicle [188]. Extensive cell–cell communication and growth factor signaling between the oocyte and somatic cells occurs at all stages of oogenesis [181, 182]. Most developing follicles do not reach the stage at which ovulation occurs. Rather, follicles at several stages undergo atresia and regress [189–191], (Figure 4).
A luteinizing hormone (LH) surge from the pituitary induces ovulation in late-stage pre-ovulatory follicles, as well as promoting the resumption of meiosis in the oocyte [192]. Meiosis progresses in ovulated oocytes through the production of the first polar body, and then arrests again in metaphase two of the second meiotic division until the time of fertilization. If fertilization occurs, then meiosis again resumes and progresses to completion with the production of the second polar body and the formation of the female pronucleus. Syngamy is the fusion of the male and female pronuclei in the newly formed zygote [193, 194]. Subsequently, the zygotic genome is activated in a carefully controlled manner to allow expression of needed genes in the newly formed individual, while suppressing expression of undesirable genes such as retrotransposons [195, 196].
Epigenetics during oogenesis and in oocytes
Information about the normal epigenetic changes that occur during oogenesis is limited. This is in part due to the difficulty of evaluating developing oocytes in ovaries, and the relatively small number of oocytes available for isolation and study compared to what can be done with male germ cells. Nonetheless, some knowledge of normal epigenomic development in female germ cells has been determined.
Primordial follicle assembly, primordial to primary follicle transition, and many subsequent stages of oogenesis have been shown to be regulated by small non-coding RNA expression, as detailed later in the non-coding RNAs section of Epigenetic Programming During Gametogenesis [197–200], (Figure 4). DNA methylation occurs at sites that are differentially imprinted between male and female gametes so that imprinted genes can be mono-allelically expressed in offspring. DNA methylation is gained gradually on imprinted genes in oocytes in developing follicles after the primary follicle stage, continuing through antral follicle stages of development [201, 202]. In the oocytes of antral stage follicles and later, H3K4Me3 histone methylation increases. This is important for normal function in mature oocytes, and is involved with establishing the DNA methylation pattern in mature oocytes [203–206].
Using in vitro xenogeneic reconstituted ovaries (xrOvaries) with mouse embryonic ovarian somatic cells, Yamashiro and collaborators studied if human primordial germ cell–like cells (hPGCLC) can undergo further development [207]. They observed around 80% of genome-wide 5mC levels in human-induced pluripotent stem cells (hiPSCs) and incipient mesoderm-like cells (iMeLCs) which decreased progressively to around 20% in hPGCLC-derived cells in culture day 77 cells, then dropped at around 13% in culture day 120 cells. Based on their data, the demethylation occurred throughout the genome. Moreover, the 5mC distribution profiles of the culture days 77 to 120 cells were comparable to those observed in the oogonia and gonocytes at weeks 7 to 10. However, they were different to those seen in the blastocysts [208] and naïve human embryonic stem cells (hESCs) [207, 209]. This study demonstrated that hPGCLC-derived cells demethylate their 5mCs similarly to that of oogonia and gonocytes but not early embryonic cells and their putative in vitro counterparts [207].
Epigenetic programming during gametogenesis
Epigenetics
Epigenetics refers to ‘the molecular factors and processes around the DNA that regulate genome activity independent of DNA sequence, and that are mitotically stable’ [3]. These molecular processes include DNA methylation, chromatin structure, histone modifications and retention, non-coding RNAs, and RNA methylation (Figure 5). The epigenome is the complex integration of epigenetic modifications. The first epigenome analysis mapped histone acetylation and methylation in yeast [210]. These epigenetic processes and factors are central for an organism to respond to its environment with changes in gene expression. Moreover, epigenetic mechanisms are required for a stem cell type to develop into a differentiated cell type, which make them an integral part of normal biology [3, 211, 212].
![Epigenetic mechanisms and processes (marks) (modified from [330]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolreprod/105/3/10.1093_biolre_ioab085/2/m_ioab085f5.jpeg?Expires=1750871241&Signature=M1Fyk15KjDgtyMrpjfAtZQTyEyKWJiJucip6xn8OSs7Qz1oRv6hFVKEMnndNuy55a07oLpAegI6xJeWVkB6vRqJXjbZAL27ZBBnqlDsppHFvrzNTblc44Xh-zBM-BIqgvMDH0ksNPrMnKAJQoZmP7t248n8U6Y1YUAb1th6YNSk-HZLhUlDGaTglEAaRq~yTDUwfaMVPdkCtuiCV5OT0a9eYhMCVEstDdjz5wuZVtVISR0s3u1KF5iazLxcw83QwkJMTTfZB~BDUGq3tbu43WjJiHRJxCT~8mXBCylNt9Dhp-kE-iof0s4LNppJ-~zslYu0dculobgOxPbk~CPlbGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Epigenetic mechanisms and processes (marks) (modified from [330]).
DNA methylation and histone modifications
De novo DNA methylation in males restarts in prospermatogonia at E14.5 in mice and is fully established at birth [213, 214]. In both female and male germlines, the factors responsible for mediating DNA methylation DNMT3A or 3B and DNMT3L at imprinted loci were identified [215–217]. In the gametes, the sequence identity and the characteristics of imprinted regions are now well characterized; however, the mechanisms targeting the de novo methyltransferases to imprinted regions remain to be further investigated [218]. In oocytes, the establishment of DNA methylation at imprinted or retrotransposon-rich sites occurs gradually in growing follicles subsequent to the primary follicle stage (reviewed in [201, 202]).
During meiosis of the gametogenesis process, germ cells stop their progress at the prophase stage to allow parental genomes to exchange genetic information through meiosis recombination [219]. At this stage, chromosomes pair in a homologous manner, and large pieces of the chromosomes can be exchanged through crossover events [219, 220]. These crossover events are necessary to maintain euploidy in gametes. An absence of crossover events has been linked to infertility and aneuploidy in the offspring [221, 222]. These meiotic crossover events happen at genomic hotspots and are enriched in regions outside of promoters that bear histone H3K4me3 peaks and established by Prdm9 [223–228]. Mutations in enzymes involved in histone posttranslational modifications observed in meiosis have been shown to have an impact (decrease or increase) on the DNA double-strand break activity, which suggests a role for histone modifications in the initiation and/or repair activity [229–232]. In oocytes, H3K4Me3 histone methylation increases from the antral follicle stage onward, and is important for meiotic recombination, oocyte maturation, oocyte transcriptional activity, and for the establishment of a normal DNA methylation pattern in mature oocytes [203–206, 233, 234].
Chromatin structure & histones
The chromatin reorganization during meiosis is largely transient. The most extensive modifications in chromatin state, structure, or composition occurs after male and female meiosis [220]. In the sperm, the vast majority of histones are replaced with sperm-specific nuclear proteins called protamines [235]. This process is facilitated by different steps: hyperacetylation of histones in round spermatids believed to weaken the interactions between histones and DNA, this will enable the eviction and replacement of histones by testes-specific histone variants, then by transition proteins to end with protamines [236–238]. Because of their endonuclease-inaccessible toroid structure, protamines manage to package the sperm DNA into a tenfold more compact structure than the heterochromatin found in somatic nuclei [239, 240]. The retained histones in the sperm were believed to be remnants of incomplete histone-to-protamine replacement, but recent studies have demonstrated that these retained histones are present at key developmental gene promoters/enhancers in mature sperm. These retained histones bear both active or repressive histone modification [241, 242].
This programmatic retention and evolutionary conservation of histone localization suggests that epigenetic information can be passed through the paternal lineage. Moreover, alteration in histone levels, or chromatin regulators involved in spermatogenesis leads to developmental defects, which can be passed on to the subsequent generation [243, 244]. Altogether, these studies imply that retained histones serve as molecular carriers of epigenetic memory; however, the mechanisms are yet to be elucidated.
Non-coding RNAs
DNA is not the only means to transmit the information between generations. Non-coding RNAs (ncRNAs) are regulatory elements of gene expression and chromatin structure [245]. The differential susceptibility to these non-coding RNAs contributes to tissue-specific gene expression. Early on, ncRNAs are important in the germline development, but they are also crucial players in posttranscriptional gene control during spermatogenesis and oogenesis. Different classes of ncRNAs exist, but this section will focus on microRNAs (miRNAs), Piwi interacting RNAs (piRNAs), and long non-coding RNAs (lncRNAs) and their role in the PGCs and the gametes.
miRNAs
During the PGC specification, some miRNAs are selectively expressed such as miR-10b, −18a, −93, −106b, −126-3p, −127, −181a, −181b, and − 301. All of them have important functions in these cells such as differentiation, migration, and apoptosis in PGCs in mice [246]. For instance, Medeiros and collaborators have shown that in mice a deficiency in miR-290-295 cluster result in an abnormal germ cell with defect in the PGC migration [247]. In the female, an upregulation of miR-29b has been shown to induce PGC development by targeting DNA methyltransferases Dnmt3a and Dnmt3b [248]. In the zebrafish, miR-202-5p has been identified as a potential germ plasma-specific biomarker due to its potential role in the germ cell development [249]. Other microRNAs have been linked to PGC migration in the zebrafish as well, such as miR-430, which regulates sdf1a and cxcr7 mRNAs key transcripts regulating migration [250].
In the early stages of spermatogenesis, different miRNAs have been described in mammals as being crucial for germ cell self-renewal and differentiation. miR-34c has been identified as promoting mouse spermatogonial stem cell (SSCs) differentiation by targeting Nanos2 [251]. Moreover, this miRNA has another role in the later stage of spermatogenesis where miR-34c is involved in apoptotic events of spermatocytes and round spermatids [252], and also in the NOTCH signaling, which is important in the control of germ cell differentiation [253]. A list of miRNAs involved in cell cycle regulation have been identified such as miR-293, 291a-5p, 290-5p and 294 [254]. Other miRNAs are involved in later stages of spermatogenesis. The Let-7 miR family is involved in the mouse spermatogonial differentiation, especially in the maturation of undifferentiated spermatogonia to A1 spermatogonia by suppressing Lin28 [255]. In contrast, some miRNAs such as miR-146 play a crucial role in keeping spermatogonia in an undifferentiated state in the mouse [256]. Other miRNAs play a role in the regulation of meiotic and postmeiotic events in the later stages of spermatogenesis such as the miR-449 cluster. During murine spermatogenesis, the upregulation of miR-449 cluster is crucial for the initiation of meiosis [257]. These miRNAs by targeting BCL2 and AFT1 are involved in germ cell apoptosis [258].
piRNAs
Another class of sncRNAs, piRNAs have been discovered in the germline. Their role is to safeguard the germline genome from retrotransposons and protect the genomic stability [259, 260]. These piRNAs are believed to be involved in pathway components of DNA methylation remodeling during early PGC specification in mammals [209]. Moreover, a loss of Piwi function in mice or zebrafish results in a decrease of germ cells by apoptosis, this underlying its role in germ cell maintenance [261].
lncRNAs
The role of long non-coding RNAs in PGC specification has not been described. Some researchers suggest their possible roles in controlling transcription factors such as BLIMP1/PRDM1 or DAZL [262, 263]. In fact, more than 300 binding sites of BLIMP1/PRDM1 in the murine PGCs are associated with non-coding genes whose functions in PGCs specification are still unknown [23, 262]. The lncRNA-Tcam1 and lncRNA-HSVIII have a crucial role in pachytene spermatocytes, which implies their potential participation in the transcriptional regulation of spermatocyte-specific gene expression [264]. LncRNAs have been linked to functions related to post-transcriptional control during spermatogenesis, such as tubulin cofactor A (TBCA), which has the ability to interact with tubulin during the microtubule rearrangement process [265]. However, most studies have been conducted in rats, so not much is known about lncRNAs in the human. In human spermatogenesis, male infertility has been associated with NLC1-C through the control of miRNA expression via RNA-binding proteins [266].
![Environmentally induced epigenetic transgenerational inheritance. Various exposures and species investigated (modified from [156]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolreprod/105/3/10.1093_biolre_ioab085/2/m_ioab085f6.jpeg?Expires=1750871241&Signature=LyIMHbS~zdL6tWJc~8vnkpKwze1-qv7cSyA0ii7qLQVZ7BESfGawYONnXTsKpCw9ZYN7QU6uvIj6ZBqUzlRuSphjbX69DovNCOUDTzgQ-nID3zaHFgOTI93HkOwLdiCBTo~vVK6VB9wjquP9PxfdxSFeEZzPSgp~oz~JeLCy4DMz1tYmMX1ix5cO0wG~i~zpDM1JKrS6CEDZmb~Lu8oknE3Dcb9iOpVuoPLfWUqU1BOmcEXKCWMRHIWj~DCOiUzctGZpsvwxDF0ePkD2MUvuBftOLh7pD3hfKl4H24LlEQBJ3e~BK7CLqlUZbZ2y4gjUC1GgJTsfI8XVhXeyDl2mnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Environmentally induced epigenetic transgenerational inheritance. Various exposures and species investigated (modified from [156]).
ncRNAs in oocytes
Most of the studies have focused on miRNAs in oogenesis and ovary function using conditional KO mice models to evaluate their involvement in the ovary. By using this approach, a clear role has been outlined for miRNAs in folliculogenesis, oocyte maturation, and ovulation. Other miRNAs are also involved in the assembly of primordial follicles, the transition from primordial to primary follicles, follicular growth, oocyte maturation, ovulation, and the formation of the corpus luteum in mammals [198, 199, 267–269]. After a conditional knockout of Dicer1 from follicular granulosa cells in mammals, abnormal oocyte maturation, disrupted follicular development and ovulation, increased follicular atresia, and infertility were reported [270–272]. Other studies have demonstrated miRNAs involvement in granulosa cells proliferation, survival, terminal differentiation, steroidogenesis, and cumulus expansion [200, 273–281]. The overexpression of miR-143 in murine 15.5 dpc ovaries has been shown to repress the formation of primordial follicles by stopping the proliferation of pre-granulosa cells. An increased number of primordial follicles were observed in transfected 18.5 dpc ovaries with miR-376a (reviewed by Grossman and Shalgi [197]).
Environmental toxicant exposures resulting in epigenetic changes in gametes
In addition to epigenetic changes being a part of the normal developmental process for gametes, it is also possible that exposure to environmental factors will induce abnormal epigenetic changes to the germ cell epigenome [282, 283]. Such changes may be heritable and affect the phenotype of subsequent generations [156, 284]. Exposure of male mice to the endocrine disruptor bisphenol A (BPA) induces changes to DNA methylation in the fetal germ cells of their developing offspring [285]. Primordial germ cells are also induced to alter DNA methylation in response to hypoglycemic conditions in the uterus [286] or from exposure to the agricultural fungicide vinclozolin [287]. Exposure to a wide variety of environmental factors can lead to DNA methylation changes in spermatozoa and mature sperm [288]. In rodents, direct exposure to arsenic [289], the fungal toxin zearalenone [290], the plastics compounds bisphenol A (i.e. BPA) [291] and phthalates [292], the agricultural fungicide vinclozolin [293], the pesticide dichlorodiphenyltrichloroethane (DDT) [294], and the herbicides glyphosate [295] and atrazine [296], all induce sperm DNA methylation changes. In humans, studies have shown that environmental factors such as exposure to phthalates [297], alcohol [298], flame retardants [299, 300], chemotherapy treatment [301], obesity [302], and exercise [303] are correlated to sperm DNA methylation changes (Figure 6). In one fish species, exposure to BPA resulted in changes to oocyte DNA methylation in the next generation [304], (Table 1).
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| BPA | DNA methylation | Fetal germ cells | Zhang et al. (2012) [285] |
| Uterine hypoglycemia | DNA methylation | PGCs | Ren et al. (2018) [286] |
| Arsenic | DNA methylation | Sperm | Nohara et al. (2019) [289] |
| Zearalenone | DNA methylation | Sperm | Gao et al. (2019) [290] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Vinclozolin | DNA methylation | Sperm | Beck et al. (2017) [293] |
| DDT | DNA methylation | Sperm | Skinner et al. (2018) [294] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| BPA | DNA methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Phthalates | DNA methylation | Human sperm | Wu et al. (2017) [297] |
| Alcohol | DNA methylation | Human sperm | Ouko et al. (2009) [298] |
| Flame retardants | DNA methylation | Human sperm | Soubry et al. (2017), Greeson et al. (2020) [299, 300] |
| Chemotherapy | DNA methylation | Human sperm | Shnorhavorian et al. (2017) [301] |
| Obesity | DNA methylation | Human sperm | Soubry et al. (2016) [302] |
| Exercise | DNA methylation | Human sperm | Denham et al. (2015) [303] |
| BPA | Histone acetylation | Fish sperm | Lombo et al. (2019) [305] |
| BPA | histone methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Carbendazim and chlorothalonil | Histone H3K9me3 | Sperm | Li et al. (2018) [306] |
| Zearalenone | Histone H3K27 methylation | Sperm | Gao et al. (2019) [290] |
| Chlordecone | Histone H3K4Me3 | Developing testes | Gely-Pernot et al. (2018) [307] |
| Chlordecone | Histone H3K4Me3 | Oocytes | Legoff et al. (2019) [308] |
| Restraint stress | Histone acetylation, methylation, phosphorylation | Oocytes | Wu et al. (2015) [309] |
| Cigarette smoke | Histone retention | Human sperm | Hamad et al. (2014) [313] |
| Smoke | Histone retention | Human sperm | Lettieri et al. (2020) [314] |
| Caloric restriction in utero | Histone retention and DNA methylation | Sperm | Radford et al. (2014) [315] |
| DDT | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | miRNA | PGCs | Brieno-Enriquez et al. (2015) [317] |
| Early life trauma | miRNA and lncRNA | Sperm | Dickson et al. (2018), Gapp et al. (2014, 2020) [318–320] |
| Early life stress | miRNA | Human sperm | Dickson et al. (2018) [318] |
| Smoking | miRNA | Human sperm | Marczylo et al. (2012) [321] |
| Obesity | miRNA | Human sperm | Lopez et al. (2018), Donkin et al. (2015) [322, 323] |
| Bariatric surgery | miRNA | Human sperm | Donkin et al. (2015) [323] |
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| BPA | DNA methylation | Fetal germ cells | Zhang et al. (2012) [285] |
| Uterine hypoglycemia | DNA methylation | PGCs | Ren et al. (2018) [286] |
| Arsenic | DNA methylation | Sperm | Nohara et al. (2019) [289] |
| Zearalenone | DNA methylation | Sperm | Gao et al. (2019) [290] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Vinclozolin | DNA methylation | Sperm | Beck et al. (2017) [293] |
| DDT | DNA methylation | Sperm | Skinner et al. (2018) [294] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| BPA | DNA methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Phthalates | DNA methylation | Human sperm | Wu et al. (2017) [297] |
| Alcohol | DNA methylation | Human sperm | Ouko et al. (2009) [298] |
| Flame retardants | DNA methylation | Human sperm | Soubry et al. (2017), Greeson et al. (2020) [299, 300] |
| Chemotherapy | DNA methylation | Human sperm | Shnorhavorian et al. (2017) [301] |
| Obesity | DNA methylation | Human sperm | Soubry et al. (2016) [302] |
| Exercise | DNA methylation | Human sperm | Denham et al. (2015) [303] |
| BPA | Histone acetylation | Fish sperm | Lombo et al. (2019) [305] |
| BPA | histone methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Carbendazim and chlorothalonil | Histone H3K9me3 | Sperm | Li et al. (2018) [306] |
| Zearalenone | Histone H3K27 methylation | Sperm | Gao et al. (2019) [290] |
| Chlordecone | Histone H3K4Me3 | Developing testes | Gely-Pernot et al. (2018) [307] |
| Chlordecone | Histone H3K4Me3 | Oocytes | Legoff et al. (2019) [308] |
| Restraint stress | Histone acetylation, methylation, phosphorylation | Oocytes | Wu et al. (2015) [309] |
| Cigarette smoke | Histone retention | Human sperm | Hamad et al. (2014) [313] |
| Smoke | Histone retention | Human sperm | Lettieri et al. (2020) [314] |
| Caloric restriction in utero | Histone retention and DNA methylation | Sperm | Radford et al. (2014) [315] |
| DDT | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | miRNA | PGCs | Brieno-Enriquez et al. (2015) [317] |
| Early life trauma | miRNA and lncRNA | Sperm | Dickson et al. (2018), Gapp et al. (2014, 2020) [318–320] |
| Early life stress | miRNA | Human sperm | Dickson et al. (2018) [318] |
| Smoking | miRNA | Human sperm | Marczylo et al. (2012) [321] |
| Obesity | miRNA | Human sperm | Lopez et al. (2018), Donkin et al. (2015) [322, 323] |
| Bariatric surgery | miRNA | Human sperm | Donkin et al. (2015) [323] |
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| BPA | DNA methylation | Fetal germ cells | Zhang et al. (2012) [285] |
| Uterine hypoglycemia | DNA methylation | PGCs | Ren et al. (2018) [286] |
| Arsenic | DNA methylation | Sperm | Nohara et al. (2019) [289] |
| Zearalenone | DNA methylation | Sperm | Gao et al. (2019) [290] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Vinclozolin | DNA methylation | Sperm | Beck et al. (2017) [293] |
| DDT | DNA methylation | Sperm | Skinner et al. (2018) [294] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| BPA | DNA methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Phthalates | DNA methylation | Human sperm | Wu et al. (2017) [297] |
| Alcohol | DNA methylation | Human sperm | Ouko et al. (2009) [298] |
| Flame retardants | DNA methylation | Human sperm | Soubry et al. (2017), Greeson et al. (2020) [299, 300] |
| Chemotherapy | DNA methylation | Human sperm | Shnorhavorian et al. (2017) [301] |
| Obesity | DNA methylation | Human sperm | Soubry et al. (2016) [302] |
| Exercise | DNA methylation | Human sperm | Denham et al. (2015) [303] |
| BPA | Histone acetylation | Fish sperm | Lombo et al. (2019) [305] |
| BPA | histone methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Carbendazim and chlorothalonil | Histone H3K9me3 | Sperm | Li et al. (2018) [306] |
| Zearalenone | Histone H3K27 methylation | Sperm | Gao et al. (2019) [290] |
| Chlordecone | Histone H3K4Me3 | Developing testes | Gely-Pernot et al. (2018) [307] |
| Chlordecone | Histone H3K4Me3 | Oocytes | Legoff et al. (2019) [308] |
| Restraint stress | Histone acetylation, methylation, phosphorylation | Oocytes | Wu et al. (2015) [309] |
| Cigarette smoke | Histone retention | Human sperm | Hamad et al. (2014) [313] |
| Smoke | Histone retention | Human sperm | Lettieri et al. (2020) [314] |
| Caloric restriction in utero | Histone retention and DNA methylation | Sperm | Radford et al. (2014) [315] |
| DDT | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | miRNA | PGCs | Brieno-Enriquez et al. (2015) [317] |
| Early life trauma | miRNA and lncRNA | Sperm | Dickson et al. (2018), Gapp et al. (2014, 2020) [318–320] |
| Early life stress | miRNA | Human sperm | Dickson et al. (2018) [318] |
| Smoking | miRNA | Human sperm | Marczylo et al. (2012) [321] |
| Obesity | miRNA | Human sperm | Lopez et al. (2018), Donkin et al. (2015) [322, 323] |
| Bariatric surgery | miRNA | Human sperm | Donkin et al. (2015) [323] |
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| BPA | DNA methylation | Fetal germ cells | Zhang et al. (2012) [285] |
| Uterine hypoglycemia | DNA methylation | PGCs | Ren et al. (2018) [286] |
| Arsenic | DNA methylation | Sperm | Nohara et al. (2019) [289] |
| Zearalenone | DNA methylation | Sperm | Gao et al. (2019) [290] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Vinclozolin | DNA methylation | Sperm | Beck et al. (2017) [293] |
| DDT | DNA methylation | Sperm | Skinner et al. (2018) [294] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| BPA | DNA methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Phthalates | DNA methylation | Human sperm | Wu et al. (2017) [297] |
| Alcohol | DNA methylation | Human sperm | Ouko et al. (2009) [298] |
| Flame retardants | DNA methylation | Human sperm | Soubry et al. (2017), Greeson et al. (2020) [299, 300] |
| Chemotherapy | DNA methylation | Human sperm | Shnorhavorian et al. (2017) [301] |
| Obesity | DNA methylation | Human sperm | Soubry et al. (2016) [302] |
| Exercise | DNA methylation | Human sperm | Denham et al. (2015) [303] |
| BPA | Histone acetylation | Fish sperm | Lombo et al. (2019) [305] |
| BPA | histone methylation | Fish oocyte | Zhu et al. (2020) [304] |
| Carbendazim and chlorothalonil | Histone H3K9me3 | Sperm | Li et al. (2018) [306] |
| Zearalenone | Histone H3K27 methylation | Sperm | Gao et al. (2019) [290] |
| Chlordecone | Histone H3K4Me3 | Developing testes | Gely-Pernot et al. (2018) [307] |
| Chlordecone | Histone H3K4Me3 | Oocytes | Legoff et al. (2019) [308] |
| Restraint stress | Histone acetylation, methylation, phosphorylation | Oocytes | Wu et al. (2015) [309] |
| Cigarette smoke | Histone retention | Human sperm | Hamad et al. (2014) [313] |
| Smoke | Histone retention | Human sperm | Lettieri et al. (2020) [314] |
| Caloric restriction in utero | Histone retention and DNA methylation | Sperm | Radford et al. (2014) [315] |
| DDT | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | miRNA | PGCs | Brieno-Enriquez et al. (2015) [317] |
| Early life trauma | miRNA and lncRNA | Sperm | Dickson et al. (2018), Gapp et al. (2014, 2020) [318–320] |
| Early life stress | miRNA | Human sperm | Dickson et al. (2018) [318] |
| Smoking | miRNA | Human sperm | Marczylo et al. (2012) [321] |
| Obesity | miRNA | Human sperm | Lopez et al. (2018), Donkin et al. (2015) [322, 323] |
| Bariatric surgery | miRNA | Human sperm | Donkin et al. (2015) [323] |
Epigenetic changes to histones in germ cells can occur after exposure to environmental factors. For example, zebrafish exposed to BPA showed decreased sperm histone acetylation, as well as impaired primordial germ cell migration, although these findings were not associated with decreased fertility [305]. BPA exposure in a minnow species resulted in changes in oocyte histone methylation in the offspring [304]. Pubertal exposure of mice to the fungicides carbendazim and chlorothalonil caused changes in H3K9me3 levels in sperm [306]. Pubertal exposure to the fungal toxin zearalenone altered mouse histone H3K27 methylation [290]. In mice, exposure to the pesticide chlordecone resulted in altered levels of H3K4Me3 in developing testes [307]. Exposure to chlordecone in mice also resulted in changes in H3K4me3 and H4ac in mature oocytes [308]. Even exposure to chronic restraint stress can alter histone acetylation, methylation and phosphorylation in germinal vesicle-stage oocytes [309].
Another way in which environmental factors can alter histones in sperm is to affect which histones are retained as male germ cells develop. As male germ cells undergo spermiogenesis, most of the histones associated with the DNA are replaced by protamines [236, 310]. Protamines help condense and package DNA into the small sperm head. However, some histones are retained, and they are often located near developmental regulatory genes that are expressed early in embryonic development [311]. Exposure to environmental toxicants has been shown to alter retention of histones in sperm [312]. Men exposed to either cigarette smoke [313] or the smoke of surrounding fires [314] have been shown to have an altered ratio of histones to protamines in sperm. In utero exposure to caloric restriction in mice has also been shown to alter histone retention in sperm [315]. In transgenerational studies in rats, it was found that exposure of gestating female F0 generation rats to either DDT or vinclozolin resulted in changes in histone retention in the subsequent transgenerational F3 generation, but interestingly not in the intervening F1 and F2 generations [294, 312, 316]. However, F1, F2 and F3 exposure-lineage generations all showed changes in DNA methylation and non-coding RNA expression (Table 1).
The expression of non-coding RNA (ncRNA) in germ cells is another epigenetic factor that can be responsive to environmental factors. Mice exposed to vinclozolin in utero exhibited changes in micro-RNAs in their primordial germ cells [317]. Rats exposed to either vinclozolin or DDT in utero have been shown to have altered levels of piwi-interacting RNAs (piRNAs) and small tRNA fragments in sperm upon reaching adulthood [294, 316]. Traumatic stress can also be an environmental factor that causes epigenetic changes in sperm. In mouse models of early life trauma, changes in miRNAs and long non-coding RNAs were seen in sperm from exposed males [318–320]. Interestingly, the behavioral and metabolic alterations seen in the resulting offspring were recapitulated by injection of sperm RNAs from traumatized males into fertilized wild-type oocytes [319]. In humans, changes in miRNA expression in sperm have been seen after exposure to early life stress [318], smoking [321], obesity [322, 323], and bariatric surgery [323] (Table 1).
Epigenetic transgenerational inheritance
Exposure to environmental factors, such as toxicants, can induce epigenetic changes in germ cells that can affect the subsequent generations. These epimutation changes are brought to the next generation at fertilization and have the possibility of altering gene expression and phenotype in the developing embryo. Since the germ cell epimutations can affect the earliest stem cells formed in the embryo, then any subsequent cell type in the embryo and adult animal may have epimutations and changes in gene expression that could affect their phenotype [156].
Epigenetic transgenerational inheritance is defined as germline-mediated inheritance of epigenetic information between generations in the absence of continued direct environmental influences that leads to phenotypic variation [3]. When a male or a non-pregnant female is exposed to an environmental factor that can induce epigenetic change, then epimutations can arise in that individual (the F0 generation), and that individual’s germ cells. The germ cells that contribute to forming the next F1 generation were directly exposed to the environmental factor, so epigenetic and phenotypic changes seen in the F1 generation are an example of direct multigenerational exposure, but not transgenerational inheritance [324], (Figure 7). If these F1 generation animals pass on epigenetic and phenotypic changes to the unexposed F2 generation, then this is an example of epigenetic transgenerational inheritance. Similarly, if a pregnant female is exposed to an environmental factor such as a toxicant, the developing F1 generation embryo is directly exposed, and the germ cells in that embryo that go on to form the F2 generation are also directly exposed. Epigenetic and phenotypic changes would have to be seen in subsequent F3 generation or later generations for this to be an example of epigenetic transgenerational inheritance (Figure 7, Table 2) [156, 157, 324].
![Environmentally induced transgenerational epigenetic inheritance. Schematic of multigenerational versus transgenerational environmental exposures (modified from [324]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolreprod/105/3/10.1093_biolre_ioab085/2/m_ioab085f7.jpeg?Expires=1750871241&Signature=Deebu-e0SQ0I68pdcwvrfBerAzD5TxSUgQrglA18XJZTpnQ110sNpilIUAcpSIi4zYAL8elbZkccgv46Cf3QdGbQHAK4GNO3nJhZp5dOzh4grWxD9yRiOhyTlM9~DizbAGE0AWSsNSrroa9lr5IuRO1Rh85eA47dv5MCje8e-aQ~0ZJ372BkDHcm7UoTnpfmH18mDiuSvyI7I7O34sTtF4A3wYp1gF92CkCde8tcHwlL0DJ~rZ4P5papOeMARRhfoTkntJf4Zo6IY2rgdbzwo2a7z3t080v~hcMnnXBvDlSqgAME2OfLTQg-8bT83A9e5kkG8kJeLAIlnsUCPkDWYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Environmentally induced transgenerational epigenetic inheritance. Schematic of multigenerational versus transgenerational environmental exposures (modified from [324]).
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| Several toxicants (review) | DNA methylation | Sperm | Nilsson et al. (2018) [156] |
| DDT | DNA methylation | Sperm | Ben Maamar et al. (2019) [176] |
| DDT | DNA methylation, non-coding RNA, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | DNA methylation | Sperm | Anway et al. (2005) [157] |
| DDT or vinclozolin | Histone retention | Sperm | Ben Maamar et al. (2020) [177] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| Methoxychlor | DNA methylation | Sperm | Manikkam et al. (2014) [331] |
| Glyphosate | DNA methylation and histone retention | Sperm | Ben Maamar et al. (2020) [332] |
| Dioxin | DNA methylation | Sperm | Manikkam et al. (2012) [333] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Jet fuel | DNA methylation | Sperm | Manikkam et al. (2012) [334] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Methylmercury | DNA methylation | Fish sperm | Carvan et al. (2017) [326] |
| Nutrition change | Gene promoter methylation | Pig liver | Braunschweig et al. (2012) [336] |
| Famine | DNA methylation | Human blood cells | Jiang et al. (2020) [337] |
| Genetic manipulation | Histone modifications | Drosophila embryos | Xia et al. (2016) [338] |
| Genetic manipulation | Histone modifications | Caenorhabditis elegans larvae | Kelly et al. (2014) [339] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| Several toxicants (review) | DNA methylation | Sperm | Nilsson et al. (2018) [156] |
| DDT | DNA methylation | Sperm | Ben Maamar et al. (2019) [176] |
| DDT | DNA methylation, non-coding RNA, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | DNA methylation | Sperm | Anway et al. (2005) [157] |
| DDT or vinclozolin | Histone retention | Sperm | Ben Maamar et al. (2020) [177] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| Methoxychlor | DNA methylation | Sperm | Manikkam et al. (2014) [331] |
| Glyphosate | DNA methylation and histone retention | Sperm | Ben Maamar et al. (2020) [332] |
| Dioxin | DNA methylation | Sperm | Manikkam et al. (2012) [333] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Jet fuel | DNA methylation | Sperm | Manikkam et al. (2012) [334] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Methylmercury | DNA methylation | Fish sperm | Carvan et al. (2017) [326] |
| Nutrition change | Gene promoter methylation | Pig liver | Braunschweig et al. (2012) [336] |
| Famine | DNA methylation | Human blood cells | Jiang et al. (2020) [337] |
| Genetic manipulation | Histone modifications | Drosophila embryos | Xia et al. (2016) [338] |
| Genetic manipulation | Histone modifications | Caenorhabditis elegans larvae | Kelly et al. (2014) [339] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| Several toxicants (review) | DNA methylation | Sperm | Nilsson et al. (2018) [156] |
| DDT | DNA methylation | Sperm | Ben Maamar et al. (2019) [176] |
| DDT | DNA methylation, non-coding RNA, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | DNA methylation | Sperm | Anway et al. (2005) [157] |
| DDT or vinclozolin | Histone retention | Sperm | Ben Maamar et al. (2020) [177] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| Methoxychlor | DNA methylation | Sperm | Manikkam et al. (2014) [331] |
| Glyphosate | DNA methylation and histone retention | Sperm | Ben Maamar et al. (2020) [332] |
| Dioxin | DNA methylation | Sperm | Manikkam et al. (2012) [333] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Jet fuel | DNA methylation | Sperm | Manikkam et al. (2012) [334] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Methylmercury | DNA methylation | Fish sperm | Carvan et al. (2017) [326] |
| Nutrition change | Gene promoter methylation | Pig liver | Braunschweig et al. (2012) [336] |
| Famine | DNA methylation | Human blood cells | Jiang et al. (2020) [337] |
| Genetic manipulation | Histone modifications | Drosophila embryos | Xia et al. (2016) [338] |
| Genetic manipulation | Histone modifications | Caenorhabditis elegans larvae | Kelly et al. (2014) [339] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Environmental exposure . | Epigenetic change . | Cell type . | Reference . |
|---|---|---|---|
| Several toxicants (review) | DNA methylation | Sperm | Nilsson et al. (2018) [156] |
| DDT | DNA methylation | Sperm | Ben Maamar et al. (2019) [176] |
| DDT | DNA methylation, non-coding RNA, and histone retention | Sperm | Skinner et al. (2018) [294] |
| Vinclozolin | DNA methylation, non-coding RNA expression, and histone retention | Sperm | Ben Maamar et al. (2018) [316] |
| Vinclozolin | DNA methylation | Sperm | Anway et al. (2005) [157] |
| DDT or vinclozolin | Histone retention | Sperm | Ben Maamar et al. (2020) [177] |
| Glyphosate | DNA methylation | Sperm | Kubsad et al. (2019) [295] |
| Atrazine | DNA methylation | Sperm | McBirney et al. (2017) [296] |
| Methoxychlor | DNA methylation | Sperm | Manikkam et al. (2014) [331] |
| Glyphosate | DNA methylation and histone retention | Sperm | Ben Maamar et al. (2020) [332] |
| Dioxin | DNA methylation | Sperm | Manikkam et al. (2012) [333] |
| BPA | DNA methylation | Sperm | Rahman et al. (2020) [291] |
| Phthalates | DNA methylation | Sperm | Prados et al. (2015) [292] |
| Jet fuel | DNA methylation | Sperm | Manikkam et al. (2012) [334] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
| Methylmercury | DNA methylation | Fish sperm | Carvan et al. (2017) [326] |
| Nutrition change | Gene promoter methylation | Pig liver | Braunschweig et al. (2012) [336] |
| Famine | DNA methylation | Human blood cells | Jiang et al. (2020) [337] |
| Genetic manipulation | Histone modifications | Drosophila embryos | Xia et al. (2016) [338] |
| Genetic manipulation | Histone modifications | Caenorhabditis elegans larvae | Kelly et al. (2014) [339] |
| Vinclozolin | tRNA halves | Sperm | Schuster et al. (2016) [335] |
Epimutations have been reported in transgenerational generation gametes after ancestral exposure to a wide variety of environmental stressors including toxicants [156] and psychological stress [325]. This phenomenon has been observed in several organisms including fish [326] and rodents [157] (reviewed in [156]) (Table 2). Epimutations have been reported in transgenerational F3 generation rat sperm after ancestral exposure to several toxicants [156]. One question that occurs is at what stage of germ cell development do these sperm epimutations arise, or are they continuously present? Two recent studies have addressed this question. The first examined differences in DNA methylation compared to controls after ancestral exposure to DDT [176]. The number of differential DNA methylation regions (DMRs) was determined for the spermatogenic stages of primordial germ cells, prospermatogonia, spermatogonia, pachytene spermatocytes, round spermatids, caput epididymal spermatozoa, and cauda epididymal sperm. It was found that, of the 265 DMRs present in cauda epididymal sperm, 26% arose in the prospermatogonia stage, 25% in spermatogonia, 18% in pachytene spermatocytes, 5% in round spermatids, 12% in caput epididymal spermatozoa, and 14% only arose in cauda epididymal sperm (Figure 3). Similar observations were made with ancestral vinclozolin exposure [327]. This shows that transgenerational changes in DNA methylation arise throughout gametogenesis.
| 1) . | Examine all stages PGCs to gametes for epigenetic and genetic transitions. . |
|---|---|
| 2) | Examine and investigate all the epigenetic processes (DNA methylation, histones, ncRNA, and chromatin structure) for the epigenetic regulation of gametogenesis. |
| 3) | Use systems biology and genome-wide analyses to integrate the epigenetics and genetics of the gametogenesis process. |
| 4) | Incorporate environmentally induced epigenetic transgenerational inheritance into our research and understanding of generational impacts of altering gamete epigenetics. |
| 1) . | Examine all stages PGCs to gametes for epigenetic and genetic transitions. . |
|---|---|
| 2) | Examine and investigate all the epigenetic processes (DNA methylation, histones, ncRNA, and chromatin structure) for the epigenetic regulation of gametogenesis. |
| 3) | Use systems biology and genome-wide analyses to integrate the epigenetics and genetics of the gametogenesis process. |
| 4) | Incorporate environmentally induced epigenetic transgenerational inheritance into our research and understanding of generational impacts of altering gamete epigenetics. |
| 1) . | Examine all stages PGCs to gametes for epigenetic and genetic transitions. . |
|---|---|
| 2) | Examine and investigate all the epigenetic processes (DNA methylation, histones, ncRNA, and chromatin structure) for the epigenetic regulation of gametogenesis. |
| 3) | Use systems biology and genome-wide analyses to integrate the epigenetics and genetics of the gametogenesis process. |
| 4) | Incorporate environmentally induced epigenetic transgenerational inheritance into our research and understanding of generational impacts of altering gamete epigenetics. |
| 1) . | Examine all stages PGCs to gametes for epigenetic and genetic transitions. . |
|---|---|
| 2) | Examine and investigate all the epigenetic processes (DNA methylation, histones, ncRNA, and chromatin structure) for the epigenetic regulation of gametogenesis. |
| 3) | Use systems biology and genome-wide analyses to integrate the epigenetics and genetics of the gametogenesis process. |
| 4) | Incorporate environmentally induced epigenetic transgenerational inheritance into our research and understanding of generational impacts of altering gamete epigenetics. |
Similarly, another study examined transgenerational differences in histone retention in sperm from rats ancestrally exposed to DDT or vinclozolin [177]. In mature sperm nuclei, most histone proteins are replaced by protamines to facilitate packaging the DNA into the small sperm head [328]. However, some histones are retained, sometimes near genes that are important to early embryo development [243, 329]. In this study [177], the sites of differential histone retention (DHR) were determined for the spermatogenic stages of round spermatids, caput epididymal spermatozoa, and cauda epididymal sperm. It was found that, of those DHRs that were present in cauda epididymal sperm, about 50% were present in round spermatids, very few arose in caput epididymal spermatozoa, and 40–50% arose at the cauda epididymal sperm stage. Again, this shows that an environmentally induced cascade of histone retention changes occurs during different stages of spermatogenic development.
Recently, two studies investigated the integration of DMR, ncRNA, and DHR by overlapping the different genomic sites between the different epimutations. Rats were ancestrally exposed to DDT or vinclozolin and the F1, F2, and F3 generations sperm epimutations examined. The chromosomal locations of the DMR, ncRNA, and DHR epimutations were distinct with few overlapping, but present in the same regions with similar genomic features such as CpG deserts. Comparing the F1, F2, and F3 generations provided insights into the integration of the different epimutations and the direct exposure versus transgenerational impacts of exposures. Interestingly, the effects observed in the F3 generation were different than those observed in the direct exposure F1 and F2 generations. Direct exposure impacted the ncRNA in the F1 generation, but to a much lesser extent in the F2 and F3 generations. DMR were predominant in the F1, F2, and F3 generations, whereas the DHRs were observed primarily in the F3 generation. Observations suggest a potential role for ncRNA-directed DNA methylation and DNA methylation-directed histone retention in the environmentally induced epigenetic transgenerational inheritance of altered gametogenesis [294, 316].
Conclusions and future research
Normal fertilization and early embryonic development require the integration of genetic and epigenetic processes in the gametes. Few developmental or physiological processes will not require the integration of these two distinct molecular mechanisms. Understanding the underlying processes and mechanisms in vivo will help us better understand the gametogenesis and formation of the sperm and egg, as well as pathologies such as idiopathic infertility. The past few decades have advanced our knowledge of primordial germ cell and gamete development. This has provided insights into the molecular mechanism involved in related disorders, such as infertility and birth defects. These findings are also promising in the field of reproductive therapies.
Although a genetic focus can identify the genes involved in a developmental process such as gametogenesis, an understanding of the epigenetics is required to clarify how the gene expression is controlled and environmental factors regulate the developmental process. The DNA methylation, histone modifications, ncRNA, and chromatin structure are the epigenetic mechanisms required to regulate the gene expression. Therefore, no development or biological process can be understood without the integration of epigenetics and genetics. This is the case for normal developmental processes like spermatogenesis and oogenesis, as well as abnormal processes involved in pathology. The ability of environmental factors such as nutrition, toxicants, or stress to impact developmental processes like gametogenesis involves alterations in the epigenetics to then impact the basic genetics and gene expression. Since the gametogenesis process produces the gametes, sperm and eggs, alterations in gametogenesis impact the future generations physiology, phenotype, and gametes. This non-genetic form of inheritance termed epigenetic transgenerational inheritance requires manipulation during gametogenesis to obtain epigenetically modified sperm and eggs. Therefore, the current review provides the basic information on the gametogenesis process, epigenetic regulation of the developmental process, and impacts on epigenetic transgenerational inheritance. As the information on epigenetic regulation of gametogenesis is a new area of research, future research is needed to expand our understanding of the integration of epigenetics and genetics in gametogenesis.
The future research suggested includes the following, Table 3. A need to examine all the gametogenesis stages from PGCs to gametes to understand the integration and transition between the different stages of development. A need to integrate all the epigenetic processes and gene expression for a more complete understanding of the epigenetic regulation of gametogenesis. Emphasis on genome-wide analysis and system biology approaches in the study of gametogenesis to fully understand the developmental process and associated pathology. A need for a generational context, to incorporate the environmentally induced epigenetic transgenerational inheritance of gametogenesis alterations into our understanding of gamete development. Without these more complete systems biology approaches, the progress of research and impacts will be diminished.
Acknowledgements
We thank Dr. Jennifer L.M. Thorson for critical review of the manuscript. We thank Dr. Stephanie King for the drawings of the stages of spermatogenesis and oogenesis. We acknowledge Ms. Amanda Quilty for assistance in editing the manuscript, and Ms. Heather Johnson for assistance in preparation of the manuscript.
Conflict of interest: The authors declare no conflict of interest.
Abbreviations
Primordial germ cells (PGCs)
Bone morphogenetic protein (BMP).
Knock-out (KO).
Stromal cell-derived factor 1 (SDF-1).
Chemokine receptor type 4 (CXCR4).
5-methylcytosine (5mC).
Germline reprogramming responsive (GRR).
Ten eleven translocation (TET).
Follicle stimulating hormone (FSH).
Androgen receptors (ARs).
Ethanedimethane sulfonate treatment (EDS).
Follistatin (FST).
Follistatin-like 3 (FSTL3).
Stem cell factor (SCF).
Luteinizing hormone (LH).
Non-coding RNAs (ncRNAs).
MicroRNAs (miRNAs).
Piwi interacting RNAs (piRNAs).
Long non-coding RNAs (lncRNAs).
Tubulin cofactor A (TBCA).
Bisphenol A (BPA).
Differential DNA methylation regions (DMRs).
Differential histone retention sites (DHRs).
Author Contributions
MBM—Writing and editing manuscript.
EN—Writing and editing manuscript.
MKS—Funding acquisition, writing and editing manuscript.
Grant support: This study was supported by John Templeton Foundation (50183 and 61174) (https://templeton.org/) grants to M.K.S. and NIH (ES012974) (https://www.nih.gov/) grant to M.K.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
Carvan MJr, Kalluvila TA, Klingler RH, Larson JK, Pickens M, Mora-Zamorano FX, Connaughton VP, Sadler-Riggleman I, Beck D, Skinner MK.



