-
PDF
- Split View
-
Views
-
Cite
Cite
Nichole Tiernan, Kyoko Nakamura, Christina Burns, Brett Jestrow, Ramona Oviedo Prieto, Javier Francisco-Ortega, Phylogenetic relationships of Cuban and Caribbean Plumeria (Apocynaceae) based on the plastid genome, Biological Journal of the Linnean Society, Volume 140, Issue 3, November 2023, Pages 397–412, https://doi.org/10.1093/biolinnean/blad042
Close - Share Icon Share
Abstract
Plumeria L. (Apocynaceae) is a Neotropical genus mostly restricted to the Caribbean, which is cultivated in tropical gardens worldwide. Almost all ~27 species are endemic to the Caribbean Islands, except for four (Plumeria inodora Jacq., Plumeria obtusa L., Plumeria pudica Jacq., and Plumeria rubra L.) that are native to Central America and northern South America. Cuba harbours the largest diversity of the genus, with 13 endemic species. We present the first molecular phylogeny of the genus based on the nucleotide sequences of the complete plastid genome. Partial plastid genomes of 11 Plumeria species and one closely related species (Himatanthus sp.) were sequenced using long-PCR and next-generation sequencing. The recovered clades mostly grouped by single islands or closely neighboring islands. The phylogenetic relationships indicate patterns of adaptive radiation, with ecological shifts within these island-based clades and a west-to-east colonization route in the region. Plumeria rubra and Himatanthus sp. were resolved as sister to the rest of the genus. One unexpected finding was that P. pudica, a species restricted to the southern Caribbean Basin mainland, was recovered within the clade containing the Caribbean Island endemics, rendering the endemic group polyphyletic. Our study provides a phylogenetic framework for future studies that will need to include nuclear molecular markers and a more extensive sampling.
Resumen
Plumeria L. (Apocynaceae) es un género Neotropical principalmente restringido al Caribe que a menudo se cultiva en jardines tropicales en todo el mundo. Casi todas sus especies (~27) son endémicas de las islas del Caribe, con la excepción de cuatro (P. inodora Jacq., P. obtusa L., P. pudica Jacq., y P. rubra L.) que son nativas de América Central y norte de América del Sur. Cuba alberga la mayor diversidad del género con 13 especies endémicas. Presentamos la primera filogenia molecular del género basada en las secuencias de nucleótidos del genoma completo del cloroplasto. Se obtuvieron secuencias para 11 especies de Plumeria así como para el grupo externo (Himatanthus sp.) utilizando métodos de secuenciación de próxima generación y PCR de larga lectura. Los clados obtenidos se agruparon mayormente por islas individuales o por islas vecinas cercanas. Las relaciones filogenéticas indican pautas de radiación adaptativa con cambios ecológicos dentro de cada isla y una ruta de colonización en la región con una dirección del oeste al este. Plumeria rubra y Himatanthus sp. se muestran como hermanas del resto de las especies de Plumeria incluidas en nuestro estudio. Un hallazgo inesperado fue que P. pudica, una especie endémica del sur de la cuenca del Caribe, forma parte del clado que contiene los endemismos insulares. Esto hace que este conjunto de endemismos formen un grupo polifilético. Nuestro estudio proporciona un marco filogenético para estudios futuros que deberán incluir marcadores moleculares nucleares y un muestreo más extenso en las islas del Caribe y en el continente.
INTRODUCTION
Nearly a century ago Robert E. Woodson Jr. (1904–1963), an American botanist and curator of the Missouri Botanical Garden (1937–1963), published the first revision of the Neotropical genera Plumeria L. and Himatanthus Willd. ex. Schult. (Apocynaceae) (Woodson, 1937). Woodson was an authority on the Apocynaceae and a prolific author (Allen et al., 1965). The only other revision for the genus was done for the single island of Cuba, where most of the diversity for the group is found (Lippold, 1979). Members of Plumeria, commonly known as frangipani, are trees and shrubs found in tropical gardens worldwide and prized for their charismatic flowers. The Caribbean Islands, as defined here, include the Greater and Lesser Antilles and the Bahamas and are the centre of diversity for Plumeria, with many species as single-island endemics (Woodson, 1937; León & Alain, 1957; Adams, 1972; Lippold, 1979; Correll & Correll, 1982; Howard, 1989; Liogier, 1989, 1995).
Apocynaceae Juss. are the second largest family in the order Gentianales, with 374 genera. The two subfamilies of the traditional Apocynaceae (Rauvolfioideae and Apocynoideae) are paraphyletic and treated as informal ranks (Simões et al., 2016; Endress et al., 2019). Endress & Bruyns (2000) were the first to provide a treatment for all genera of the Apocynaceae s.l. with the incorporation of molecular data. Since the study by Endress & Bruyns (2000), Plumeria has consistently been placed in the informal Rauvolfioids and tribe Plumerieae E. Mey. (Endress et al., 2007, 2014, 2019; Simões et al., 2007). Endress et al. (2014) further placed Plumeria in the subtribe Plumeriinae Pichon ex Leeuweb. together with Mortoniella Woodson and Himatanthus. Although the intergeneric relationships still need molecular support (Endress et al., 2019), morphologically the three genera (Himatanthus, Mortoniella and Plumeria) are recognizable from the rest of the tribe owing to their partly inferior ovary (Endress et al., 2019).
Linnaeus (1753) first described Plumeria obtusa, referring to Catesby’s (1743) morphological accounts and illustration. The protologue has a very brief morphological diagnosis (‘Plumeria foliis lanciolatis petiolatis obtusis’) and does not provide a precise locality (‘Habitat in America calidiore’). Dandy (1958) designated Catesby’s (1743) illustration as the lectotype, an illustration that was based on material from the Bahamas. The typification of P. obtusa is discussed in depth by Tiernan et al. (2021). Himatanthus remained monotypic until Woodson (1937) transferred several species of Plumeria to this genus. Morphologically, the two genera are similar, but Himatanthus, with approximately nine recognized species, is restricted to South America (Spina et al., 2013; Endress et al., 2019), and the majority of Plumeria are naturally distributed across the Caribbean Islands, specifically the Greater and Lesser Antilles plus the Bahamas Archipelago (Tiernan et al., 2020). Himatanthus can be distinguished from Plumeria by its large, showy bracts; however, the generic limits are deserving of further revision (Woodson, 1937; Endress et al., 2019). Mortoniella includes only Mortoniella pittieri Woodson, which is restricted to Belize, Nicaragua and Costa Rica (Endress et al., 2019).
Plumeria is recognizable by its corky periderm and conspicuous leaf scars (Woodson, 1937). Leaves are alternate and usually clustered at the ends of the branches. Inflorescences are terminal, cymose and thyrsiform, with small calyx lobes that are glandular at the apex. The corolla is salverform, thick, waxy and white, usually with a yellow throat (except for Plumeria rubra L., which can be pink or red). The ovary is partly inferior, with numerous ovules. Fruits are large follicles with winged seeds (Endress et al., 2019).
Plumeria has 49 names published for the Caribbean Islands (Tiernan et al., 2020, 2021). The genus had not received a thorough revision until Woodson’s (1937) treatment, where he accepted only three species as confined to the Caribbean Islands (Plumeria alba L., Plumeria filifolia Griseb. and Plumeria subsessilis A.D.C.) and three others to South America (Plumeria inodora Jacq., Plumeria pudica Jacq., and P. rubra) (Tiernan et al., 2020). However, the natural distribution of P. rubra is poorly understood owing to its wide cultivation in the Neotropics. Furthermore, Woodson (1937) recognized a P. obtusa L. complex, restricted to the Caribbean Islands, Belize, Swan Islands (Honduras) and Mexico (Yucatan), synonymizing 37 names under P. obtusa.
Woodson (1937) suggested that this species not only occurs in the Caribbean Islands [Bahamas, Cuba, Hispaniola, Jamaica and Puerto Rico, and Swan Island (Honduras)], but also on the mainland [Mexico (Yucatan) and Belize]. Regional floras treated P. obtusa as occurring in the Bahamas archipelago (Correll & Correll, 1982), Cayman Islands (Proctor, 2012), Cuba (León & Alain, 1957), Hispaniola (Liogier, 1989), Jamaica (Adams, 1972) and Puerto Rico (Liogier, 1995). Recently, Acevedo-Rodríguez & Strong (2012) recognized P. obtusa as occurring in the Bahamas, Cayman Islands, Cuba, Hispaniola, Jamaica, Puerto Rico and Central America. However, Lippold (1979), who conducted the most recent and thorough single-island assessment of Plumeria in Cuba, did not consider P. obtusa to occur on this island.
We loosely recognize 27 species as a working taxonomy, as outlined by Tiernan et al. (2020). Plumeria species display high morphological and ecological variations. Many have obovate leaves [e.g. Plumeria krugii Urb. (Puerto Rico), Plumeria lanata Britton (Cuba), Plumeria cubensis Urb. (Cuba), Plumeria jamaicensis Britton (Jamaica) and P. pudica (Leeward Antilles, Northern South America and Panama)]. Several have very distinct morphologies; for instance, the long filiform or needle-like leaves of P. filifolia (Cuba), the very narrow-petalled flowers of Plumeria stenopetala Urb. (Hispaniola) and Plumeria montana Britton & P.Wilson (Cuba) or the distinct tuberculate stems of Plumeria tuberculata G.Lodd. (Hispaniola). Furthermore, most species of Plumeria occur in lowland habitats [e.g. Plumeria emarginata Griseb. (Cuba), P. stenopetala, P. subsessilis (Hispaniola) and P. jamaicensis (Jamaica)]. However, the genus occurs in a wide variety of ecological zones, from high-elevation montane rainforests [e.g. Plumeria marchii Urb. (Jamaica) and Plumeria montana (Cuba)] to serpentine montane slopes [e.g. P. krugii (Puerto Rico) and P. cubensis (Cuba)] and limestone mountainous regions [e.g. Plumeria clusioides Griseb. (Cuba)] (Tiernan et al., 2020).
The Caribbean Islands are considered a global hotspot of biodiversity, containing an extraordinary concentration of endemic plant species, exceptionally endangered by habitat loss and vulnerable to extinction (Myers et al., 2000; Mittermeier et al., 2004; Santiago-Valentin & Olmstead, 2004). As such, there is an urgency to completing an assessment for Plumeria, which has several threatened taxa that have: (1) not been collected since they were described (e.g. Plumeria barahonensis Urb., Plumeria confusa Britton and Plumeria paulinae Urb.); (2) a narrow distribution range (e.g. P. clusioides, P. krugii and P. montana); and (3) poorly understood taxonomic placement (e.g. P. tuberculata and P. obtusa). In the present study, we present the first phylogeny for Plumeria based on the use of extensive sequences of the plastid (cp) genome. A phylogeny for Plumeria might offer new insights on the evolutionary history and diversity patterns, which, in turn, provide scientifically based plant conservation rationale.
Recent studies of angiosperms have shown that large chloroplast DNA (cpDNA) data sets have resulted in well-resolved topologies (Barrett et al., 2016; Areces-Berazain et al., 2020; Celiński et al., 2020). Within the genus Plumeria, only one complete plastid genome had been sequenced before this study, Plumeria rubra (GenBank: MN812495, 153 912 bp; Wang et al., 2020); and one partial plastid genome, Plumeria cubensis (GenBank: MG963231.1, 124 103 bp; Fishbein et al., 2018). See the Material and Methods for P. cubensis collection information, because the authors agree with this identification although they did not see the herbarium specimen. Several partial plastid genomes have also been sequenced previously, representing each subtribe within Plumerieae that occurs in tropical America [Allamanda schottii Pohl (128 603 bp), P. cubensis (124 103 bp), Skytanthus acutus Meyen (128 408 bp) and Cascabela thevetia (L.) Lippold (in GenBank as Thevetia peruviana (Pers.) K.Schum.) (128 309 bp)] (Fishbein et al., 2018). All five of these sequences were included in our analysis. The objective of this study was to identify and interpret the main clades of Plumeria based on a molecular phylogenetic study of nucleotide sequences of the cp genome. We included a representative sampling for the geographical distribution and the morphological diversity of the genus.
MATERIAL AND METHODS
Taxon sampling
There are 27 taxa generally accepted for the genus Plumeria (Tiernan et al., 2020). Our sampling covered around half, including 13 Plumeria taxa and four closely related outgroups. To consider the biogeographical affinities of the genus in the Caribbean Islands, representative taxa from each island where the genus occurs were chosen, including 11 endemic taxa (Table 1). We aimed to represent the genus properly and, as such, we sequenced a broad selection of species spanning the entirety of the geographical and ecological range, while also including species to represent substantial morphological variation.
List of species names, biogeography of species, type of plant material used in the study, voucher information and GenBank accession numbers for individuals sequenced for this study
| Species . | Biogeography . | Provenance . | Plant material used in study . | FTBG accession number/FTBG herbarium voucher number . | GenBank accession number . |
|---|---|---|---|---|---|
| Plumeria alba L. | Puerto Rico, Virgin Islands, Lesser Antilles | Cultivated at FTBG from material acquired from Gemini Botanical Garden | Fresh | 20541 2014-0917/Tiernan NT125 | MW392089 |
| Plumeria filifolia Griseb. | Cuba | Cultivated at FTBG (plots 17, 46 and 49) from material sent from Palmetum of Santa Cruz de Tenerife Botanic Garden, Canary Islands | Fresh | 2019–0067/Tiernan NT122 | MW392092 |
| Plumeria jamaicensis Britton. | Jamaica | Cultivated at FTBG (plot 172), collected at Hector’s River, Portland Parish, Jamaica | Fresh | 90250A/Tiernan NT126 | MW392090 |
| Plumeria krugii Urb. | Puerto Rico | Cultivated at FTBG (plot 172), collected near Sabana Grande, Puerto Rico | Fresh | 2020-0311 A/Tiernan NT127 | MW392093 |
| Plumeria lanata Britton | Cuba | Collected in Parque Nacional Desembarco del Granma, province of Granma, Cuba | Dried | -/Tiernan NT59 | MW392094 |
| Plumeria magna Zanoni & M. Mejía | Dominican Republic | Cultivated at FTBG, collected west of Las Galeras, province of Samaná, Dominican Republic | Fresh | 2014-0461/Jestrow 2013-DR-42 | MW392095 |
| Plumeria montana Britton & Wilson | Cuba | Collected in Parque Nacional Turquino, province of Santiago de Cuba, Cuba | Dried | -/Tiernan NT60 | MW392096 |
| Plumeria obtusa L. | Bahamas, Greater Antilles | Cultivated in FTBG (plot 164), collected on Mangrove Cay, Andros Island, The Bahamas | Fresh | 73213A/Fantz 3672 | MW392097 |
| Plumeria pudica Jacq. | Leeward Antilles, Northern South America, Panama | Unknown origin | Fresh | 2002-0667A/Tiernan NT124 | MW392100 |
| Plumeria subsessilis A.DC. | Hispaniola | Cultivated at FTBG, collected in Boquerón, province of Azua, Dominican Republic | Fresh | 2015-0143/Tiernan DR2015-NT02 | MW392098 |
| Plumeria tuberculata G.Lodd. | Hispaniola, Cuba | Cultivated at FTBG (plot 158), collected above Labadee, Port-au-Prince, Haiti | Fresh | 992117D/Burman, 2012-028 | MW392099 |
| Himatanthus sp. | Brazil | Cultivated at FTBG, acquired from Gemini Botanical Garden | Fresh | 2011-1085.1/- | MW392091 |
| Species . | Biogeography . | Provenance . | Plant material used in study . | FTBG accession number/FTBG herbarium voucher number . | GenBank accession number . |
|---|---|---|---|---|---|
| Plumeria alba L. | Puerto Rico, Virgin Islands, Lesser Antilles | Cultivated at FTBG from material acquired from Gemini Botanical Garden | Fresh | 20541 2014-0917/Tiernan NT125 | MW392089 |
| Plumeria filifolia Griseb. | Cuba | Cultivated at FTBG (plots 17, 46 and 49) from material sent from Palmetum of Santa Cruz de Tenerife Botanic Garden, Canary Islands | Fresh | 2019–0067/Tiernan NT122 | MW392092 |
| Plumeria jamaicensis Britton. | Jamaica | Cultivated at FTBG (plot 172), collected at Hector’s River, Portland Parish, Jamaica | Fresh | 90250A/Tiernan NT126 | MW392090 |
| Plumeria krugii Urb. | Puerto Rico | Cultivated at FTBG (plot 172), collected near Sabana Grande, Puerto Rico | Fresh | 2020-0311 A/Tiernan NT127 | MW392093 |
| Plumeria lanata Britton | Cuba | Collected in Parque Nacional Desembarco del Granma, province of Granma, Cuba | Dried | -/Tiernan NT59 | MW392094 |
| Plumeria magna Zanoni & M. Mejía | Dominican Republic | Cultivated at FTBG, collected west of Las Galeras, province of Samaná, Dominican Republic | Fresh | 2014-0461/Jestrow 2013-DR-42 | MW392095 |
| Plumeria montana Britton & Wilson | Cuba | Collected in Parque Nacional Turquino, province of Santiago de Cuba, Cuba | Dried | -/Tiernan NT60 | MW392096 |
| Plumeria obtusa L. | Bahamas, Greater Antilles | Cultivated in FTBG (plot 164), collected on Mangrove Cay, Andros Island, The Bahamas | Fresh | 73213A/Fantz 3672 | MW392097 |
| Plumeria pudica Jacq. | Leeward Antilles, Northern South America, Panama | Unknown origin | Fresh | 2002-0667A/Tiernan NT124 | MW392100 |
| Plumeria subsessilis A.DC. | Hispaniola | Cultivated at FTBG, collected in Boquerón, province of Azua, Dominican Republic | Fresh | 2015-0143/Tiernan DR2015-NT02 | MW392098 |
| Plumeria tuberculata G.Lodd. | Hispaniola, Cuba | Cultivated at FTBG (plot 158), collected above Labadee, Port-au-Prince, Haiti | Fresh | 992117D/Burman, 2012-028 | MW392099 |
| Himatanthus sp. | Brazil | Cultivated at FTBG, acquired from Gemini Botanical Garden | Fresh | 2011-1085.1/- | MW392091 |
Abbreviation: FTBG, Fairchild Tropical Botanic Garden.
List of species names, biogeography of species, type of plant material used in the study, voucher information and GenBank accession numbers for individuals sequenced for this study
| Species . | Biogeography . | Provenance . | Plant material used in study . | FTBG accession number/FTBG herbarium voucher number . | GenBank accession number . |
|---|---|---|---|---|---|
| Plumeria alba L. | Puerto Rico, Virgin Islands, Lesser Antilles | Cultivated at FTBG from material acquired from Gemini Botanical Garden | Fresh | 20541 2014-0917/Tiernan NT125 | MW392089 |
| Plumeria filifolia Griseb. | Cuba | Cultivated at FTBG (plots 17, 46 and 49) from material sent from Palmetum of Santa Cruz de Tenerife Botanic Garden, Canary Islands | Fresh | 2019–0067/Tiernan NT122 | MW392092 |
| Plumeria jamaicensis Britton. | Jamaica | Cultivated at FTBG (plot 172), collected at Hector’s River, Portland Parish, Jamaica | Fresh | 90250A/Tiernan NT126 | MW392090 |
| Plumeria krugii Urb. | Puerto Rico | Cultivated at FTBG (plot 172), collected near Sabana Grande, Puerto Rico | Fresh | 2020-0311 A/Tiernan NT127 | MW392093 |
| Plumeria lanata Britton | Cuba | Collected in Parque Nacional Desembarco del Granma, province of Granma, Cuba | Dried | -/Tiernan NT59 | MW392094 |
| Plumeria magna Zanoni & M. Mejía | Dominican Republic | Cultivated at FTBG, collected west of Las Galeras, province of Samaná, Dominican Republic | Fresh | 2014-0461/Jestrow 2013-DR-42 | MW392095 |
| Plumeria montana Britton & Wilson | Cuba | Collected in Parque Nacional Turquino, province of Santiago de Cuba, Cuba | Dried | -/Tiernan NT60 | MW392096 |
| Plumeria obtusa L. | Bahamas, Greater Antilles | Cultivated in FTBG (plot 164), collected on Mangrove Cay, Andros Island, The Bahamas | Fresh | 73213A/Fantz 3672 | MW392097 |
| Plumeria pudica Jacq. | Leeward Antilles, Northern South America, Panama | Unknown origin | Fresh | 2002-0667A/Tiernan NT124 | MW392100 |
| Plumeria subsessilis A.DC. | Hispaniola | Cultivated at FTBG, collected in Boquerón, province of Azua, Dominican Republic | Fresh | 2015-0143/Tiernan DR2015-NT02 | MW392098 |
| Plumeria tuberculata G.Lodd. | Hispaniola, Cuba | Cultivated at FTBG (plot 158), collected above Labadee, Port-au-Prince, Haiti | Fresh | 992117D/Burman, 2012-028 | MW392099 |
| Himatanthus sp. | Brazil | Cultivated at FTBG, acquired from Gemini Botanical Garden | Fresh | 2011-1085.1/- | MW392091 |
| Species . | Biogeography . | Provenance . | Plant material used in study . | FTBG accession number/FTBG herbarium voucher number . | GenBank accession number . |
|---|---|---|---|---|---|
| Plumeria alba L. | Puerto Rico, Virgin Islands, Lesser Antilles | Cultivated at FTBG from material acquired from Gemini Botanical Garden | Fresh | 20541 2014-0917/Tiernan NT125 | MW392089 |
| Plumeria filifolia Griseb. | Cuba | Cultivated at FTBG (plots 17, 46 and 49) from material sent from Palmetum of Santa Cruz de Tenerife Botanic Garden, Canary Islands | Fresh | 2019–0067/Tiernan NT122 | MW392092 |
| Plumeria jamaicensis Britton. | Jamaica | Cultivated at FTBG (plot 172), collected at Hector’s River, Portland Parish, Jamaica | Fresh | 90250A/Tiernan NT126 | MW392090 |
| Plumeria krugii Urb. | Puerto Rico | Cultivated at FTBG (plot 172), collected near Sabana Grande, Puerto Rico | Fresh | 2020-0311 A/Tiernan NT127 | MW392093 |
| Plumeria lanata Britton | Cuba | Collected in Parque Nacional Desembarco del Granma, province of Granma, Cuba | Dried | -/Tiernan NT59 | MW392094 |
| Plumeria magna Zanoni & M. Mejía | Dominican Republic | Cultivated at FTBG, collected west of Las Galeras, province of Samaná, Dominican Republic | Fresh | 2014-0461/Jestrow 2013-DR-42 | MW392095 |
| Plumeria montana Britton & Wilson | Cuba | Collected in Parque Nacional Turquino, province of Santiago de Cuba, Cuba | Dried | -/Tiernan NT60 | MW392096 |
| Plumeria obtusa L. | Bahamas, Greater Antilles | Cultivated in FTBG (plot 164), collected on Mangrove Cay, Andros Island, The Bahamas | Fresh | 73213A/Fantz 3672 | MW392097 |
| Plumeria pudica Jacq. | Leeward Antilles, Northern South America, Panama | Unknown origin | Fresh | 2002-0667A/Tiernan NT124 | MW392100 |
| Plumeria subsessilis A.DC. | Hispaniola | Cultivated at FTBG, collected in Boquerón, province of Azua, Dominican Republic | Fresh | 2015-0143/Tiernan DR2015-NT02 | MW392098 |
| Plumeria tuberculata G.Lodd. | Hispaniola, Cuba | Cultivated at FTBG (plot 158), collected above Labadee, Port-au-Prince, Haiti | Fresh | 992117D/Burman, 2012-028 | MW392099 |
| Himatanthus sp. | Brazil | Cultivated at FTBG, acquired from Gemini Botanical Garden | Fresh | 2011-1085.1/- | MW392091 |
Abbreviation: FTBG, Fairchild Tropical Botanic Garden.
Thirteen Plumeria samples were included, ten of which were from the living collection at Fairchild Tropical Botanic Garden (FTBG) in Miami, FL, USA. Accessions originating from wild-collected material included P. filifolia, P. jamaicensis, P. krugii, Plumeria magna Zanoni & M.M. Mejía, P. obtusa, P. subsessilis and P. tuberculata. Accessions originating from cultivated material from other botanical gardens included P. alba and P. pudica, in addition to the closely related outgroup (Himatanthus sp.). Fresh leaf material was collected from all taxa. Samples for two species (P. lanata and P. montana) were from collections made by N. Tiernan and R. Oviedo during fieldwork in eastern Cuba in August of 2016; leaf material of these species was dried on Drierite desiccant and later frozen. Herbarium vouchers are deposited at FTBG (Table 1). The remaining two Plumeria sequences were retrieved from GenBank: P. rubra (GenBank: MN812495), cultivated at Guangxi Botanical Garden, China (voucher at GXMG), and P. cubensis (GenBank: MG963231.1), cultivated at the National Tropical Botanical Garden, Kauai, Hawaiian Islands; the Endress s.n. voucher of P. cubensis listed in GenBank appears to correspond to Lorence 9520, housed in PTBG (M. E. Endress, personal communication). The three outgroup sequences were retrieved from GenBank: A. schottii (GenBank: MG963232.1), S. acutus (GenBank: MG963271.1) and C. thevetia (GenBank: MG963240.1) (Fishbein et al., 2018).
Eight of the 13 taxa studied are single-island endemics from Cuba (P. cubensis, P. filifolia, P. lanata and P. montana), Hispaniola (P. magna and P. subsessilis), Jamaica (P. jamaicensis) or Puerto Rico (P. krugii). Two are endemic in more than one island [i.e. P. alba (Puerto Rico, Virgin Islands and Lesser Antilles) and P tuberculata (Cuba and Hispaniola)]. Two of the ingroup species occur both on the mainland and on the islands {i.e. P. obtusa [Bahamas, Greater Antilles, Swan Island (Honduras), Mexico (Yucatan), Belize and northern Guatemala] and the cultivated P. pudica [Leeward Antilles, Northern South America and Panama]}. Finally, P. rubra has an undetermined natural range, because it is widely cultivated in the region. The four outgroup taxa included are native to the Neotropics. Himatanthus is a small genus (approximately nine species) restricted to South America. Skytanthus acutus is native to Chile. Allamanda schottii is native to Brazil, and C. thevetia is native to Mexico and Central America; however, both are widely cultivated and naturalized throughout the tropics.
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from leaf samples using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocols. The quality and integrity of isolated DNA were determined using gel electrophoresis. The total genomic DNA yield was quantified using the Qubit dsDNA HS Assay Kit and Qubit 2.0 fluorometer (Thermo Fisher Scientific, Invitrogen).
For all 12 samples, a long-range PCR-based approach was used to generate templates of plastid DNA for next-generation sequencing (Uribe-Convers et al., 2014). The PCR was performed using overlapping primers that cover the whole plastid genome; the 16 universal angiosperm primers used were previously published by Uribe-Convers et al. (2014). This protocol resulted in a plastome divided into 16 sequential regions that Uribe-Convers et al. (2014: fig. 1) labeled as Sections 1–16. In our study, we follow the same terminology when referring to regions of the cp genome.
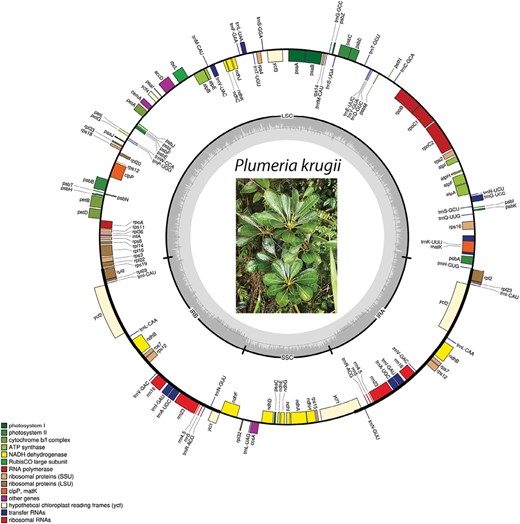
Gene map of the Plumeria krugii plastid genome. Genes on the outside of the outer black circle are forward direction and those on the inside are reverse. Gene function is displayed by different colours, as indicated by the legend. The black circle corresponds to IRa and IRb, which separates the genome into the small and large single-copy regions. The inner grey circle represents the within-plastome GC content.
The best results were obtained when PCR was performed with two high-quality Taq polymerases (QIAGEN Taq DNA Polymerase and QIAGEN HotStar HiFidelity DNA Polymerase; QIAGEN, Valencia, CA, USA) and TBT-PAR (Samarakoon et al., 2013). The resulting amplicons ranged in size from 4 to 11 kb, with 135–2059 bp overlap. Region 2 was split up into 2a and 2b, with the smaller fragments being 6.3 and 4 kb, respectively, to facilitate amplification (Uribe-Convers et al., 2014). Amplicons were visualized on a 1% agarose gel with appropriate size standards and quantified with a Qubit dsDNA HS Assay Kit and Qubit 2.0 fluorometer.
Amplicon concentrations were standardized at equal volume ratios by taxa before library preparation, which was performed according to the manufacturer’s protocol using the Nextera DNA Flex Library Preparation Kit following the manufacturer’s protocol (Illumina, San Diego, CA, USA). The library quality was assessed with a BioAnalyzer using the Agilent High Sensitivity DNA assay (Agilent Technologies, CA, USA). Purified libraries were loaded onto a high-output kit cartridge for 2 × 150 paired end sequencing on the Illumina MiniSeq platform.
Genome assembly and annotation
Following DNA sequencing, unindexed reads were downloaded via FASTQ files from BaseSpace Sequence Hub (Illumina). FastQC was used to evaluate the quality of the sequence data. Base calls with a quality score < 32 were trimmed from the raw reads using Trimmomatic v.0.36 (Bolger et al., 2014), and duplicate reads were removed with Dedupe software in the BBMap package v.36.92 (Bushnell, 2014). Quality-filtered reads of P. krugii were aligned against the plastid genome of P. rubra (Wang et al., 2020) using BWA 0.7.12-r1039, with the MEM algorithm (Li & Durbin, 2009) and other default settings. The resulting alignment BAM file was imported, visually inspected and manually corrected in Geneious Prime v.2020.0.5 (Kearse et al., 2012). The consensus sequence with 0%-majority threshold was extracted from the alignment, against which the quality-filtered reads of P. krugii were aligned to fill any gaps that might have resulted from mismatches between P. rubra and P. krugii. This step was repeated until no more additional reads were aligned to the reference. For each subsequent taxon of Plumeria, the above procedure was repeated using the P. krugii plastome sequences for the initial reference.
The organelle annotation programs we used produced inconsistent results (e.g. GeSeq, DOGMA); therefore, we initially aligned our experimental plastome sequences with the published annotated sequences of P. rubra (NC_046018), Arabidopsis thaliana (L.) Heynh. (Brassicaceae) (NC_000932) and Nicotiana tabacum L. (Solanaceae) (NC_001879) using the Auto algorithm of MAFFT (Katoh & Standley, 2013) in Geneious Prime (v.2020.0.5). Exon boundaries of all genes containing introns were verified based on the study by Sugita & Sugiura (1996). Gene and exon boundaries of transfer RNAs were verified using tRNAscan-SE (Schattner et al., 2005). After verification, the most appropriate annotations were transferred from one of the three reference sequences above to the experimental sequences using the Transfer Annotations function in Geneious Prime. The graphical representation of the P. krugii circular annotated plastome was created in OGDRAW (Greiner et al., 2019).
Phylogenetic reconstruction
For four species, we were unable to obtain sequences for several of the cp genome regions. The troublesome species included P. jamaicensis (region 9), P. magna (regions 6 and 9), P. montana (regions 10, 11 and 15) and P. pudica (regions 9, 10 and 15). Additionally, owing to low sequencing coverage, there were areas with no sequence data. Therefore, we followed two different strategies for the phylogenetic reconstructions. The first was based on the analysis of a data matrix that excluded those regions that did not have robust data. The second phylogenetic strategy used a larger data matrix that included data for all the regions, even those with missing data. Both strategies produced the same topologies; however, nodes were better resolved and had stronger support for the data matrix that included the missing data. Consequently, we herein present and discuss only the results from the second phylogenetic analyses, which had all the available data. Notably, previous studies using plastomes have shown identical phylogenetic results regardless of the partitioning strategy (Areces-Berazain et al., 2020).
The plastome sequences were aligned using the MAFFT v.1.4.0 plugin for Geneious, with default parameters (Algorithm = Auto; Scoring matrix = 200PAM/k = 2; Gap open penalty = 1.53; Offset value = 0.123). Inverted repeat A was removed to prevent a duplication of data. Phylogenetic inference was conducted on the unpartitioned alignment using both maximum parsimony (MP) and Bayesian inference (BI) analyses.
The MP analysis was performed with PAUP* v.4.0 build 169 (Swofford, 2002) using a branch and bound search, with gaps coded as uninformative. Phylogenetic support values were estimated through a bootstrap analysis using 10 000 replicates in PAUP*, with the same settings. Branch lengths were assigned with the Deltran algorithm (Agnarsson & Miller, 2008). Allamanda schottii, Himatanthus sp., Skytanthus acutus and Cascabela thevetia were used as the outgroup. The consistency index (CI), retention index (RI) and homoplasy index (HI) were calculated.
The BI analyses were performed using the MrBayes plugin for Geneious v.2.2.4. A GTR substitution model and gamma rate variation were selected according to Akaike’s information criterion as the best substitution model. The Markov chain Monte Carlo algorithm was run for 1 000 000 generations, with a 20% burn-in. The remaining analysis parameters were set as follows: heated chains = 4 and heated chain temperature = 0.2; unconstrained branch lengths = GammaDir (1;0.1;1;1), with shape parameter exponential = 10. Allamanda schottii was used as the outgroup.
Reconstructing the biogeographical history
Biogeographical analyses were based on our BI tree, with outgroups removed. Analyses were conducted using the dispersal–extinction–cladogenesis (DEC) method (Ree et al., 2005; Ree & Smith, 2008) implemented in RASP (Yu et al., 2015). Ancestral range estimation was done using the nested DEC and DEC+j models in BioGeoBEARS (Matzke, 2013, 2014, 2018). Taxa were coded as present or absent using the range distribution of eight different areas. The regions were as follows: (A) Puerto Rico and Virgin Islands; (B) Hispaniola; (C) Cuba; (D) Bahamas; (E) Jamaica; (F) Central America; (G) South America; and (H) Lesser Antilles. BioGeoBEARS was run with default settings, with no more than eight areas for each node. Species distribution data follow Acevedo-Rodríguez & Strong (2012) except for P. jamaicensis, which we recognize here as distinct from P. obtusa.
RESULTS
Table 1 contains the voucher and GenBank accession information for the 12 plastomes generated for this study. They were produced during one run on an Illumina MiniSeq, which resulted in 4.87 gb output, comprising 8 081 163 reads from 12 samples (after quality control). The plastomes of Plumeria are very similar and largely conserved. All exhibited typical quadripartite organization, each with a large single copy (LSC), small single copy (SSC) and two inverted repeat (IR) regions. A circular map of the P. krugii plastome, which was used as a reference for genome assembly for the remaining taxa, is presented in Figure 1.
The plastome data set of the 17 taxa consisted of 138 794 aligned sites. There were 2232 potentially parsimony-informative characters. The MP search generated five trees, with a best tree score of 10 579 (CI = 0.954; RI = 0.860; HI = 0.046). The majority rules consensus tree of the BI analysis is shown in Figure 2; branches that collapsed in the strict consensus tree are indicated by the filled square. The MP tree topologies were congruent with the majority-rule consensus of the BI analysis. Major clades were well supported with both bootstrap values and posterior probabilities, and no conflicting clades were observed between the model-based and parsimony analyses. Mainland P. rubra was resolved in a clade with the closely related Himatanthus. The Jamaican endemic P. jamaicensis was the next diverging lineage. Plumeria pudica, a species native to the Leeward Antilles and the northern South American mainland, was resolved as sister to a clade composed of the remaining ten species. Within this clade there are two monophyletic groups. The first one paired endemics from Puerto Rico or Puerto Rico/Virgin Islands/Lesser Antilles with endemics from Hispaniola or Hispaniola/Cuba. The second group paired a clade of Cuban endemics with P. obtusa, a species from the Caribbean Islands, Mexico and Central America. In the BI strict consensus tree within the Cuban clade, the branches collapsed into an unresolved polytomy. In BioGeoBEARS, our analysis showed a broad distribution at the early nodes that then segregated by island at each node (Fig. 5).
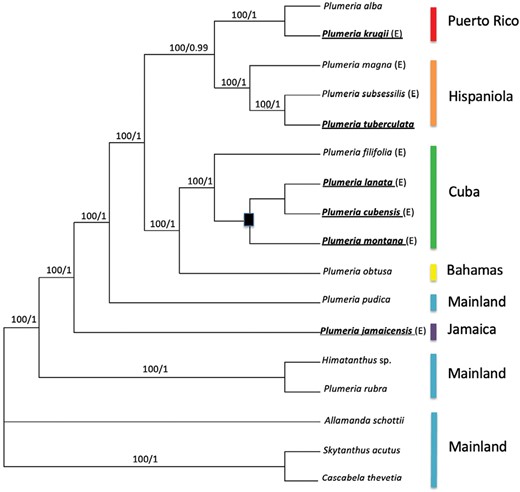
The majority rules consensus tree. Nodes are labelled with bootstrap values and posterior probabilities. The black square represents the branches that collapsed in the strict consensus tree. Endemic species are indicated with ‘(E)’. Species merged with Plumeria obtusa by Woodson (1937) are in bold and underlined. Coloured bars indicate the island of origin sampled for this study.
DISCUSSION
Taxonomy
Plumeria rubra and Himatanthus were resolved as sister to the rest of Plumeria. Himatanthus, also a mainland group, has been shown to be the closest relative of Plumeria in previous studies (Potgieter & Albert 2001; Spina et al., 2013). The two genera have a complicated taxonomic history, and Woodson (1937) distinguished Himatanthus from Plumeria by its large petaloid bracts. The position of P. rubra as sister to Himatanthus renders the genus Plumeria paraphyletic. The analysis presented here is based only on cpDNA, and although the two genera are nested with high bootstrap support, no taxonomic change is recommended here without further phylogenetic studies.
Plumeria pudica and P. rubra are both widely cultivated species with distinct morphologies and are grown in gardens worldwide. Plumeria pudica (Fig. 3A) has always been considered a distinct species within the genus, and this is the first reported association of P. pudica with Caribbean endemics. Although this species shares its white flowers with the rest of the clade, it exhibits unique pandurate, or fiddle-shaped, leaves, with a wide lobe at the distal end and a pointed apex. Interestingly, the mainland-occurring species (P. obtusa, P. pudica and P. rubra) did not resolve together forming a distinct monophyletic assemblage.
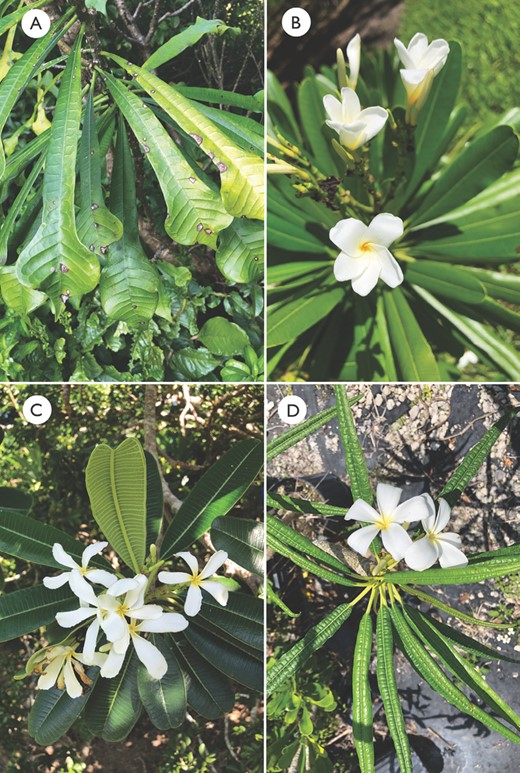
Plants growing at Fairchild Tropical Botanic Garden. A, pandurate leaves of Plumeria pudica. B, obovate leaves and white flowers with a yellow centre of Plumeria obtusa. C, densely pubescent abaxial leaf surface with a defined marginal vein of Plumeria tuberculata. D, elongate leaves with revolute margins of Plumeria alba.
Cuba holds the greatest diversity of the genus, and the four endemic species included in this analysis form a strongly supported monophyletic group, the only group in this study restricted to a single island. Plumeria filifolia was resolved as sister to the other three species, which collapsed into a polytomy in a strict consensus of the tree. Arguably the most distinctive of the genus is P. filifolia (Fig. 4D), with its long linear leaves. Plumeria lanata (Fig. 4B) is characterized by a felt-like pubescence found on the underside of the elliptic–obovate leaf and its small flowers (~4 cm wide) for the genus. Plumeria montana (Fig. 4C) is recognizable with its obovate–elongate leaves with revolute margins and flowers with long, slender petals. The morphology of P. cubensis is similar to that of P. obtusa (Fig. 3B), with its obovate leaves that have a round apex and cuneate base. Lippold’s (1979) classification was the only one to assign informal ‘Groups’ to closely related species for the Cuban-occurring taxa, combining morphological traits and biogeography. Without a previous comprehensive formal infrageneric and supraspecific taxonomic classification, and with our limited sampling, it is beyond the scope of this study to describe and assign taxonomic sections confidently.

Field photographs. A, cuneate leaves of Plumeria krugii (San Germán, Puerto Rico). B, felt-like abaxial leaf pubescence of Plumeria lanata (Parque Nacional Desembarco del Granma, province of Granma, Cuba). C, narrow-petalled flowers of Plumeria montana (Parque Nacional Turquino, province of Santiago de Cuba, Cuba). D, filiform leaves of Plumeria filifolia (Pilón, province of Granma, Cuba).
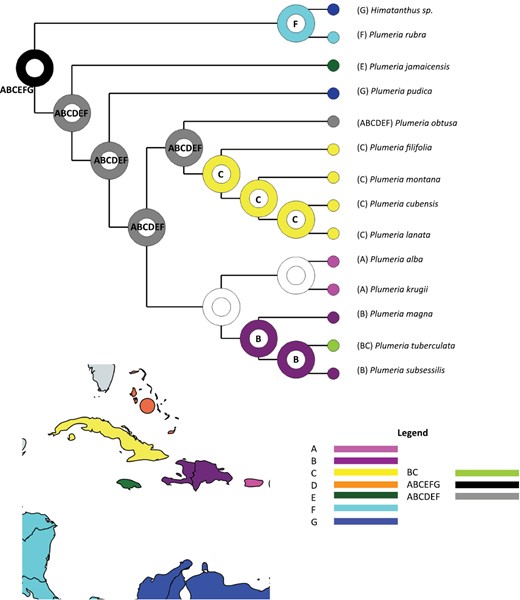
Estimates of ancestral ranges for Plumeria under the DEC+j model. Nodes are coloured with most likely states only. The regions are as follows: (A) Puerto Rico and Virgin Islands; (B) Hispaniola; (C) Cuba; (D) Bahamas; (E) Jamaica; (F) Central America; (G) South America; and (H) Lesser Antilles.
Plumeria alba and P. krugii formed a well-supported clade. Morphologically, P. alba (Fig. 3D) is unlike any other species in the genus; it is distinctive, with elongate leaves, extremely revolute leaf margins and dense pubescence on the abaxial leaf surface. In contrast, P. krugii (Fig. 4A), endemic to Puerto Rico, is characterized by cuneate to ovate–spatulate leaves that are completely glabrous and are most similar in shape to those of P. magna. However, P. magna was resolved in another clade mostly composed of species restricted to Hispaniola.
Sister to the Plumeria alba–P. krugii clade is an assemblage composed of three species: P. magna, P. subsessilis and P. tuberculata. Plants of P. magna are large and display spatulate leaves with a rounded apex and an angled base. The leaves are glabrous throughout and clustered at the ends of the branches. Plumeria subsessilis is recognizable by its subsessile leaves, lacking or nearly lacking a petiole, and prominent lateral veins. Lastly, P. tuberculata (Fig. 3C) is characterized by a densely pubescent abaxial leaf surface and a defined marginal vein, but most distinctive is the tuberculate stem, which is not found in other species of the genus.
The systematic boundaries of P. obtusa are one of the most taxonomically difficult challenges for the genus. Of the taxa included in the phylogeny, Woodson (1937) considered P. cubensis, P. jamaicensis, P. krugii and P. montana as synonyms of P. obtusa. The phylogenetic placement of P. filifolia, P. jamaicensis and P. pudica renders P. obtusa sensu Woodson as paraphyletic (Fig. 2), because he treated P. filifolia and P. pudica as distinct species, while considering P. jamaicensis as merged with P. obtusa. Woodson (1937) created a P. obtusa complex with many synonyms based solely on leaf morphology, "leaves obovate to obovate–oblong, rounded, emarginate or very broadly obtuse at the apex, rather than very shortly and abruptly acuminate–submucronulate, coriaceous to subcoriaceous, rarely firmly membranaceous, secondary veins anastomosing distally to form a well-developed marginal vein". He merged most of the Caribbean Island morphs into this species complex, while disregarding their ecological and morphological peculiarities. Woodson (1937) accepted only one infraspecific taxon, P. obtusa var. sericifolia (C. Wright) Woodson, with two synonyms (P. lanata and P. tuberculata), distinguished by pubescence on the adaxial leaf surface.
Biogeography
Beyond the taxonomy, the biogeographical relationships among the species of Plumeria are poorly understood. This is largely attributable to the range and circumscription of P. obtusa (Tiernan et al., 2020). Plumeria obtusa was resolved as paraphyletic in our phylogenetic analysis, and therefore we disagree with Woodson’s (1937) narrow species concept of P. obtusa. For our biogeographical analysis, we scored P. obtusa as occurring on every island where Plumeria occurs.
The only Jamaican endemic included in our study (P. jamaicensis) formed a lineage that was sister to the clade that included the rest of the Caribbean Island endemics and P. pudica. Plumeria jamaicensis has a widespread coastal distribution on limestone substrates and is morphologically distinct from the typical P. obtusa morph also found in this habitat. The palaeogeographical history of Jamaica supports the distinct phylogenetic placement of P. jamaicensis. Jamaica is an island that was below sea level until the middle–late Miocene (Robinson, 1994; Draper, 1998; Graham, 2003).
Fossils of Plumeria have been documented twice: (1) Plumiera evidens Hollick from Collazo Shale (Puerto Rico), estimated to be from the mid-Oligocene (23–33 Mya); (2) Plumiera alia Hollick from Moruga (Trinidad), estimated to be from the Miocene and maybe as early as Pliocene (3–23 Mya) (Hollick, 1924). During the late Oligocene (34 Mya) there was a major sea-level drop, and land bridges were established between Eastern Cuba, Hispaniola and Puerto Rico (Iturralde-Vinent & MacPhee, 1999). With fossil evidence estimated as early as 33 Mya and Jamaica emerging ~10 Mya, it is likely that Woodson’s (1937) concept of P. obtusa was misconstrued.
The P. alba–P. krugii clade had the two species restricted to the eastern-most Caribbean Islands (Lesser Antilles, Puerto Rico and Virgin Islands). This monophyletic group included P. krugii, a species confined to the premontane serpentine soils of western Puerto Rico, and the lowland species P. alba. The latter is a coastal species commonly found in Puerto Rico, the Virgin Islands and the Lesser Antilles. It grows on both limestone and volcanic substrates.
This eastern Caribbean Island group (P. alba–P. krugii clade) is sister to a clade mostly restricted to Hispaniola, a neighbouring island to Puerto Rico. Two Hispaniolan endemics were recovered in this clade, P. magna and P. subsessilis. The former is confined to the north-eastern Dominican Republic and to the Cordillera Central, where it can be found mostly on limestone, but also on sandy and serpentine soils. Plumeria subsessilis is commonly widespread throughout the island in semi-dry forest at low to mid elevations. This clade also includes P. tuberculata, another common species for the island, mostly from dry lowlands. The sample of P. tuberculata used in this study was from Haiti; however, the full range of this species is unknown. Some authors consider P. tuberculata as also occurring in Cuba (León & Alain, 1957; Liogier, 1989). In our biogeographical analyses, we scored P. tuberculata as occurring in both Hispaniola and Cuba.
The Cuban clade included four endemic species (P. cubensis, P. filifolia, P. lanata and P. montana) that have radiated into different habitats. Cuba is the largest island in the Greater Antilles, with a mosaic of soil substrates and plant formations that have created a complex environment harbouring a large number of endemic species (Borhidi, 1996). Plumeria filifolia (Fig. 4D) occurs in eastern Cuba in coastal scrublands, usually on limestone. Plumeria lanata (Fig. 4B) is also found on limestone substrate in south-eastern Cuba. Plumeria montana (Fig. 4C) is found in the eastern part of the island, at high elevations in the rainforests of the Sierra Maestra mountain range, mostly occurring on volcanic soils, and is the only species in the genus to occur in this habitat in Cuba. Plumeria cubensis is widespread across the island and favours serpentine soils.
One of the more puzzling results of our study concerns the biogeography of P. pudica (Fig. 3A) and its phylogenetic arrangement. The P. pudica lineage is nested inside a clade confined to the Caribbean Islands. Within this monophyletic group, P. pudica is sister to the clade that includes those species endemic to the Bahamas, Cuba, Hispaniola, Puerto Rico, the Virgin Islands and the Lesser Antilles. Plumeria pudica does not naturally reach either the Antilles or the Bahamas, because it is restricted to the southern shores of the Caribbean Basin (northern South America and islands located offshore of the coast of Venezuela). The placement of this species in the phylogeny renders the Caribbean endemics paraphyletic. Our ancestral area estimation for P. pudica and others (not including P. jamaicensis and P. rubra) includes Central America and the Caribbean Islands. It is possible that the distribution of P. pudica is attributable to a dispersal event. Further phylogenetic analyses that include additional samples of P. obtusa from the Yucatan peninsula, Bahamas, etc. would help to resolve the estimation of the ancestral area for P. pudica.
During the evolutionary process there were probably independent ecological shifts to areas with serpentine soils of Cuba (P. cubensis), Hispaniola (P. subsessilis) and Puerto Rico (P. krugii). These ecological shifts presumably encompassed island-specific adaptive radiation groups that targeted Cuba, Hispaniola and Puerto Rico–Virgin Islands–Lesser Antilles. Furthermore, it appears that across the Caribbean Islands the genus followed a west-to-east biogeographical track from Cuba to the Bahamas and to the eastern Antilles.
Often, radiations happen that result in later diversification on islands (Lavin, 1993; Lavin et al., 2001; Majure et al., 2021). Previous phylogenetic studies of Caribbean Island plants have also detected a pattern in which clades are mostly confined to single islands or to islands that are in close proximity to one another. In some cases, these clades do not involve ecological shifts and their species occur only in a particular ecosystem. Examples of this phylogenetic trend include the Euphorbiaceae genera Leucocroton Griseb. and Garciadelia Jestrow & Jiménez Rodr. (Jestrow et al., 2012). With 16 species, Leucocroton forms a clade restricted to serpentine areas of Cuba. The three species of Garciadelia also form a monophyletic group; however, it is confined to Hispaniola, where it occurs only in mid-elevation areas with limestone substrate.
Conclusion
This study provides the foundational phylogeny for Plumeria. Our sampling covered approximately half of the 27 taxa generally accepted for the genus and, most importantly, the key endemics (Tiernan et al., 2020). Despite some gaps and the need for future studies, several new findings are presented here. The resolution of P. rubra in a clade with Himatanthus and sister to the rest of the genus renders Plumeria paraphyletic. Given that further studies are needed, no taxonomic changes are presented here.
As is seen in the taxonomic history of Plumeria, relationships can be obscured when only morphological patterns are considered. Plumeria obtusa was not resolved as monophyletic and is likely to have a much narrower range than previously thought. Our study did not include samples of P. obtusa from the mainland. Having them and samples from other islands would have helped us to gain additional insights regarding the evolutionary and biogeographical history of the genus, in addition to its taxonomy.
Other species that should be included in future phylogenetic studies to test the presence of clades restricted to insular groups are the other two Bahamian taxa (Plumeria bahamensis Urb. and Plumeria inaguensis Britton) that have been described for the Bahamas.
It will also be interesting to determine whether samples of P. tuberculata from Cuba and Hispaniola form a monophyletic group. In addition to P. tuberculata from Cuba there are ~11 other species from this island that were not included. With the biogeographical links between Cuba and Hispaniola observed in the present study, it would be imperative to explore the species not included from Hispaniola, including P. stenopetala, which is morphologically distinguished by its long, lanceolate petals and conduplicate oblong–spatulate leaves. Plumeria stenopetala is found widespread across the island on limestone substrates. Lastly, Plumeria marchii Urb. from the high-elevation rainforests of Jamaica should be included in future molecular phylogenetic studies. Although many authors consider P. marchii to be a synonym of P. obtusa (Woodson, 1937; Adams, 1972; Acevedo-Rodríguez & Strong, 2012), it is a species in the P. obtusa complex that stands out as the most morphologically distinct, a canopy tree with the largest leaves of the genus (Tiernan et al., 2020).
The cpDNA topologies that we have recovered through this study provide an initial phylogenetic framework for future research that should include other Caribbean Island endemics, mainland and insular samples of P. inodora, P. obtusa and P. tuberculata from Cuba. Furthermore, additional phylogenetic reconstruction should explore the use of nuclear DNA markers. A more detailed analysis of the biogeography of Plumeria will be feasible with further sampling that will allow the determination of relationships between Caribbean and mainland species.
ACKNOWLEDGEMENTS
Financial support for this work was provided by Fairchild Tropical Botanic Garden (FTBG), the Botany in Action Fellowship at Phipps Conservatory and Botanical Garden, the Southern California Plumeria Society, the Garden Club of America, the International Center for Tropical Botany, the Tinker Foundation, the Kelly Foundation, the American Society of Plant Taxonomy, and Judith Evans Parker. This study is part of the PhD research of N.T. under the advisement of J.F.-O. at Florida International University (FIU). N.T. gratefully acknowledges her graduate study committee: H. Bracken-Grissom, B. Jestrow, S. Koptur and H. Liu. We are grateful to L. González-Oliva, R. Verdecia, J. L. Gómez, J. Montes de Oca, G. Brull and Z. Acosta Ramos for their invaluable help during our field studies and the arrangement of the required collecting, export and research permits in Cuba; A. Veloz and F. Jiménez-Rodríguez in the Dominican Republic; W. Cinea and P. Theogene in Haiti; T. Commack and K. Campbell in Jamaica; and E. Santiago-Valentín and J. Sustache-Sustache in Puerto Rico. FTBG and BioTECH @ Richmond Heights Botany Magnet High School kindly provided MiniSeq research facilities, which also facilitated molecular biology research mentoring of BioTECH students by N.T. Additionally, we thank the volunteers in the FTBG molecular laboratory, in particular Dr Michael Hass. We acknowledge Dr Rob Nicholson and Dr Jimmy Grogan at Smith College for sharing the material of Plumeria krugii. We thank laboratory member Jonathan Flickinger, who provided the translated version of Lippold’s treatment of Plumeria L. (Apocynaceae) in Cuba (Lippold, 1979) and for sharing fieldwork. Finally, the authors would like to thank Dr Mary Endress for her comments on an earlier version of this manuscript and the two anonymous reviewers for the comments that helped to improve the manuscript.
This a contribution to a Special Issue of the Biological Journal of the Linnean Society entitled Cuba: biodiversity, biogeography and evolution, edited by John A. Allen, Bernardo Reyes Tur, Roberto Alonso Bosch, Eldis R. Bécquer and José Ángel García Beltrán.
DATA AVAILABILITY
The data sets generated during this study are available from the corresponding author upon reasonable request.



