-
PDF
- Split View
-
Views
-
Cite
Cite
Yuhan Fu, Mengdi Nan, Qing Ren, Xiang Chen, Jie Gao, A multi-view graph convolutional network framework based on adaptive adjacency matrix and multi-strategy fusion mechanism for identifying spatial domains, Bioinformatics, Volume 41, Issue 4, April 2025, btaf172, https://doi.org/10.1093/bioinformatics/btaf172
Close - Share Icon Share
Abstract
Spatial transcriptomics (ST) addresses the loss of spatial context in single-cell RNA-sequencing by simultaneously capturing gene expression and spatial location information. A critical task of ST is the identification of spatial domains. However, challenges such as high noise levels and data sparsity make the identification process more difficult.
To tackle these challenges, STMGAMF, a multi-view graph convolutional network model that employs an adaptive adjacency matrix and a multi-strategy fusion mechanism is proposed. STMGAMF dynamically adjusts the edge weights to capture complex spatial structures during training by implementing the adaptive adjacency matrix and optimizes the embedded features through the multi-strategy fusion mechanism. STMGAMF is evaluated on multiple ST datasets and outperforms existing algorithms in tasks like spatial domain identification, visualization, and spatial trajectory inference. Its robust performance in spatial domain identification and strong generalization capability position STMGAMF as a valuable tool for unraveling the complexity of tissue structures and underlying biological processes.
Source code is available at Github (https://github.com/Fuyh0628/STMGAMF) and Zenodo (https://zenodo.org/records/15103358).
1 Introduction
The spatial distribution of cells within tissues is important for understanding their biological functions and their expression in pathological states (Longo et al. 2021). Spatial transcriptomics (ST), as a revolutionary and cutting-edge tool, is capable of capturing both gene expression and spatial location information in a tissue context. This technology breaks through the limitations of traditional transcriptomics technology on tissue context and spatial resolution and has achieved remarkable results in the study of various biological systems. Spatial domain identification is one of the core tasks in analyzing ST data, which identifies and delineates functional regions with similar gene expression characteristics and spatial consistency by analyzing the spatial patterns of gene expression in tissues. Through spatial domain identification, the spatial distribution patterns of different cell types can be analyzed, the microenvironmental relationship network at the cellular level can be constructed, the interactions between cells can be revealed in-depth, and the mechanism of multicellular collaboration can be analyzed, which provides important clues for the study of cellular heterogeneity and tissue dynamics in complex diseases such as tumors (Galeano Niño et al. 2022).
The spatial domain identification task faces three significant challenges: high noise, data sparsity, and resolution limitation. In recent years, researchers have proposed various algorithms to improve the accuracy and stability of spatial domain identification. High noise in ST data mainly comes from low-throughput signal dilution, spatial coding impairments, and background noise generated by sequencing errors. SEDR combined variational graph autoencoder and deep masked autoencoder network to optimize the low-dimensional representation and enhance noise immunity (Xu et al. 2024). spaVAE was based on a negative binomial model-based decoder to minimize the effect of noise (Tian et al. 2024). sGAE innovatively transformed single-cell gene expression matrices into stable gene association network entropy matrices and enhanced data stability through dynamic network entropy analysis (Zhong et al. 2023). IRIS integrated single-cell RNA sequencing data to infer cell-type composition to minimize the impact of technical noise and improve bio-interpretability (Ma and Zhou 2024). Data sparsity is another key factor limiting ST data analysis. Insufficient sequencing depth or low gene expression in a specific region can lead to information loss and affect the integrity of the null domain. STAGATE learned spatial neighbor relationships through the graph attention mechanism to improve feature learning in low-expression regions (Dong and Zhang 2022). Spatial-MGCN (Wang et al. 2023) and SpaNCMG (Si et al. 2024) utilized a multi-view graph convolutional network (GCN) to integrate gene expression and spatial structural information, enabling neighborhood information to enhance sparse data. MuCoST (Zhang et al. 2024) and stCluster (Wang et al. 2024a) combined contrastive learning (CL) to optimize feature extraction and learning for low-expression data. In addition, different ST platforms (e.g. 10×Visium, Slide-seq, MERFISH, and Stereo-seq) have different spatial resolutions, which affects the finesse of spatial domain identification. GAAEST improved the ability to identify spatial domains at cellular resolution through the synergistic optimization of graph attention network and self-supervised learning (Wang et al. 2024b). DeepST introduced the domain adversarial neural network to effectively solve the cross-platform resolution difference problem (Xu et al. 2022). GraphST used self-supervised learning and contrast loss strategy within a graph neural network framework to improve resolution adaptation (Long et al. 2023). CytoCommunity proposed a differentiable graph pooling technique, which effectively solves the problem of cross-sample graph alignment through a supervised learning framework (Hu et al. 2024). SpatialGlue improved spatial domain identification by integrating multiple spatial histology data from the same tissue and slices (Long et al. 2024). Although these algorithms have made significant progress in dealing with high noise, data sparsity, and resolution limitations, they still suffer from generally high computational complexity, sensitivity to data quality, and limited ability to generalize across datasets. Therefore, developing practical algorithms to adapt to complex data characteristics remains a central research goal of ST.
We propose STMGAMF, a multi-view graph convolutional network model that employs an adaptive adjacency matrix and a multi-strategy fusion mechanism. STMGAMF first considers spatial and gene expression similarity comprehensively by constructing spatial and feature adjacency graphs, thus overcoming the problem that a single adjacency cannot adequately capture the interactions between gene expression data and spatial information. Second, STMGAMF introduces an adaptive adjacency matrix to optimize the graph structure based on the data characteristics, which overcomes the limitations of static, predefined adjacencies in traditional algorithms when dealing with complex spatial structures, thus better adapting to the complexity of the data. Subsequently, in designing the multi-view graph convolutional network encoder, STMGAMF introduces adaptive adjacency matrices in the spatial convolution and feature convolution modules, respectively. In contrast, the joint convolution module uses the original adjacency matrix. This strategy enhances the model’s flexibility to extract spatial and feature information while preserving the global commonality information in the data. Finally, STMGAMF proposes a multi-strategy fusion mechanism that combines the adaptive ability of the attention mechanism with the stability of the fixed learnable parameters, overcoming the problem that simple splicing or fixed weighted averaging to fuse different features cannot dynamically adjust the importance of the features according to the differences in the data distribution, and improves the model’s stability and generalization ability. We evaluate STMGAMF on several downstream analysis tasks, including spatial domain identification, visualization, spatial trajectory inference, gene expression imputation, and differential expression analysis. The results indicate that STMGAMF performs competitively compared to seven other popular algorithms.
2 Materials and methods
2.1 Data preprocessing
2.2 Graph construction
To integrate gene expression and spatial location information effectively, we employ various adjacency graph construction methods based on spatial proximity and gene expression similarity. First, spatial proximity is assessed by analyzing the locations of adjacent spots within the tissue. We construct a spatial adjacency graph , where is the spatial adjacency matrix of spots and denotes the normalized gene expression matrix, where is the number of filtered genes. If both spot and are within a predefined radius , then we set , and 0 otherwise (for the DLPFC, , while for the other datasets ). The spatial adjacency graph accurately represents the tissue’s spatial structure and ensures that the model reflects direct proximity based on physical location within the tissue. The graph takes into account only the spatial proximity of cells, but it does not effectively represent the biological significance of variations in gene expression.
To overcome the limitations of the spatial adjacency graph, we construct a feature adjacency graph based on gene expression similarity. This graph identifies cellular areas within a tissue that have similar functions or states by assessing the similarity of gene expression vectors, even if these areas are physically far apart. Specifically, we construct the k-nearest neighbor feature adjacency graph , where is the feature adjacency matrix of spots. The edge weights of the feature adjacency matrix are calculated based on the cosine similarity. If spot is a neighbor of spot , then we set , and 0 otherwise ( by default). The feature adjacency graph effectively identifies cells or areas with similar gene expression patterns. However, it may sometimes mistakenly group spatially distant spots in adjacent areas, overlooking the spatial structure. By building both a spatial adjacency graph and a feature adjacency graph, we enable the model to leverage spatial proximity and gene expression similarity simultaneously.
2.3 Adaptive adjacency matrix construction
2.4 Multi-view graph convolutional network encoder
In this model, we develop a multi-view learning framework based on a GCN. The goal is to fully integrate the spatial and feature information within the graph structure while capturing the dependencies and interrelationships between various spots. The framework consists of three main modules: the spatial convolution module, the feature convolution module, and the joint convolution module.
2.4.1 Spatial convolution and feature convolution
2.4.2 Joint convolution
2.5 Multi-strategy fusion
To enhance identification accuracy, we design a multi-strategy fusion mechanism that adaptively integrates these features. The mechanism combines an attention-based fusion method and a fixed learnable parameter-based fusion method.
2.5.1 Fusion based on the attention mechanism
2.5.2 Fusion based on fixed learnable weight parameters
2.5.3 Final embedding
This fusion merges the adaptive capability of the attention mechanism with the stability of fixed learnable weight parameters, enabling flexibility in capturing the significance of different embeddings while maintaining representation stability.
2.6 Zero-inflated negative binomial decoder
2.7 Spatial regularization constraints
By maximizing the similarity between adjacent spots and minimizing the similarity between non-adjacent spots, the model can create a spatial representation in potential space that aligns with the biological structure.
2.8 Model loss function
During the model training process, we jointly optimize the multi-view GCN encoder, the ZINB decoder, and the spatial regularization constraints to enhance the model’s performance.
In this loss function, we set weight parameters , , and to balance the effects of consistency loss, reconstruction loss, and spatial regularization loss.
Detailed methodological procedures are presented in Supplementary Section S1.
3 Results
3.1 Overview of STMGAMF
STMGAMF is a novel spatial domain identification algorithm that utilizes a multi-view GCN with an adaptive adjacency matrix and a multi-strategy fusion mechanism (Fig. 1). In order to more effectively integrate gene expression with spatial location information and fully explore adjacency information, STMGAMF constructs both a spatial adjacency graph and a feature adjacency graph . To enhance the capacity of the adjacency graph to capture spatial structural complexity, STMGAMF incorporates an adaptive adjacency matrix. This matrix is built upon the spatial adjacency relationship and feature adjacency relationship among spots (Fig. 1A). During model training, the adaptive adjacency matrix dynamically adjusts the weights of the edges, enabling the model to learn the adjacency graph automatically. To extract information from specific perspectives, STMGAMF employs a multi-view GCN that performs graph convolution on each adjacency matrix separately. Furthermore, STMGAMF contains a joint convolution module that extracts the joint embedding from both the spatial graph and the feature graph using a parameter-sharing strategy, which maximizes the use of common information between the two graphs (Fig. 1B). Given that different embedding features contribute variably to the model’s overall performance, STMGAMF adaptively integrates these features using a multi-strategy fusion mechanism to achieve the final embedding representation (Fig. 1D). In order to align with the characteristics of ST data, STMGAMF utilizes a ZINB decoder structure, which allows for the reconstruction of the gene expression matrix and captures the complexity of the data from a holistic perspective (Fig. 1E). At the same time, in order to further maintain the integrity of the spatial structure, STMGAMF introduces a spatial regularization constraint (Fig. 1C). This constraint significantly enhances the embedding’s ability to express spatial structures by strengthening the similarity among adjacent spots while suppressing similarities between non-adjacent spots. Finally, STMGAMF jointly optimizes the multi-view GCN encoder, ZINB decoder, and spatial regularization constraints, resulting in learned embedding representations that can be used for various downstream analysis tasks (Fig. 1F). We apply STMGAMF to the human dorsolateral prefrontal cortex (DLPFC), human breast cancer, anterior tissue of mouse brain datasets obtained by the 10× Visium platform, and coronal mouse olfactory bulb dataset obtained by the Stereo-seq platform, respectively, of which the results of the latter three datasets are described in detail in Supplementary Section S3.
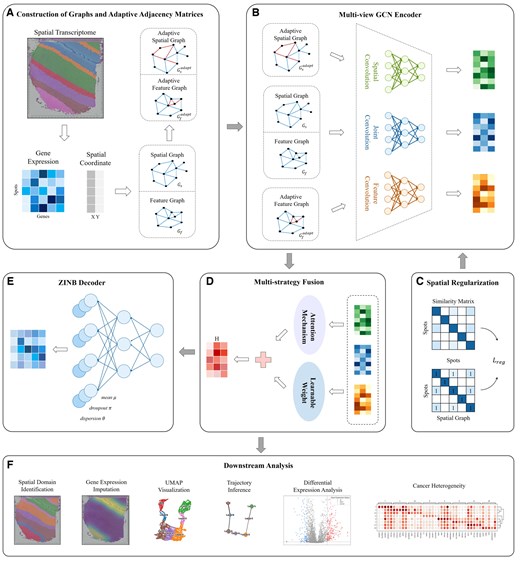
Overview of STMGAMF. (A) Construction of graphs and adaptive adjacency matrices. The spatial and feature adjacency graphs are constructed based on different criteria, and the adaptive adjacency matrix is introduced to adjust the edge weights dynamically. (B) Multi-view GCN encoder. STMGAMF integrates the spatial, feature and their common information in the graph structure using a multi-view GCN encoder. (C) Spatial regularization. STMGAMF preserves the spatial structure using spatial regularization constraints. (D) Multi-strategy Fusion. STMGAMF adaptively integrates spatial, feature and joint embeddings through a multi-strategy fusion mechanism to obtain the final embedding representation. (E) ZINB Decoder. STMGAMF reconstructs gene expression using the ZINB decoder. (F) Downstream analysis. STMGAMF clusters the learned embedding representations and implements downstream analysis tasks.
3.2 STMGAMF enhances the accuracy of spatial domain identification
To quantitatively assess the spatial clustering performance of STMGAMF, we select the 10× Visium dataset of the DLPFC, which includes spatial expression information from 12 slices (Maynard et al. 2021). This dataset is manually annotated based on morphological features and genetic markers by Maynard et al. (2021) who categorized the six cortical layers (L1–L6) of the DLPFC as well as the white matter (WM) (Fig. 2). These manual annotations provide a reliable reference standard for evaluating model performance. In our study, we test the accuracy of STMGAMF against seven other mainstream spatial clustering algorithms: STAGATE, DeepST, GraphST, Spatial-MGCN, SEDR, MuCoST, and GAAEST. The goal is to identify biologically meaningful spatial domains. STMGAMF efficiently identifies the expected cortical layer structure and demonstrates significant improvements compared to the other seven algorithms. A visual comparison of the spatial structural domains identified by these algorithms across tissue sections #151507-#151510 illustrates the differences. STAGATE and GAAEST only recover the clustering for cortical layer L1. DeepST struggles to distinguish between cortical layers L4–L6, failing to achieve clear separations and leading to numerous mixing spots within those areas. Although GraphST and MuCoST generate cortical layers that resemble the annotations, they inaccurately represent the thickness of these layers and mixed in other layers with the clustering for L1. None of these algorithms correctly identifies the full number of cortical layers, resulting in a division of the layers into only five categories. Spatial-MGCN, SEDR, and STMGAMF successfully map the complete structure of cortical layers L1–L6 and white matter. However, Spatial-MGCN fails to accurately distinguish between cortical layer L6 and white matter in slice #151510. At the same time, SEDR has confounding errors in identifying layers L2 and L3 in slice #151507, and the boundaries are not smooth enough. In contrast, STMGAMF is the only algorithm to identify the cortical layers L1–L6 and WM in high agreement with the manual annotations and establishes neuroscience definitions. It clearly captures the ‘sandwich’ morphology of cortical layers L1–L3 as indicated in the annotations and effectively detects the deeper L4–L6, which the other algorithms struggle to differentiate. Additionally, STMGAMF presents a relatively clear and intact representation of the white matter.
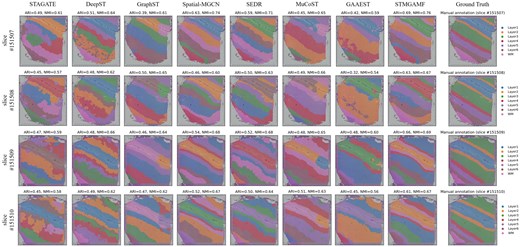
Manual annotation results and spatial domain identification results in the DLPFC for STMGAMF and the seven baseline algorithms, focusing on slices #151507-#151510.
For quantitative evaluation, we assess the clustering performance of each algorithm using several metrics: Adjusted Rand Index (ARI), Normalized Mutual Information (NMI), Homogeneity Score (HS), Completeness Score (CS), and V-measure. These algorithms are applied to all slices of the DLPFC (Supplementary Figs S1 and S2). STMGAMF achieves the best performance, with the highest median ARI of 0.61 and the highest median NMI of 0.68. Spatial-MGCN and SEDR follow closely with median ARI values of 0.56 and 0.55 and median NMI values of 0.67 and 0.68, respectively. MuCoST yields a median ARI of 0.53 and a median NMI of 0.67, while GraphST achieves a median ARI of 0.51 and a median NMI of 0.66. Notably, the other three algorithms do not achieve a median ARI above 0.50. In particular, for slices #151507, STMGAMF clearly delineates the layer boundaries and achieves the highest clustering accuracies, with an ARI of 0.69 and NMI of 0.76 (Fig. 2). This outcome indicates that STMGAMF can capture the complexity of spatial structure more accurately.
Furthermore, in order to thoroughly assess the performance of STMGAMF and its capability to generalize to downstream tasks, we visualize the 2D Uniform Manifold Approximation and Projection (UMAP) space of the embeddings generated by various algorithms applied across 12 slices of DLPFC. This visualization is combined with partition-based graph abstraction (PAGA) to infer spatial trajectories (Supplementary Fig. S3). In particular, for slices #151507, the UMAP generated from STMGAMF embeddings clearly demonstrates a structured layering, with spots belonging to the same manually annotated layer clustering together (Supplementary Fig. S4A). Additionally, spots from different layers are distinctly separated, exhibiting well-defined boundaries. Both HS and CS achieve the score of 0.76, marking the best performance. This indicates that STMGAMF effectively and accurately reveals the spatial organization of the DLPFC. Furthermore, the PAGA maps illustrate a developmental pathway consistent with the temporal developmental order of the cortical layers (Supplementary Fig. S4B). This finding aligns with the functional similarities between adjacent cortical layers and their temporal progression (Gilmore and Herrup 1997). These results surpass the performance of other baseline algorithms. In the UMAP visualization results produced by GAAEST, the spots of different layers are mixed and not separated. Cortical layers L4–L6 of STAGATE are not correctly distinguished as laminae. Similarly, DeepST and MuCoST struggle to clearly separate spots from different layers, and spots from the same layer lack tight clustering. Additionally, GraphST has difficulty accurately identifying cortical layer L6 and some spots in cortical layer L4 are partially fused with those in cortical layer L5. The boundaries between the different cortical layers of the SEDR are not distinct enough and are slightly mixed. In contrast, both STMGAMF and Spatial-MGCN reflect a consistent relationship between spatial trajectories and spatial maps. However, Spatial-MGCN produces UMAP visualizations with slightly blended speckles in cortical layers L4 and L5, whereas STMGAMF presents a clearer delineation with a biologically consistent organization embedded in space. This experiment validates STMGAMF’s ability to enhance the spatial domain identification performance of ST data while preserving the underlying biological features necessary for effective representation in downstream tasks.
Additionally, the density heatmap based on UMAP further confirms the advantages of STMGAMF in the embedding space (Supplementary Fig. S4C). It is evident that the density distributions of different clusters generated by the STMGAMF model are continuous, with good intraclass compactness observed in high-density areas. This clearly demonstrates the hierarchical structure, indicating that the model can accurately reflect the internal consistency of the clusters in terms of feature representation. The heatmap also shows that the hierarchical structure of the spatial domain is preserved not only in the local density distribution but also in the clarity and separation of boundaries between different clusters. The hierarchical structure in the density heatmap aligns with the identification results of the spatial domains, demonstrating that the model preserves the topological relationship between spatial domains in the feature embedding. These results further verify that STMGAMF employs a multi-strategy fusion mechanism to ensure high embedding quality and to fully learn the representation of spatial information and inter-domain boundaries.
Raw ST data are often affected by high levels of noise and significant loss events, which can hinder the accuracy of gene expression analysis. To address this, robust representation learning algorithms should effectively distinguish between irrelevant confounding factors and the critical information regarding tissue distribution present in the raw data. In our study, we apply the STMGAMF to the DLPFC to uncover the spatial expression patterns of genes with greater accuracy and resolution. We select layer marker genes in each of the six layers—GFAP, HPCAL1, ENC1, NEFH, B3GALT2, and MBP—from DLPFC section #151507 and compare their raw gene expression levels with the expression reconstructed using STMGAMF (Fig. 3A). In the analysis of raw gene expression, we observe significant confounding effects impacting all layer marker genes. For instance, NEFH exhibits a spatially dispersed distribution that lacked clear pattern characteristics. However, after reconstructing the expression data, NEFH reveals high expression areas within cortical layer L5 as well as adjacent layers, clearly showcasing layer-specific expression patterns. To further validate the denoising capabilities of STMGAMF, we analyze the original expression data alongside the reconstructed expression using violin plots (Fig. 3B). The results indicate that the spatial expression patterns of these layer-tagged genes are significantly enhanced following reconstruction. Overall, our findings suggest that STMGAMF, which utilizes a ZINB decoder, is better suited for adapting to the features of ST data, effectively reducing noise and data loss while preserving significant biological information.
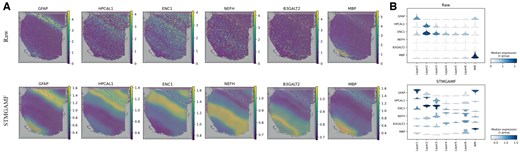
STMGAMF reconstructs the expression of layer marker genes on slice #151507 in the DLPFC. (A) Visualization of the original and reconstructed expression patterns of layer marker genes on slice #151507 in the DLPFC. (B) Violin plots of original layer marker gene expression and violin plots of STMGAMF reconstructed layer marker gene expression.
4 Conclusion
Spatial domain identification is a key challenge in ST due to high noise levels and data sparsity. To address this, we propose STMGAMF, a novel spatial domain identification algorithm. STMGAMF integrates spatial and feature adjacency graphs, considering both spatial and gene expression similarity, overcoming the limitations of relying on a single adjacency relationship. Unlike traditional methods relying on static adjacency relationships, STMGAMF introduces the adaptive adjacency matrix to optimize graph structures dynamically for complex tissue architectures. Furthermore, its multi-view GCN enhances model flexibility and robustness, while the multi-strategy fusion mechanism dynamically adjusts feature importance, improving stability and generalization.
We verify the superior performance of STMGAMF in spatial transcriptome data analysis and compare the results with seven mainstream algorithms. The results show that STMGAMF exhibits excellent spatial domain identification capabilities. In the DLPFC, the results in Fig. 2 and Supplementary Fig. S4 show that STMGAMF not only successfully distinguishes the six cortical layers (L1–L6) and WM, accurately captures the layer structure that conforms to the neuroscience definition, but also performs best in all evaluation indicators. The median ARI is 0.61, and the median NMI is 0.68. In addition to its excellent spatial domain identification performance, STMGAMF effectively removes high noise in ST data and retains key biological features. Regarding gene expression pattern reconstruction, the results in Fig. 3 show that STMGAMF significantly enhances the spatial expression specificity of layer marker genes and reveals significant patterns of genes in the organizational hierarchy. In the human breast cancer, STMGAMF verifies its performance in highly heterogeneous cancer tissues with the highest spatial domain identification accuracy. ARI improves by about 0.10–0.35, and NMI improves by about 0.07–0.13 compared with other algorithms. The results in Supplementary Figs S5 and S6 show that STMGAMF clearly distinguishes different spatial domains and enhances gene expression through denoising, revealing important cancer biological characteristics. For example, the differentially expressed gene analysis in DCIS/LCIS and IDC regions provides new clues for cancer mechanism research and clinical diagnosis, demonstrating the practical application potential of STMGAMF. In the mouse anterior brain, the results in Supplementary Fig. S7 show that STMGAMF exhibits a clear hierarchical structure and natural smooth boundaries, accurately depicting the spatial heterogeneity of tissues, achieving an optimal ARI of 0.53 and a suboptimal NMI of 0.71. In addition, in the coronal mouse olfactory bulb obtained by the high-resolution Stereo-seq platform, the results in Supplementary Fig. S8 show that STMGAMF further demonstrates its adaptability to complex spatial structures. Compared with the limitations of other algorithms in hierarchical structure identification, STMGAMF can accurately parse the anatomical domain of the olfactory bulb, successfully distinguish the inner and outer tissues, and highly match the known tissue annotations. These results suggest that STMGAMF can be used to identify ST organizational structures at different spatial resolutions. We also perform ablation experiments on DLPFC and human breast cancer, and the results are shown in Supplementary Section S5, where STMGAMF achieves the best performance among all variants.
In conclusion, STMGAMF not only demonstrates excellent advantages in analyzing complex biological data but also promotes an in-depth understanding of life micro-mechanisms by its ability to accurately reveal the spatial structure and functional properties of biological tissues, providing powerful support for life science research and clinical medicine.
Acknowledgements
The authors would like to express their sincere appreciation in advance to the editors and reviewers for their time and efforts in evaluating this manuscript and for their valuable and constructive feedback that will help improve the quality of this work.
Author contributions
Yuhan Fu contributed in concept, methodology, software, writing the original manuscript and visualization. Qing Ren and Mengdi Nan contributed to the survey and data organization. Xiang Chen participated in the biology knowledge survey and flowchart design. Jie Gao reviewed, edited, validated, and guided this study.
Supplementary data
Supplementary data are available at Bioinformatics online.
Conflict of interests: No competing interest is declared.
Funding
This work was supported by the National Natural Science Foundation of China [12271216, 11831015, and 92370131].
Data availability
The DLPFC is available on the spatialLIBD website (http://research.libd.org/spatialLIBD/). The human breast cancer is available on the 10× Genomics website (https://support.10xgenomics.com/spatial-gene-expression/datasets). The mouse brain is available on the 10× Genomics website (https://www.10xgenomics.com/resources/datasets). The processed mouse olfactory bulb is available on the website (https://github.com/JinmiaoChenLab/SEDR_analyses/).
Code availability
Source code is available at Github (https://github.com/Fuyh0628/STMGAMF) and Zenodo (https://zenodo.org/records/15103358).



