-
PDF
- Split View
-
Views
-
Cite
Cite
Ketrin Gjoni, Katherine S Pollard, SuPreMo: a computational tool for streamlining in silico perturbation using sequence-based predictive models, Bioinformatics, Volume 40, Issue 6, June 2024, btae340, https://doi.org/10.1093/bioinformatics/btae340
Close - Share Icon Share
Abstract
The increasing development of sequence-based machine learning models has raised the demand for manipulating sequences for this application. However, existing approaches to edit and evaluate genome sequences using models have limitations, such as incompatibility with structural variants, challenges in identifying responsible sequence perturbations, and the need for vcf file inputs and phased data. To address these bottlenecks, we present Sequence Mutator for Predictive Models (SuPreMo), a scalable and comprehensive tool for performing and supporting in silico mutagenesis experiments. We then demonstrate how pairs of reference and perturbed sequences can be used with machine learning models to prioritize pathogenic variants or discover new functional sequences.
SuPreMo was written in Python, and can be run using only one line of code to generate both sequences and 3D genome disruption scores. The codebase, instructions for installation and use, and tutorials are on the GitHub page: https://github.com/ketringjoni/SuPreMo.
1 Introduction
Many machine learning (ML) models have been developed that predict cellular profiles from input DNA sequences (Supplementary Table S1). These sequence-to-profile models can predict biological features—including gene expression [Enformer (Avsec et al. 2021a), ExPecto (Zhou et al. 2018), Xpresso (Agarwal and Shendure 2020)], genome folding [Akita (Fudenberg, Kelley and Pollard 2020), C.origami (Tan et al. 2023), DeepC (Schwessinger et al. 2020), ORCA (Zhou 2022)], chromatin accessibility [Basenji (Kelley 2020), Basset (Kelley, Snoek and Rinn 2016)], and epigenetic marks [(DeepFIGV (Hoffman et al. 2019), HyenaDNA (Nguyen et al. 2023), Sei (Chen et al. 2022a)]—with incredible accuracy. These approaches are becoming increasingly popular for exploring biological questions at lower cost and higher throughput than experimental methods allow, and for addressing questions that are not possible to test experimentally. One exciting potential is to use sequence-to-profile models in tandem with in silico mutagenesis (ISM), in order to investigate how genomic alterations affect cellular profiles. This strategy generates testable, causal hypotheses about genotype-phenotype relationships (Chen et al. 2022b). ISM has been applied to the genomes of modern humans, archaic hominins (McArthur, Rinker and Capra 2021), and other species (Keough et al. 2023) to prioritize putative pathogenic variants for experimental studies (Benegas, Batra and Song 2023), decode the grammar of noncoding DNA sequences (Deng et al. 2023), discover new sequence motifs (Avsec et al. 2021b), design tissue-specific enhancers (Gosai et al. 2023), and uncover novel roles of sequence elements (Gunsalus, Keiser and Pollard 2023).
In theory, ISM is very high-throughput, making it feasible to quantify the effects of a large set of sequence perturbations, such as all variants in an individual’s genome or a cohort of patients. However, the application of ISM at scale is currently limited by the process of generating sequences with and without perturbations. While several tools exist to perform analogous tasks, such as creating synthetic haplotypes [bcftools consensus (Li 2011), GATK FastaAlternateReferenceMaker (Van der Auwera and O’Connor 2020), perEditor (Rivas-Astroza et al. 2011), etc] or randomly mutating sequences [SNP mutator (Davis et al. 2015), BBMap mutate.sh (Bushnell 2014), etc], they are not compatible with ISM. One of the biggest limitations is that they incorporate all variants from an input variant call format (vcf) (Danecek et al. 2011) file into a single output fasta file, making it very difficult to isolate the effects of individual variants. Workarounds, such as generating an independent vcf file for each variant (or variant combination) and looping over these or post-processing the output fasta file to include one variant per locus, are extremely inefficient. Second, existing tools are made largely for SNPs or small insertions or deletions (indels), and cannot accommodate symbolic alleles—annotations in vcf files for structural variants (SVs). A possible workaround is to convert symbolic alleles into sequences by extracting them from a reference genome, but this becomes infeasible with large structural variants due to limitations with both variant complexity and memory allocation. One tool [perEditor (Rivas-Astroza et al. 2011)] is compatible with some complex variants but is not comprehensive and has stringent requirements. Finally, existing tools require the perturbations to be in a vcf format, which means that pseudo input files must be generated if one wishes to apply ISM to custom or simulated sequences (e.g. deleting all motifs for a given transcription factor or creating synthetic enhancers).
Due to these limitations, it is common practice for ISM practitioners to write their own code to generate input sequences for ISM studies. Indeed, the codebases for several ML models include code examples or frameworks for performing ISM [Enformer, Sei, Basset], but these are restricted to simple variants (SNPs and indels) and do not generate sequence files for input into other models. SVs make good candidates for ISM since they span larger regions and are more likely to be damaging to the genes, regulatory regions, or active sites they overlap or neighbor. For example, noncoding SVs have been shown to lead to cancer and developmental disorders by disrupting genomic contacts of key genes (Paik et al. 2021). SVs also alter more base pairs of the genome than any other type of genetic variation (1000 Genomes Project Consortium et al. 2015). One major challenge with SVs is that, to adhere to the fixed length input requirements of most ML models, input sequences must be padded, and consequently, model outputs require un-padding and masking. Another consideration is that—due to both biological effects and model artifacts related to making predictions for fixed width genomic windows—models can be highly sensitive to small changes in the input, such as masking, padding, and variant position in the window. Therefore, it is important to make predictions for augmented input sequences (shifted and/or reverse complement sequences) and evaluate them consistently across perturbations. Thus, incorporating perturbations into a reference genome becomes increasingly complicated and error-prone as variants get larger and more complex.
2 Tool description
To address these challenges, we developed SuPreMo, a framework for generating perturbed sequences for input into predictive models that is scalable, flexible, and comprehensive (Fig. 1A). SuPreMo, which incorporates variants into the human reference genome one at a time and generates model-ready sequences (Supplementary Fig. S1A), was extended to SuPreMo-Akita, which inputs those sequences into Akita (Fudenberg et al. 2020), an ML model that predicts chromatin contact maps, and generates scores that measure variants’ disruption to those maps (Supplementary Fig. S1C).
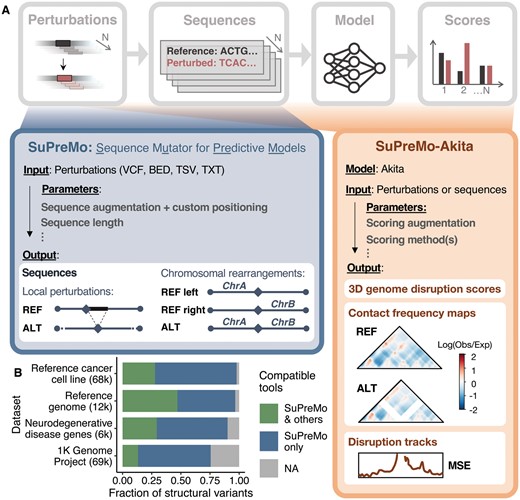
(A) Schematic representation of SuPreMo. SuPreMo generates sequences by incorporating perturbations into the hg38 human reference genome. SuPreMo-Akita applies Akita to those sequences and generates 3D genome disruption scores (effect size of each perturbation) and, optionally, disruption tracks and predicted contact frequency maps. Parameters and outputs are specified. REF: derived from reference allele; ALT: derived from alternate allele; Log(Obs/Exp): log of observed over expected contacts; MSE: mean squared error. (B) Categorization of SVs based on the ability of SuPreMo and other existing tools to incorporate them into a reference genome. SVs that other tools can already process include small indels (green); SVs that only SuPreMo can process include deletions, duplications, inversions, and chromosomal rearrangements (navy); SVs that no tool can process include insertions and copy number variants (CNVs) because the exact sequence is not provided by upstream variant calling pipelines (gray). Datasets are WGS/WES from healthy and disease individuals: a reference cancer cell line (Talsania et al. 2022), a reference genome (Zook et al. 2020), neurodegenerative disease gene sequences (Kaivola et al. 2023), and the 1K Genome Project (Mahmoud et al. 2019).
Both tools accept a variety of variant file types—vcf (version 4.1 and 4.2), txt, bed-like, and tsv [generated from AnnotSV (Geoffroy et al. 2018)]—making them flexible for use with real or synthetic perturbations (Supplementary Text). The following variant types [marked by their Manta (Chen et al. 2016) abbreviations] are supported: SNPs, indels, deletions (DEL), duplications (DUP), inversions (INV), and complex rearrangements with breakends (BNDs). Across a variety of datasets, including the 1K Genome Project, SuPreMo makes it possible to analyze over 50% of SVs that would not be accessible with existing tools (Fig. 1B).
In particular, symbolic alleles are now easily and uniformly processed (Fig. 1B, navy). On the other hand, insertions, which make up <20% of SVs, remain inaccessible for sequence-based models since the precise inserted sequence is not provided by SV calling methods (Fig. 1B, gray).
SuPreMo provides flexibility through various parameters. While the perturbation is by default centered in the generated sequence, the shift parameter slides the window around the perturbation by the given number of base pairs, to the right for positive shift and to the left for negative shift (Supplementary Fig. S2). The seq_len parameter determines the length of the output sequence, providing compatibility across models (Supplementary Table S1). The limit parameter sets a maximum variant length to be processed, with the default set to two thirds of seq_len. The revcomp parameter takes the reverse complement of the output sequence. Since the position of the perturbation can vary based on its length, the shift parameter, or if the perturbation is near chromosome arm ends, generated sequences are accompanied by the relative position of the perturbation in each sequence. This value is relevant because when a variant is too close to the chromosome arm end– meaning near centromeres or telomeres– it will be positioned near the edge of the sequence (Supplementary Fig. S1B), which can have worse prediction accuracy than the rest of the sequence (Kuang and Pollard 2023). Thus, SuPreMo is a flexible tool for performing ISM that can be applied across sequence-based ML models.
SuPreMo-Akita generates an array of 3D genome disruption scores, predicted contact frequency maps for reference (wild type) and alternate (perturbed) sequences, and genomic tracks of disruption scores across the prediction window. Akita predicts contact frequency maps for a ∼1 Mb input sequence at a ∼2 kb resolution. SuPreMo-Akita inputs variants as described above and optionally also takes in already generated sequences. Since methods for scoring contact maps are biased and sometimes only target certain features, we have made available 13 different predefined metrics (Gunsalus et al. 2023) to use with this tool, with the defaults being the most common measures: mean squared error (MSE) and Spearman’s rank correlation coefficient (referred to here as just correlation). To assess the robustness of the generated disruption scores, the augmentation parameter optionally provides averages of scores from standard sequences, sequences with −1 bp and +1 bp shifts, and reverse complement sequences, or any other augmentations specified. Each generated map will be accompanied by the start genomic position and the relative bin that the variant lies in.
Lastly, we considered computational efficiency. To enable customization to different hardware, the user can choose the number of rows to be processed at a time from the input file and what outputs to request, keeping in mind storage and memory limitations. We measured the run time, peak memory, and size of outputs on 3 GHz CPUs using a set of 100–1000 SVs of different types from the reference cancer cell line in Fig. 1B. SuPreMo-Akita is fast and easily scaled up—with the augmentation parameter it takes approximately 19 seconds per variant and reaches ∼527 Mb of peak memory (Supplementary Table S2).
We implemented SuPreMo using two models, although our framework is extendable to any model utilizing genome sequences as input. First, we used SuPreMo with DeepSEA (Zhou and Troyanskaya 2015) to rank a set of CTCF deletions based on their predicted effect on epigenetic marks. Second, we used SuPreMo-Akita on cancer SVs (Supplementary Fig. S3). SVs were scored using MSE and correlation, and the top 3 scoring variants for each SV type and scoring method were selected (Supplementary Fig. S3A–B). We separately ranked variants by their type because their 3D genome disruption scores vary, and by the scoring method because each has unique biases. Using SuPreMo-Akita, contact frequency maps and disruption tracks were generated for these selected SVs and the most interesting variants, based on the structures they disrupt, were chosen (Supplementary Fig. S3C). This method prioritized a deletion of an insulated site that is predicted to cause increased contact frequency between neighboring regions (Supplementary Fig. S3C, left panel). Step-by-step instructions for both implementations are available on Github.
3 Conclusion
SuPreMo is a software tool that facilitates ISM with predictive models and extends this principle with Akita to predict scores for 3D genome folding disruption. Potential use cases include scoring all variants in an individual or cohort for disruption to genome folding, generating predicted contact frequency maps to explore the effects of noncoding variants on regulatory interactions, performing ISM to evaluate or discover sequence motifs using Akita, and, more broadly, generating sequences for input into predictive models of interest to evaluate variant effects. SuPreMo is scalable to a large number of variants and only limited by the storage capacity the user has for the expected outputs. Overall, SuPreMo allows for easy, fast, and broadly applicable analysis of simple variants, SVs, and chromosomal rearrangements in the context of sequence-based predictive models.
Acknowledgements
We thank Shu Zhang for helpful discussion and feedback and for reviewing the manuscript. We thank Daniel Miller and others for helpful advice and test running the tool as well as Miguel Brown for useful suggestions on SuPreMo input compatibilities. We thank Maureen Pittman, Laura Gunsalus, and Shuzhen Kuang for helpful insight on using Akita.
Supplementary data
Supplementary data are available at Bioinformatics online.
Conflict of interest
None declared.
Funding
This work was supported by the National Institutes of Health [grant number R03OD034499 and grant number U01HL157989], Additional Ventures, and Gladstone Institutes.
Data availability
All code and data used for this study is posted on GitHub in the provided repository.



