-
PDF
- Split View
-
Views
-
Cite
Cite
Asad Naeem, Andrew P. French, Darren M. Wells, Tony P. Pridmore, High-throughput feature counting and measurement of roots, Bioinformatics, Volume 27, Issue 9, May 2011, Pages 1337–1338, https://doi.org/10.1093/bioinformatics/btr126
Close - Share Icon Share
Abstract
Summary: The original RootTrace tool has proved successful in measuring primary root lengths across time series image data. Biologists have shown interest in using the tool to address further problems, namely counting lateral roots to use as parameters in screening studies, and measuring highly curved roots. To address this, the software has been extended to count emerged lateral roots, and the tracking model extended so that strongly curved and agravitropic roots can be now be recovered. Here, we describe the novel image analysis algorithms and user interface implemented within the RootTrace framework to handle such situations and evaluate the results.
Availability: The software is open source and available from http://sourceforge.net/projects/roottrace.
Contact: [email protected]
Supplementary information: Supplementary data are available at Bioinformatics online.
1 INTRODUCTION
High-throughput measurement of plant roots is a key requirement in systems biology. The nature of biological experiments means the observation of many plants is necessary in order to understand growth processes, e.g. responses to stimuli and hormones. Traditionally, changes in growth were monitored by manual measurement of plants. Today, image analysis algorithms are being developed which allow such measurements as root length, curvature and/or architectural properties (Armengaud et al., 2009; Basu et al., 2007; Chavarría-Krauser et al., 2007; Le Bot et al., 2010) to be taken semi-automatically from an image. Some software also enables multiple measurements on sequences of images with little interaction from the user (French et al., 2009; Kolukisaoglu and Thurow, 2010; Nagel et al., 2009). Such analysis opens the door to high throughput quantification of plant growth.
Previous work has seen the release of such a tool, RootTrace (French et al., 2009) which is able to measure primary length of many individual roots, given standard digital camera images of plants grown on agar plates in growth chambers, as is common practice. RootTrace is able to record the growth of these roots over time-lapse image sequences. It is also able to record the curvature properties of each root trace, with user input only required on the first frame. In this article, we describe important extensions to the RootTrace algorithms, which additionally allow the counting of lateral roots, and a new tracking model which is able to track increasingly curved roots, the addition of which allows different biological questions to be answered. Both these extensions add to the high-throughput screening capabilities of the software.
2 METHODS
2.1 Tracing highly curved roots
The RootTrace framework uses top-down, particle filter-based tracking methods to trace from a user-defined start point to the root tip. The current estimate of growth direction present in the tracking model predicts the root location at each step forward. An appearance model is then used to evaluate hypotheses of location.
RootTrace version 1(RT1) assumed the root to be growing nominally in the direction of gravity. The tracker's motion model was set to move downwards one pixel (∼0.05 mm) at each step, reflecting the effect gravity has on root growth. Gravitropic responses could be observed, but the root could not be tracked if the root tip bent >90○. Additionally, some pre-rotating of images was required to enable RT1 to track the complete gravitropic response. There also exist agravitropic mutants (which can grow in any direction) and some chemical treatments which can invalidate the original gravity-based growth assumption. A new tracking model is required to examine these biological effects, which allows roots growing in any direction to be successfully traced. A polynomial fit of previous locations is now used to predict the position of the next portion of root (see Supplementary Video 1), combined with a ‘tabooing’ of previous locations to prevent backtracking.
The user tunes the tracking model via a single, intuitive, ‘blender bar’. With the bar in the leftmost position, RT1's gravity dependent model is employed. As the bar is moved to the right, fewer and more recent points are used to fit the prediction model, the order of the polynomials is gradually increased and the prediction is made closer to the current location (Fig. 1a and b). This improves tracking of more curved roots (Fig. 1h–j). A RootTrace version 2 (RT2) user is able to match the tracking model to the data to be analyzed without having to enter a numerical parameter.
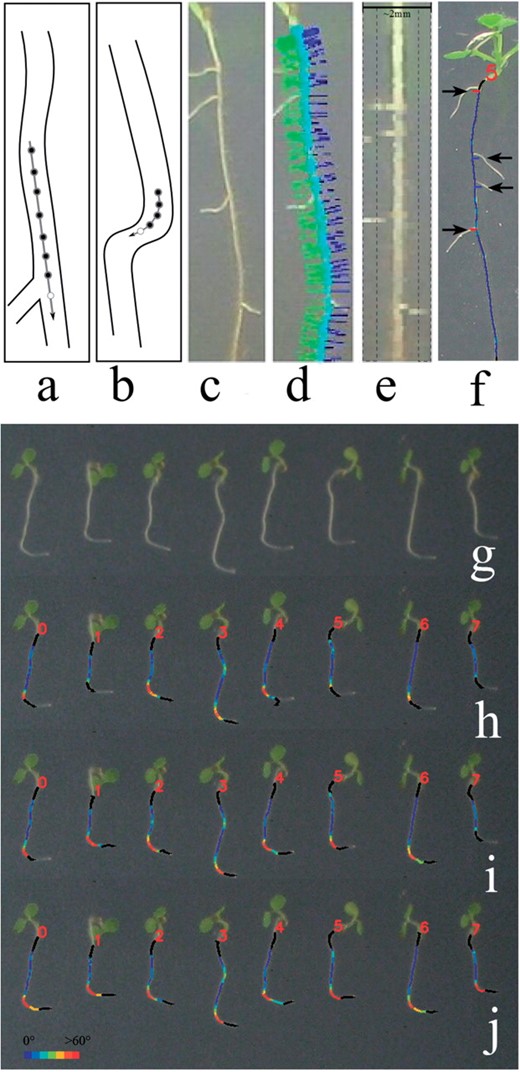
(a) Tracking prediction using a low-bending model and (b) a high-bending model, which uses a higher order polynomial, fits fewer points and predicts less far into the future; open circle is the current prediction. Processing: (c) original root, (d) 40 pixel mask based on angle of growth (e) mask contents, lateral detection regions marked. Outputs: (f) lateral detection, (g) original image showing a sharp gravitropic response. (h–j) Results of applying increasingly tracking-capable models using the blender bar; color indicates local curvature (red for high, blue for low).
2.2 Counting lateral roots
RootTrace has been extended to count the lateral roots on typically straighter primary roots, which is useful for high-throughput screening. As the tracker traverses the main root, its orientation is maintained. At each point a linear mask, 40 pixels long and perpendicular to the root, is read from the image (Fig. 1d). Plotting all the masks produces a straightened image of the main root with laterals on each side (Fig. 1e). The average intensity of this image is computed and anything over a threshold is considered to be root. Connected components are extracted from the regions to the side of the main root (Fig. 1e) and sufficiently large areas labeled as lateral roots (Fig. 1f). Laterals need to be >0.5 mm to enter the detection region. Also, the nature of connected components means very dense laterals may generate merged detections at this stage.
3 RESULTS
Figure 1h shows roots tracked using RT1's motion model; note the poor tracking around the bend, especially where the roots appear to grow upwards. Figure 1i and j show results obtained with the blender set to use models optimized for increasingly curved roots; tracking improves steadily. The mean absolute percentage error in lengths measured in Figure 1j versus a human-measured groundtruth is 2%.
Lateral root detection (e.g. Fig. 1f) was compared with manual identification of emerging laterals by a biologist (Table 1). A true positive marks a lateral at approximately the right position, on the correct side. The test was challenging, as the ground truth included emerging laterals only a few pixels in size whose existence could only be inferred with expert prior knowledge. Considering this, the results, especially the ratio of total lateral counts, are encouraging. This metric is important for high-throughput screening.
| Software . | Laterals detected / manual lateral count . | Sensitivity . | False alarm rate . |
|---|---|---|---|
| RT2 | 0.84 | 0.70 | 0.16 |
| Software . | Laterals detected / manual lateral count . | Sensitivity . | False alarm rate . |
|---|---|---|---|
| RT2 | 0.84 | 0.70 | 0.16 |
n = 24 plants.
| Software . | Laterals detected / manual lateral count . | Sensitivity . | False alarm rate . |
|---|---|---|---|
| RT2 | 0.84 | 0.70 | 0.16 |
| Software . | Laterals detected / manual lateral count . | Sensitivity . | False alarm rate . |
|---|---|---|---|
| RT2 | 0.84 | 0.70 | 0.16 |
n = 24 plants.
4 CONCLUSION
The top-down model-based approach used by RootTrace can now be intuitively tailored to meet the individual requirements of the biological data being analyzed. A new, gravity-independent tracking framework has been included, and a method of detecting lateral roots on typically straighter roots has been implemented.
ACKNOWLEDGEMENTS
We would like to thank Julien Lavenus for lateral root images.
Funding: The authors acknowledge Engineering and Physical Sciences Research Council, Biotechnology and Biological Sciences Research Council, Centres for Integrative Systems Biology programme funding to CPIB.
Conflict of Interest: none declared.
REFERENCES
Author notes
Associate Editor: John Quackenbush



