-
PDF
- Split View
-
Views
-
Cite
Cite
Semin Lee, Tom L. Blundell, Ulla: a program for calculating environment-specific amino acid substitution tables, Bioinformatics, Volume 25, Issue 15, August 2009, Pages 1976–1977, https://doi.org/10.1093/bioinformatics/btp300
Close - Share Icon Share
Abstract
Summary: Amino acid residues are under various kinds of local environmental restraints, which influence substitution patterns. Ulla,1 a program for calculating environment-specific substitution tables, reads protein sequence alignments and local environment annotations. The program produces a substitution table for every possible combination of environment features. Sparse data is handled using an entropy-based smoothing procedure to estimate robust substitution probabilities.
Availability: The Ruby source code is available under a Creative Commons Attribution-Noncommercial License along with additional documentation from http://www-cryst.bioc.cam.ac.uk/ulla.
Contact: [email protected]
Supplementary information: Supplementary data are available at Bioinformatics online.
1 INTRODUCTION
In the evolution of proteins, individual amino acid residues are under various kinds of local environmental restraints such as secondary structure type, solvent accessibility and hydrogen bonding patterns. Previous study of amino acid substitutions as a function of local environment has showed that there are clear differences among substitution patterns under various environmental restraints (Overington et al., 1992). The unique patterns of amino acid substitutions have been successfully exploited to predict the stability of protein mutants (Topham et al., 1993), to identify potential interaction sites (Chelliah et al., 2004; Gong and Blundell, 2008) and to detect remote sequence-structure homology (Chelliah et al., 2005).
However, estimating amino acid substitution probabilities is not a trivial problem, especially when there are a very small number of observations in specific combinations of environments. To cope with the sparse data problem, an algorithm was developed by Sali (1991) as an extension of the method used by Sippl (1990) to derive robust potentials of mean force. Several variants of the generalized procedure such as Makesub (Topham et al., 1993) and SUBST (Mizuguchi, unpublished results) have been subsequently implemented for smoothing substitution probabilities. Nevertheless, each lacks crucial features implemented in the other, and they use slightly different procedures for smoothing substitution probabilities, which may lead to very different amino acid substitution matrices.
To overcome these problems, we developed Ulla, a program for calculating environment-specific substitution tables (ESSTs), to unify all the major features of the previously developed programs and to provide additional functionalities. The program also generates heat maps from substitution tables to visualize the degree of conservation of amino acids under the environmental restraints.
2 DESCRIPTION
Ulla reads multiple sequence alignments and annotations for local environments in JOY template format (Mizuguchi et al., 1998a). Users can provide their own definition of environment features, and an environment feature can be constrained to count substitutions only when the environment of residues is conserved. Ulla also supports confining percent identity (PID) range of sequence pairs to be considered and uses BLOSUM-like weighting scheme (Henikoff and Henikoff, 1992) to minimize sampling bias from highly similar sequences.
Ulla uses entropy-based smoothing procedures to reduce problems caused by sparse data. It is an iterative procedure that estimates probability distribution by perturbing the previous probability distribution with the successive measurement (Sali, 1991; Sippl, 1990). Hence, starting from a uniform frequency distribution, the estimated probability distribution at each step serves as an approximation for the next probability distribution (see Supplementary Material for details).
3 EXAMPLE USAGE
As an illustration, we generate ESSTs from HOMSTRAD alignments (Mizuguchi et al., 1998b) with environment feature definitions of secondary structure type and solvent accessibility (Fig. 1a):
Actual annotations for the environment features need to be provided in PIR format: 
# name of feature (string);\\
# values adopted in .tem (alignment) file (string);\\
# class labels assigned for each value (string);\\
# constrained or not (T or F);\\
# silent (used as masks)? (T or F) secondary structure and phi angle;HEPC;HEPC;F;F solvent accessibility;TF;Aa;F;F
JOY (Mizuguchi et al., 1998a) is useful to annotate the alignments with the structural environments, but Ulla recognizes any environment feature definition which conforms to the format above. Paths for an environment definition file and a file containing the list of environment feature annotated alignments are given to Ulla as input: Ulla produces three different types of substitution tables: raw counts tables, substitution probability tables and log-odds ratio tables. Heat maps also can be generated to visualize resultant substitution tables (Fig. 1b).
$ ulla -c feature.def -l alignments.lst
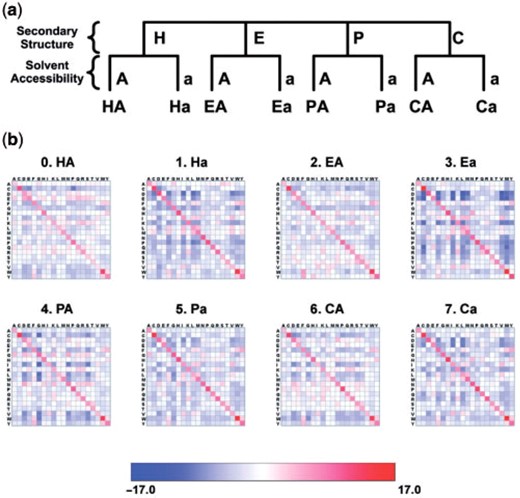
Environment feature combinations and ESST generation. (a) The environment features are secondary structure type (H: helix, E: beta sheet, P: positive phi, C: coil) and solvent accessibility (A: solvent accessible, a: solvent inaccessible). Eight sets of combinations of environment features are generated. (b) Heat maps from each of resultant ESSTs. Blue to red indicates log-odds ratio of substitution probabilities.
4 CONCLUSION
Ulla generates ESSTs from a sparse data set using entropy-based smoothing procedures. It allows us to conduct analyses of amino acid substitution patterns under various environmental restraints. The resultant ESSTs can be exploited in many ways such as binding site prediction, remote homology detection, and protein stability estimation.
Ulla is publicly available on the web site http://github.com/semin/ulla, where the code is maintained in a Git repository, and its pre-built RubyGems package can be obtained from http://rubyforge.org/projects/ulla.
1Ulla is a traditional Korean percussion instrument.
ACKNOWLEDGEMENTS
We thank Juok Cho for statistical advice; Dan Bolser and Duangrudee Tanramluk for review of the manuscript; Richard Bickerton and Bernardo Ochoa for thorough beta testing.
Funding: Mogam Science Scholarship Foundation (to S.L., partial); The Wellcome Trust (to T.L.B.)
Conflict of Interest: none declared.
REFERENCES
Author notes
Associate Editor: Anna Tramontano



