-
PDF
- Split View
-
Views
-
Cite
Cite
Naomi Dunning, Leishmania vaccines: from leishmanization to the era of DNA technology, Bioscience Horizons: The International Journal of Student Research, Volume 2, Issue 1, March 2009, Pages 73–82, https://doi.org/10.1093/biohorizons/hzp004
Close - Share Icon Share
Abstract
Leishmania are obligate intracellular vector-borne parasites that cause significant morbidity and mortality in many countries worldwide. There are several species of the parasite which vary according to geographical location and cause a variety of clinical syndromes ranging from self-limiting cutaneous lesions to potentially fatal infection of the viscera. The disease manifested is dependent on both the species of the parasite and the immune response of the host. Depending on the species of the parasite, resistance to infection is generally associated with a T-helper-1 immune response that activates macrophages to kill intracellular Leishmania in a nitric oxide-dependent manner. Conversely, disease progression is generally associated with a T-helper-2 response that activates humoral immunity. Chemotherapeutic treatments for leishmaniasis exist but are expensive, toxic and ineffective against resistant strains. A vaccine against leishmaniasis is feasible since most individuals that were once infected become resistant to clinical infection when later exposed. However, despite the wealth of information regarding the genetics of the parasite and the experimental immunology of the disease, there is currently no vaccine against Leishmania. A multitude of vaccine strategies have been pursued including the use of killed and genetically modified parasites. Immunization with naked plasmid DNA encoding Leishmania antigens represents a new approach to a Leishmania vaccine and confers several advantages over the more traditional vaccination methods. In order to develop an effective vaccine against leishmaniasis, it is important to understand the mechanisms of the immune response to Leishmania infection. This review discusses such immune mechanisms in detail and also explores several of the Leishmania vaccination strategies employed to date, with particular emphasis on DNA vaccines.
Introduction
Leishmaniasis has been identified as a category 1 disease by the World Health Organisation (WHO) and a rising cause for concern as an emerging disease with the advent of HIV-Leishmania coinfection.1, 2Leishmania spp. cause a wide variety of diseases that range in severity from self-healing cutaneous leishmaniasis to fatal disseminated visceral leishmaniasis.3 The disease manifested is determined by both the species of Leishmania and the host immune system, although certain species are associated with specific clinical conditions. For example, visceral leishmaniasis usually results from infection with either L. donovani or L. infantum.4
Although chemotherapeutic treatments for the leishmaniases exist, the drugs are costly, limited and toxic.5 Furthermore, available treatments are threatened by drug resistance, which is reviewed by Croft et al.6 Even with treatment, various disease forms can cause lifelong disfigurement and scarring. Thus, as with all infectious diseases, prevention of leishmaniasis is superior to a cure. A prophylactic vaccination would prove to be the most effective strategy to control infection and spreading of this group of diseases.7 However, despite substantial effort spent in developing a vaccine, there is currently no licensed vaccine against human leishmaniasis.1 In order to develop an effective vaccine, it is important to understand the mechanisms of the immune response to Leishmania infection so that the vaccine can be engineered to induce a protective response rather than one that could result in susceptibility to the parasite.
The Immune Response to Leishmania infection
The Immune response to Leishmania infection is dependent on both the species of the parasite infecting the host and the genetics of the host. The severity of disease caused by a particular species may vary markedly between individuals; hence, one species of Leishmania can cause more than one clinical syndrome.8 There are several key components involved in the immune response to Leishmania. The outcome of infection is largely dependent on the ability of the host to mount a protective T-helper-1 (Th1) response versus the ability of the parasite to evade and manipulate the host's immune system.4
Macrophages and effector molecules, dendritic cells (DC), T-helper cells (CD4+ T cells), cytotoxic T cells (CD8+ T cells), natural killer (NK) cells and cytokines are all, in one way or another, considered to play important roles in the immune response to Leishmania infection.9
Macrophages are phagocytic antigen-presenting cells (APC) that play host to Leishmania when promastigotes bind to their specific receptors and are internalized by receptor-mediated phagocytosis in a process which is crucial for the establishment of infection in the host.10 The primary mechanism for the elimination of Leishmania occurs upon activation of macrophages by IFN-γ, secreted by NK cells and T-helper-1 cells (Th1), which enables them to kill intracellular Leishmania amastigotes in a nitric oxide (NO)-dependent manner11 (Fig. 1). Studies have demonstrated that the inhibition of nitric oxide production from inducible nitric oxide synthase (iNOS) (by Leishmania) renders macrophages powerless against Leishmania infection.12

Antagonistic Th1 and Th2 responses that confer either resistance or susceptibility to Leishmania. APC, antigen-presenting cell; Th, T-helper cell; IL, interleukin; TGF-β, transforming growth factor; TFN-α/β, tumour necrosis factor-α/β; IFN-γ, interferon-gamma; Ig, immunoglobulin.
Opsonization, the process whereby a pathogen becomes coated with antibody or complement to render it more readily ingested by phagocytic cells, is a protective immune response. However, studies have demonstrated that opsonization of Leishmania major promastigotes with the third component of complement (C3) dramatically enhances their survival inside macrophages.10 Providing that the parasitized macrophages do not become activated, they provide a safe breeding ground for Leishmania parasites which are capable of enhancing macrophage survival after infection.12 Therefore, it is evident that macrophages play an important role in both resistance and susceptibility to Leishmania infection.
Sanabria et al.13 emphasized the importance of the interaction between NK cells and DC in the immune response to Leishmania amazonensis. They found that NK cells promoted the activation of L. amazonensis infected DC and that in turn, the activated DC stimulated NK activation. These findings imply differential roles of DC–NK cross-talk at various stages of Leishmania infection and present new insight on the interactions between innate immune components during infection with this parasite.13
Vanloubbeeck and Jones4 outlined the unique and central role of DC in pathogen-specific immunity in that they present antigens to CD4+ T cells (T-helper cells) on MHC class II as well as presenting them to CD8+ T cells (cytotoxic T cells) on MHC class I. This is known as cross-priming and is important because both CD8+ T cells and CD4+ T cells are considered to be involved in the immune defence against Leishmania.4 DC play an essential role in conferring resistance or susceptibility to Leishmania by driving the differentiation and proliferation of T-helper cells (CD4 + ) to either T-helper-1 cells (Th1) or T-helper-2 cells (Th2).14 On presentation of Leishmania antigens to CD4+ T cells, the concomitant secretion of IL-12 drives the proliferation of (IFN-γ secreting) Th1 cells and NK cells, which activate macrophages and inhibit Th2 responses.15 Conversely, the secretion of IL-4 during antigen presentation to CD4+ T cells drives Th2 cell development that inhibits Th1 responses and promotes B lymphocyte growth and development14 (Fig. 1). Therefore, it is evident that DC play an essential role in both initiation and regulation of antimicrobial immune responses to Leishmania.16
The role of Langerhans cells (LC), a specific subset of skin DC located in the supra-basal epidermal layer, has recently been revised by Ritter et al.17 Although they were previously thought to fulfil the same role as other DC, Ritter et al.17 proposed that LC have a regulatory function and may be responsible for the suppression of the inflammatory response against L. major. This is of great importance as it indicates that LC could induce Leishmania-specific immunosupression instead of parasite control and therefore has major implications against the use of LC in vaccine studies.18
It has been postulated that the type of immune response to Leishmania (Th1 versus Th2) is dependent on the type of leishmanial antigen presented and recognized by T cells. Therefore, Th1 responses may be initiated by different antigens to Th2 responses.8 However, various animal studies have implicated that the same parasite epitope may induce a Th1 response in animals with resolving infection or a Th2 response in others susceptible to the disease.2
Many have observed that a Th2 response to Leishmania infection, which inhibits inflammatory responses, renders the host more susceptible to disease and that, conversely, resistance is associated with a Th1 response.4, 5, 8, 14, 19, 20
Macatonia et al.21 demonstrated that while DC were capable of induction and clonal expansion of T-helper cells, they were unable to induce T cell differentiation towards Th1 or Th2 without IL-12 and IL-4, respectively. This emphasizes the importance of these cytokines in the immune response to Leishmania. Remarkably, the roles of IL-12 and IL-4 in antagonistic Th1 and Th2 responses were uncovered based upon observations with L. major.20
The protective role of IL-12 has been demonstrated. In one study IL-12-deficient mice, originally derived from a strain genetically resistant to infection with L. major, were susceptible to infection with this parasite.22 However, Vanloubbeeck and Jones4 found that promoting Th1 polarization of CD4+ T cells, using IL-12 as an adjuvant, was not enough to offer resistance to L. amazonensis.
The role of IL-4 in disease progression has been implicated in several studies in which the administration of anti-CD4+ and anti-IL-4 antibodies healed Leishmania infection.8 Furthermore, Kopf et al.23 demonstrated that the disruption of the IL-4 gene in susceptible BALB/c mice rendered them resistant to infection with L. major, a finding that clearly reveals the effects of this cytokine on disease progression.
Figure 1 demonstrates the antagonistic Th1 and Th2 responses and the role of each cytokine in response to Leishmania infection.
Cytotoxic T lymphocytes (CTL) are essential in the defence against viruses yet their function with respect to intracellular parasites, such as Leishmania, remains somewhat elusive.15
Experiments conducted using mice deficient in MHC class II/CD4+ T cells and mice deficient in MHC class I/CD8+ T cells have indicated that CD8+ T cells are not required for controlling Leishmania infection, as mice deficient in these cells were able to control the infection with L. major. This was in contrast to MHC class II/CD4+ T cell deficient mice that suffered fatal and uncontrolled infection.8 Since Leishmania reside in the parasitophorous vacuole within macrophages, the route of peptide loading onto MHC class I and the mechanism of CD8+ T cell activation remain unclear.15 Therefore, it is tempting to speculate that these cells play only a minor role in immunity to Leishmania. However, activation of cytotoxic T cells may occur as a result of cross-priming, a phenomenon which enables DC to take up extracellular antigens and present them on MHC class I to CD8+ T cells.4
The cytokine-producing capability of CTL may play a role in the defence against Leishmania infection as they participate through IFN-γ production that is essential in the host defence against Leishmania.15 Muller et al.24 found that CTL were involved in the elimination of L. major as well as establishment and maintenance of immunity as the inhibition of CTL with monoclonal antibodies rendered resistant mice susceptible to L. major. Basu et al.25 demonstrated that CD8+ T cells are vital in a protective response against L. donovani following hybrid cell vaccination, as the depletion of these cells resulted in a higher parasite burden in the spleen and liver. Contrary to these studies, others have shown that CD8+ T cells may contribute towards disease progression as large numbers of these cells have been isolated in acute phase lesions and in the peripheral blood.15
A recent study by Ali et al. (personal communication, School of Science and Technology, NTU) has implicated that L. mexicana are capable of MHC class I downregulation. This is a significant finding and may perhaps explain why the role of CTL in the immune response to Leishmania has been difficult to establish.
Currently little is known about the role of cytotoxic T cells in the hosts defence against Leishmania infection. Therefore, further investigation must be undertaken in order to fully establish their role. Like viruses, Leishmania are intracellular organisms; therefore, it is feasible that CTL could target and eliminate parasitized macrophages in the manner in which they locate and eliminate virus-infected cells.
Immune Evasion by Leishmania
Survival of Leishmania within the mammalian host depends on their ability to evade and manipulate the anti-leishmanial immune response, thus enabling them to reside long enough to establish infection.19 Since Leishmania are intracellular parasites, once inside the host cell, they evade the humoral immune response but become susceptible to attack by cell-mediated immunity. However, despite the presentation of Leishmania antigens and the initiation of inflammatory responses, Leishmania can reside within the host tissues for long periods by exploiting several mechanisms of evasion.26 There are numerous strategies utilized by Leishmania to survive within mammalian hosts, some of which are common to other protozoan parasites such as Toxoplasma gondii and Trypanosoma cruzi.12 The various strategies employed by the invading pathogens demonstrate how Leishmania parasites have evolved to successfully evade the immune responses designed to target and destroy them.26 Such evasion strategies include the modulation of macrophage function and the inhibition of antigen presentation and T cell stimulation. Investigation of this parasite's interaction with the host's immune system may lead to a better understanding of how Leishmania infection can be resisted or the evasion strategies of these parasites which influence susceptibility of the host. Improved understanding of these areas should assist in the development of an effective prophylactic vaccine, capable of inducing the specific immune responses required to battle this parasite.
Vaccination against Leishmaniasis
Evidence that most individuals that were once infected with Leishmania are resistant to clinical infections, when later exposed, provides the justification for vaccine development.1 Although there is no licensed vaccine against any form of leishmaniasis for general human use, a vaccine against different forms should, in theory, be possible. This is considering the abundance of genetic and biological information about the parasite, clinical and experimental immunology of the disease, and the availability of vaccines that offer protection in experimental animals against challenge with different Leishmania species.27 The leishmaniases are unique among parasitic diseases because a single vaccine could successfully prevent and treat disease and has the potential to protect against more than one species (disease).28 To date, there have been numerous attempts at developing a successful vaccine against leishmaniasis and there are several categories of vaccine candidates.
Table 1 demonstrates the main categories of Leishmania vaccines in development, according to Khamesipour et al., 27 with some specific subcategories and examples of each.
| Category . | Subcategory . | Principle . | Example . | Result . |
|---|---|---|---|---|
| Live Leishmania (virulent or attenuated) | Live virulent | Vaccination with live, virulent Leishmania | Leishmanization (using L. major)29 | • Life-long protection against L. major after cure |
| Live non-pathogenic to humans | Vaccination with a live species that is non-pathogenic to humans | Leishmania tarentolae31 | • L. tarentolae induced a protective immune response against L. donovani | |
| Knock-out parasites | Removal/blocking/replacement of parasite genes essential for survival | DHFR-TS (enzyme gene) L. major knock-out27 | • Survival of knock-out in mice for about 2 months without producing a lesion. • Conferred short-term protection against wild type | |
| Suicidal cassettes | Genetic modification of parasite to induce suicide in response to external signals/to produce biological substances that activate immune attack against them | L. major strains producing biologically active granulocyte-macrophage colony-stimulating factor (GM-CSF)28 | • Enhanced parasite killing • Delayed lesion development in susceptible BALB/c mice | |
| Killed Leishmania or fractions | Whole killed parasite | A single species of killed promastigotes or more than one species | Killed single strain of L. amazonensis27 | • Significant protection from natural infection • Long lasting Th1 responses |
| Whole killed parasite and adjuvant | Use of whole dead promastigotes and an adjuvant to stimulate immune response | Killed L. mexicana/L. braziliensis promastigotes and BCG29 | • Used immunotherapeutically it induced a high cure rate even in severe cases • Cure accompanied by development of Th1 immune response | |
| Fraction of a killed parasite and adjuvant | Use of Leishmania antigens and an adjuvant to stimulate an immune response | Fructose Mannose Ligand (FML) antigen from surface of parasite and saponin adjuvant (Leishmune®)39 | • First vaccine against canine visceral leishmaniasis • Potential transmission-blocking vaccine | |
| Recombinant and synthetic | DNA vaccination | Introduction of bacterial plasmid DNA encoding antigens into host cells in vivo | Multi-antigenic DNA vaccine encoding KMPII, TRYP, LACK and CP636 | • Unable to induce protection in dogs against L. infantum |
| DNA vaccine against parasite enzyme gamma-glutamylcysteine synthetase (γ-GCS)48 | • Production of IgG1 and IgG2a • Increased cell-mediated immunity | |||
| Recombinant protein vaccination | Vaccination with recombinant proteins | Recombinant hydrophilic acylated surface protein B1 (HASPB1)35 | • Protection against experimental challenge with L. donovani |
| Category . | Subcategory . | Principle . | Example . | Result . |
|---|---|---|---|---|
| Live Leishmania (virulent or attenuated) | Live virulent | Vaccination with live, virulent Leishmania | Leishmanization (using L. major)29 | • Life-long protection against L. major after cure |
| Live non-pathogenic to humans | Vaccination with a live species that is non-pathogenic to humans | Leishmania tarentolae31 | • L. tarentolae induced a protective immune response against L. donovani | |
| Knock-out parasites | Removal/blocking/replacement of parasite genes essential for survival | DHFR-TS (enzyme gene) L. major knock-out27 | • Survival of knock-out in mice for about 2 months without producing a lesion. • Conferred short-term protection against wild type | |
| Suicidal cassettes | Genetic modification of parasite to induce suicide in response to external signals/to produce biological substances that activate immune attack against them | L. major strains producing biologically active granulocyte-macrophage colony-stimulating factor (GM-CSF)28 | • Enhanced parasite killing • Delayed lesion development in susceptible BALB/c mice | |
| Killed Leishmania or fractions | Whole killed parasite | A single species of killed promastigotes or more than one species | Killed single strain of L. amazonensis27 | • Significant protection from natural infection • Long lasting Th1 responses |
| Whole killed parasite and adjuvant | Use of whole dead promastigotes and an adjuvant to stimulate immune response | Killed L. mexicana/L. braziliensis promastigotes and BCG29 | • Used immunotherapeutically it induced a high cure rate even in severe cases • Cure accompanied by development of Th1 immune response | |
| Fraction of a killed parasite and adjuvant | Use of Leishmania antigens and an adjuvant to stimulate an immune response | Fructose Mannose Ligand (FML) antigen from surface of parasite and saponin adjuvant (Leishmune®)39 | • First vaccine against canine visceral leishmaniasis • Potential transmission-blocking vaccine | |
| Recombinant and synthetic | DNA vaccination | Introduction of bacterial plasmid DNA encoding antigens into host cells in vivo | Multi-antigenic DNA vaccine encoding KMPII, TRYP, LACK and CP636 | • Unable to induce protection in dogs against L. infantum |
| DNA vaccine against parasite enzyme gamma-glutamylcysteine synthetase (γ-GCS)48 | • Production of IgG1 and IgG2a • Increased cell-mediated immunity | |||
| Recombinant protein vaccination | Vaccination with recombinant proteins | Recombinant hydrophilic acylated surface protein B1 (HASPB1)35 | • Protection against experimental challenge with L. donovani |
| Category . | Subcategory . | Principle . | Example . | Result . |
|---|---|---|---|---|
| Live Leishmania (virulent or attenuated) | Live virulent | Vaccination with live, virulent Leishmania | Leishmanization (using L. major)29 | • Life-long protection against L. major after cure |
| Live non-pathogenic to humans | Vaccination with a live species that is non-pathogenic to humans | Leishmania tarentolae31 | • L. tarentolae induced a protective immune response against L. donovani | |
| Knock-out parasites | Removal/blocking/replacement of parasite genes essential for survival | DHFR-TS (enzyme gene) L. major knock-out27 | • Survival of knock-out in mice for about 2 months without producing a lesion. • Conferred short-term protection against wild type | |
| Suicidal cassettes | Genetic modification of parasite to induce suicide in response to external signals/to produce biological substances that activate immune attack against them | L. major strains producing biologically active granulocyte-macrophage colony-stimulating factor (GM-CSF)28 | • Enhanced parasite killing • Delayed lesion development in susceptible BALB/c mice | |
| Killed Leishmania or fractions | Whole killed parasite | A single species of killed promastigotes or more than one species | Killed single strain of L. amazonensis27 | • Significant protection from natural infection • Long lasting Th1 responses |
| Whole killed parasite and adjuvant | Use of whole dead promastigotes and an adjuvant to stimulate immune response | Killed L. mexicana/L. braziliensis promastigotes and BCG29 | • Used immunotherapeutically it induced a high cure rate even in severe cases • Cure accompanied by development of Th1 immune response | |
| Fraction of a killed parasite and adjuvant | Use of Leishmania antigens and an adjuvant to stimulate an immune response | Fructose Mannose Ligand (FML) antigen from surface of parasite and saponin adjuvant (Leishmune®)39 | • First vaccine against canine visceral leishmaniasis • Potential transmission-blocking vaccine | |
| Recombinant and synthetic | DNA vaccination | Introduction of bacterial plasmid DNA encoding antigens into host cells in vivo | Multi-antigenic DNA vaccine encoding KMPII, TRYP, LACK and CP636 | • Unable to induce protection in dogs against L. infantum |
| DNA vaccine against parasite enzyme gamma-glutamylcysteine synthetase (γ-GCS)48 | • Production of IgG1 and IgG2a • Increased cell-mediated immunity | |||
| Recombinant protein vaccination | Vaccination with recombinant proteins | Recombinant hydrophilic acylated surface protein B1 (HASPB1)35 | • Protection against experimental challenge with L. donovani |
| Category . | Subcategory . | Principle . | Example . | Result . |
|---|---|---|---|---|
| Live Leishmania (virulent or attenuated) | Live virulent | Vaccination with live, virulent Leishmania | Leishmanization (using L. major)29 | • Life-long protection against L. major after cure |
| Live non-pathogenic to humans | Vaccination with a live species that is non-pathogenic to humans | Leishmania tarentolae31 | • L. tarentolae induced a protective immune response against L. donovani | |
| Knock-out parasites | Removal/blocking/replacement of parasite genes essential for survival | DHFR-TS (enzyme gene) L. major knock-out27 | • Survival of knock-out in mice for about 2 months without producing a lesion. • Conferred short-term protection against wild type | |
| Suicidal cassettes | Genetic modification of parasite to induce suicide in response to external signals/to produce biological substances that activate immune attack against them | L. major strains producing biologically active granulocyte-macrophage colony-stimulating factor (GM-CSF)28 | • Enhanced parasite killing • Delayed lesion development in susceptible BALB/c mice | |
| Killed Leishmania or fractions | Whole killed parasite | A single species of killed promastigotes or more than one species | Killed single strain of L. amazonensis27 | • Significant protection from natural infection • Long lasting Th1 responses |
| Whole killed parasite and adjuvant | Use of whole dead promastigotes and an adjuvant to stimulate immune response | Killed L. mexicana/L. braziliensis promastigotes and BCG29 | • Used immunotherapeutically it induced a high cure rate even in severe cases • Cure accompanied by development of Th1 immune response | |
| Fraction of a killed parasite and adjuvant | Use of Leishmania antigens and an adjuvant to stimulate an immune response | Fructose Mannose Ligand (FML) antigen from surface of parasite and saponin adjuvant (Leishmune®)39 | • First vaccine against canine visceral leishmaniasis • Potential transmission-blocking vaccine | |
| Recombinant and synthetic | DNA vaccination | Introduction of bacterial plasmid DNA encoding antigens into host cells in vivo | Multi-antigenic DNA vaccine encoding KMPII, TRYP, LACK and CP636 | • Unable to induce protection in dogs against L. infantum |
| DNA vaccine against parasite enzyme gamma-glutamylcysteine synthetase (γ-GCS)48 | • Production of IgG1 and IgG2a • Increased cell-mediated immunity | |||
| Recombinant protein vaccination | Vaccination with recombinant proteins | Recombinant hydrophilic acylated surface protein B1 (HASPB1)35 | • Protection against experimental challenge with L. donovani |
Live-Non-attenuated Vaccines
‘Leishmanization’, the deliberate inoculation of virulent Leishmania from the exudate of a cutaneous lesion, is the oldest vaccination against cutaneous leishmaniasis and has been practiced for centuries.29 This is because it has long been well established that recovery from cutaneous leishmaniasis is followed by long lasting immunity to the disease.30 Therefore, the parasite could be deliberately introduced to a concealed area of the body rather than risking multiple lesions to the face or other exposed regions.27 This approach was later developed and live virulent L. major promastigotes were harvested and used in large-scale vaccination trials during the 1970s and 1980s in Israel, Iran and the Soviet Union.27, 29 Although still practiced in Uzbekistan, the observation of adverse side effects, including the development of large persistent lesions, psoriasis and immunosuppression, led to the discontinuation of Leishmanization in many countries and the focus of vaccine development consequently shifted towards killed organisms.28, 29
Nowadays, the development of a new vaccine must meet several strict criteria where safety, reproducibility and efficacy are of utmost importance.27 Although Leishmanization gave a high percentage of successful lesion development and subsequent immunity to L. major infection, it was neither reproducible nor safe. Furthermore, the viability and infectivity of the injected parasites varied and organisms without virulence induced delayed-type hypersensitivity.29 It is not surprising therefore that Leishmanization is not recommended by WHO.1
Another approach to live-non-attenuated vaccines has been taken by Breton et al.31 who used a non-pathogenic species, Leishmania tarentolae, to immunize mice. The high level of immunological cross-reactivity between species at both the humoral and cellular level provides the rationale for using non-pathogenic Leishmania species to immunize against virulent species.32 Breton et al.31 believe it is a promising approach to vaccination against visceral leishmaniasis caused by L. donovani as BALB/c mice were able to elicit a protective immune response after only a single peritoneal vaccination. Although the results to this study appear promising, vaccination with live-non-attenuated parasites is not an appealing prospect and the attenuation of live parasites to render them avirulent offers a safer, more stable approach to live vaccination.
Live-Attenuated Vaccines
Genetic modification of Leishmania parasites to reduce virulence, yet maintain immunogenicity, is of current interest in Leishmania vaccine research. It is an appealing approach as attenuated parasites closely mimic natural infection that may lead to similar immune responses without the danger associated with infection with live virulent parasites.29 Due to advances in molecular biology and the genomic sequencing of L. major Friedlin, the attenuation of Leishmania parasites by removing, blocking or replacing essential genes is a possibility.28, 32 Such parasites are referred to as ‘knock-out parasites’. Leishmania can be engineered without the genes required for long-term survival in the host, thus making them safe to immunize with.29 The first Leishmania knock-out was a dihydrofolate reductase thymidylate synthase gene (DHFR-TS) in L. major. This knock out induced significant, but temporary, protection in mice when challenged with the wild type but gave disappointing results during further studies in monkeys.27 The production of Leishmania knock-outs can identify essential virulence genes. Therefore, as well as providing vaccine candidates these studies can lead to a better understanding of the parasite. Furthermore, studies using knock-out parasites may be key in identifying specific genes that can then be applied to DNA vaccines. For example, work by Selvapandiyan et al., 33, 34 which highlighted the importance of the centrin proteins for the duplication and cell cycle progression of Leishmania amastigotes, provided the justification for the use of the centrin genes as DNA vaccine candidates by Ali et al. (2008, personal communication).
Another approach to attenuating Leishmania parasites is the addition of suicide cassettes that lead to the death of the parasite in response to external stimuli.27 An example of which is the introduction of drug-sensitive genes such as Saccharomyces cerevisiae cytosine deaminase gene which is sensitive to 5-fluorocytosine.35 It has been suggested that parasites carrying drug-sensitive cassettes could provide suitable candidates for Leishmanization as an effective treatment of non-resolving lesions could be guaranteed.35 Alternatively, parasites can be modified to produce biological substances that activate immune attack, such as granulocyte macrophage colony stimulating factor (GM-CSF).36
Attenuated vaccines offer a novel approach to immunization against leishmaniasis however, there are fears that the parasite may revert back to a virulent form. Furthermore, targeted deletion of essential virulence genes can result in complete destruction of the parasite or mutants that only delay lesion development.31 Such problems are not a concern with the use of killed parasites for vaccine candidates.
Killed Vaccines
Killed Leishmania is an appealing vaccine candidate in terms of its stable biochemical composition and antigenicity, low cost and safety.37 There have been several studies on killed Leishmania, with or without adjuvants, as a vaccine. Early trials began in Brazil during the 1940s; however, these yielded conflicting results.29 Most killed vaccine studies in America have used L. amazonensis autoclaved lysate or a mixture of native species, whereas L. major is used in most vaccine studies against Old World leishmaniasis.35 In Venezuela, Convit and his colleagues introduced an autoclaved L. mexicana + BCG for immunotherapy and/or immunochemotherapy, which proved unsuccessful as the results were either inconclusive or demonstrated low protection induced by the vaccine.27 Similarly, killed Leishmania vaccines failed to confer protection in persons in the Middle East.29 However, studies in the 1980s revolutionized the use of killed Leishmania as a vaccine by demonstrating excellent protection in mice injected intravenously or intraperitoneally (but not subcutaneously) and subsequently several formulations of killed vaccines have been developed.27 This emphasizes that the site of administration affects the efficacy of a vaccine; thus, it is therefore important to investigate the most effective method of inoculation. In Venezuela, autoclaved L. mexicana is currently used immunotherapeutically to treat patients with cutaneous leishmaniasis.27
In countries with a rudimentary biotechnology industry and where a cold-chain distribution of vaccines is not possible, autoclaving of the killed vaccine is the recommended method of sterilization and preservation.35 However, as demonstrated by De Luca et al.,38 autoclaving lowers the immunogenicity of the parasite by destroying most of the proteins. Therefore, while offering a safer and more stable alternative to live vaccination, vaccination with killed Leishmania does not mimic natural infection and is also less immunogenic.
Vaccines of Leishmania Fractions
Leishmune®, the first vaccine against canine visceral leishmaniasis, consists of a purified L. donovani fraction, named fructose mannose ligand (FML) and a saponin adjuvant.39 FML has been characterized as a major antigenic complex of L. donovani and the main antigen in this complex is NH36, an essential enzyme involved in the construction of the parasite's DNA.35 Leishmune® is considered a promising tool for the prevention of canine visceral leishmaniasis and furthermore its potential as a transmission-blocking vaccine is promising for the control of zoonotic visceral leishmaniasis.39
Recombinant Protein Vaccines
Recombinant protein vaccines are produced by genetically engineered cells to produce foreign genes that encode antigenic proteins.27 They are relatively new and can be administered as purified proteins or as bacteria manufacturing the proteins in situ.29 Various proteins have been tested as possible vaccine candidates including recombinant hydrophilic acylated surface protein B1 (HASPB1) that conferred protection against experimental challenge with L. donovani.40
Novel Vaccine Strategies
To date, there have been many attempts at designing an effective vaccine against Leishmaniasis, most of which have fallen into the categories previously described. However, in addition to these there have been several novel approaches that have also proven to be promising. Such approaches include the use of DC pulsed with Leishmania antigens and vaccines comprised of components of sand fly saliva.16, 35
In light of their crucial role in immunity to Leishmania, recent studies have implicated DC as potential vaccine candidates serving as vectors for Leishmania antigens. Moll and Berberich16 observed that DC pulsed ex vivo with L. major antigen induced protection, in otherwise susceptible mice, against live challenge with L. major.
Since sand fly saliva is documented to enhance the infectivity of Leishmania, vaccines have been engineered against its components. One such component is the SP15 antigen that was shown to induce significant resistance in mice to challenge with L. major.35
DNA Vaccination
The concept of DNA vaccination is relatively new and was established by Wolff et al. in 1990 when direct intramuscular injection of plasmid DNA, encoding reporter genes, resulted in expression of the proteins in myocytes.41–43 Their study demonstrated that purified recombinant nucleic acids could be delivered to cells in vivo to direct protein expression endogenously.32 DNA vaccines consist of antigenic proteins encoded on naked plasmid DNA vectors that allow their expression in eukaryotic cells.3, 5 They represent a promising approach to vaccine development and have been shown to generate protective responses against infectious diseases such as leishmaniasis.44, 45
DNA vaccines present a multitude of advantages over other vaccine strategies and several features have made them an appealing alternative.46 Not only are they fast, simple and cheap to produce on a large scale, they are also temperature stable making storage and transport easier and cheaper as there is no need for a cold-chain of distribution.44, 35 This is an important advantage for a Leishmania vaccine candidate considering that leishmaniasis is a major health concern in many developing countries. DNA vaccines provide an extremely flexible method of vaccination as a single plasmid can encode several antigens and multiple plasmids, encoding different antigens, can be delivered in a single administration.44 Therefore, DNA vaccination could potentially provide protection against more than one species. As leishmaniasis can be caused by several different species, this is another feature that makes DNA vaccination an appealing prospect.
Genetic vaccination has been shown to induce both humoral and cellular immune responses and these responses can be tailored through modifications of the vector or incorporation of cytokine genes with adjuvant properties.42 Therefore, the immune response to the antigen encoded on the plasmid can be geared towards one that will confer resistance to the parasite.
DNA vaccines may provide better protection against Leishmania than killed or live-attenuated vaccines as they can induce the expression of Leishmania antigens, which are unaltered in their protein structure and antigenicity.44 Furthermore, bacteria-derived DNA plasmids are naturally immunogenic as their backbones contain unmethylated cytosine-phosphate-guanosine (CpG) motifs which have been shown to readily induce Th1 cytokine expression and enhance CD8+ T cell responses.42, 43 This adjuvant property is of great use for a Leishmania vaccine as these motifs would ensure the induction of cell-mediated immunity which is known to confer protection against the parasite.
Administration of DNA Vaccines
Several methods of DNA vaccine administration have been tested and it is thought that the method and site of immunization may influence that nature of the immune response elicited. Many studies have observed variation in vaccine efficacy depending on the site and method of administration despite the use of the same DNA vaccine.47 To date, successful DNA vaccination has been achieved using a number of different routes including intramuscular, intravenous, intraepidermal, intraperitoneal, intravaginal, intranasal, intrasplenic, intrahepatic, subcutaneous and oral.44 Of these routes, intramuscular administration is most commonly employed.43, 45 However, Méndeza et al.45 demonstrated that the dose of DNA vaccine required to induce full protection in C57BL/6 mice against L. major was five times smaller using the gene gun (particle-mediated epidermal delivery) than subcutaneous or intramuscular injection. Furthermore, Ali et al. (personal communication) found that 1 µg of DNA encoding L. mexicana gp63, administered using a gene gun, conferred more protection in susceptible BALB/c mice than 100 µg of vaccine administered intramuscularly. These findings clearly demonstrate that DNA vaccine efficacy is higher when administered using a gene gun than it is when administered intramuscularly. This may be because gene gun administration can directly transfect APC, such as DC, with the plasmid DNA.44 Although myocytes are capable of inducing CTL responses by presenting peptides (from the encoded antigen) via MHC class I molecules, unlike APC they are not professional antigen presenters and do not carry the co-stimulatory molecules required to prime T-cells.45, 46
Possible Modes of Action of DNA Vaccines
Figure 2 illustrates some of the possible immune mechanisms induced by gene gun delivery of DNA vaccines. The gene gun fires DNA-coated gold particles at high speed directly into cells of the epidermis, which include skin cells, LC and dermal DC. Upon entry into the cells, the plasmid is transported to the nucleus where the encoded gene is transcribed and the protein is subsequently produced, processed into peptides by host proteases and then presented in the context of MHC class I which then stimulates CD8+ T lymphocytes.44 DC directly transfected with the DNA vaccine can prime CD8+ T cells by presenting the DNA encoded antigen in the context of MHC class I.4 Conversely, there is evidence to suggest that immature DC can endocytose soluble proteins and debris from apoptotic transfected cells and express the coded antigen through MHC class I and/or MHC class II after differentiating into mature DC.4, 46 Therefore, DNA vaccination can result in the stimulation of both CD4+ T cell and CD8+ T cell populations. The unique ability of DC to present extracellular antigens in the context of MHC class I and MCH class II is known as cross-priming, as a result of this phenomenon it is likely that DC play a key role in the induction of both humoral and cell-mediated immunity following DNA vaccination.4
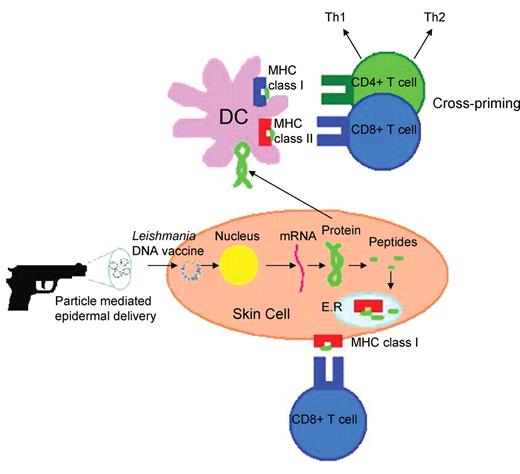
Possible immune mechanisms induced by particle-mediated epidermal delivery of a Leishmania DNA vaccine (adapted from Encke et al.44).
There have been several studies conducted on potential DNA vaccines against Leishmania. Handman et al.47 demonstrated that DNA vaccines can be used therapeutically to treat cutaneous leishmaniasis caused by L. major in both genetically resistant C3H/He mice and susceptible BALB/c mice. This is an important finding, which demonstrates that DNA vaccines may have a role to therapeutically cure disease in both susceptible and resistant individuals. Rodriguez-Cortes et al.7 found that their multiantigenic DNA vaccine encoding KMII, TRYP, LACK and Gp63 did not protect dogs against L. infantum experimental challenge, despite the hypothesis that an effective immune response was more likely to be generated following exposure to more than one antigen. Conversely, Carter et al.48 found that intramuscular DNA vaccination against the parasite enzyme gamma-glutamylcysteine synthetase conferred protection against L. donovani in BALB/c mice. The results of these studies indicate that multiantigenic vaccines do not necessarily confer better protection against Leishmania infection. However, these studies used different species of Leishmania so it is not possible to directly compare the findings.
Conclusion
Leishmaniasis is a major cause of morbidity and mortality worldwide. An effective vaccine has the potential to control this disease. However, despite great effort, there is currently no licensed vaccine available. To date, there have been numerous attempts at developing a successful vaccine against leishmaniasis and Table 1 demonstrates only a few of these. Further work in this field should not only enable the improvement of current vaccine strategies but it is hoped the development of novel methodologies will quickly follow suit. Immunization with plasmid DNA encoding Leishmania antigens represents a promising approach to vaccination against leishmaniasis in that it has intrinsic adjuvant properties, induces both humoral and cell-mediated immune responses and results in long lasting immunity.7 In light of its many advantages over other Leishmania vaccine strategies, DNA vaccination could prove to be the best approach to both treat and prevent human leishmaniasis.
References
Author notes
Supervisor: Dr Selman Ali, School of Science and Technology, Nottingham Trent University, Nottingham NG11 8NS, UK.


