-
PDF
- Split View
-
Views
-
Cite
Cite
Victoria Sebbage, Cell-penetrating peptides and their therapeutic applications, Bioscience Horizons: The International Journal of Student Research, Volume 2, Issue 1, March 2009, Pages 64–72, https://doi.org/10.1093/biohorizons/hzp001
Close - Share Icon Share
Abstract
The process of introducing drugs into cells has always proved a major challenge for research scientists and for the pharmaceutical industry. The cell membrane is selectively permeable and supports no generic mechanism for their uptake. A drug must be either highly lipophilic or very small to stand a chance of cellular internalization. These restrictions mean that the repertoire of possible drug molecules is limited. Similarly, novel therapeutic approaches such as gene and protein therapy also have limited potential due to the cell-impermeable nature of peptides and oligonucleotides. The existing methods for delivery of macromolecules, such as viral vectors and membrane perturbation techniques, can result in high toxicity, immunogenicity and low delivery yield. However, in 1988 the remarkable ability of a peptide to traverse a cell's plasma membrane independent of a membrane receptor was revealed. Known as Tat, the transcription activator of the human immunodeficiency virus type 1 (HIV-1) viral genome was shown to enter cells in a non-toxic and highly efficient manner. In light of such properties Tat became known as the first ‘cell-penetrating peptide’ (CPP). CPPs have demonstrated themselves to be capable of delivering biologically active cargo to the cell interior and the vehicular capabilities of CPPs have already been harnessed for use as laboratory tools. However, it is believed that their true potential lies within the field of therapeutics. Attached to a CPP, therapeutic cargo could be delivered to an intracellular target, thus overcoming the entry restrictions set by the plasma membrane. Since the discovery of Tat, the number of known peptides with cell-penetrating capabilities has grown and in 2003, the first CPP-based drug reached phase II clinical trials. This review discusses the controversial mechanism of entry employed by CPPs, their potential applications in vitro and in vivo, and the ways in which CPP properties have been optimized to maximize their potential as future therapeutics.
Cell-penetrating peptides (CPPs) have the ability to enter cells independent of a membrane receptor,1 and they show no cell-type specificity.2 They are small (10–30 residues in length), often positively charged sequences of amino acids.3 A consensus as to what defines a CPP is yet to be agreed upon, as there seem to be a number of diverse criteria that can define a peptide as ‘penetrating’. Current research is focused on two CPPs in particular; Tat, originating from the retrovirus human immunodeficiency virus type 1 (HIV-1) and pAntp, derived from the transcription factor Antennapedia.4 Their penetrating capabilities were revealed unexpectedly, in 1988 and 1991, respectively.
In the late 1980s, research in the field of AIDS and its causative agent HIV-1 was focused on potential targets for AIDS therapy. One such target was identified as Tat, the 86 amino acid transcriptional activator coded for by the HIV-1 genome, which is expressed upon HIV-1 infection of a host cell.5 Studies carried out in 1988 aimed to characterize the structural and functional domains of Tat.6, 7 Unexpectedly, researchers discovered two things: that Tat could be taken up from the surrounding medium by HeLa cells in vitro; and that it could directly trans-activate its target HIV LTR gene located in the nucleus7 (Fig. 1). Using mutagenesis studies, the biological activity and penetrating capability of Tat was found to reside in the region between residues 48–60.7 In this report, unless otherwise stated, ‘Tat’ refers to this commonly used peptide region.
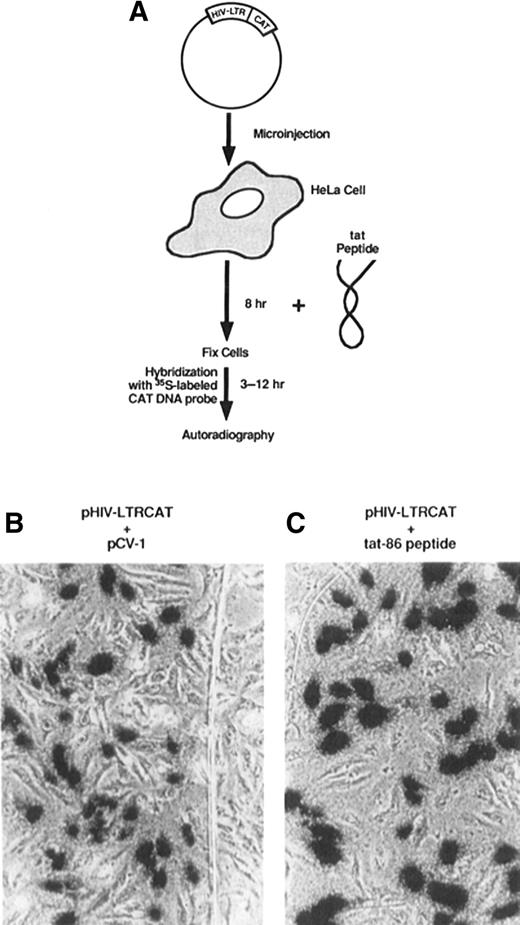
Transactivation of the LTR gene in HeLa cells by the Tat protein. (A) HeLa cells were transformed with a plasmid coding for the CAT gene fused to an HIV-LTR promoter (pHIV-LTRCAT) and incubated with Tat before fixation and analysis. Trans-activation of pHIV-LTRCAT by (B) coinjection with a plasmid encoding Tat cDNA (pCV-1) or (C) by incubation with Tat protein. Source: Taken from Green and Loewenstein 1988.
In 1991 it was established that the properties conferred by Tat were not unique. The receptor-independent penetrating capabilities of the Antennapedia transcription factor were discovered during investigations into the neural development of Drosophila.8 The 1–60 amino acid region of the transcription factor (known as the ‘homeodomain’) was shown to enter live, cultured neuronal cells, undergo targeting to the nucleus and cause cellular morphological changes.8 Specifically residues 43–58, the ‘internalization helix’ or ‘pAntp’, were responsible for cell penetration.9 Since then, the number of known natural and synthetic peptides with cell-penetrating capabilities has continued to grow (Table 1).10
| Name . | Origin . | Sequence . |
|---|---|---|
| Tat family | ||
| Tat (48-60) | HIV-1 protein | GRKKRRQRRRPPQQ |
| Oligoarginine | Tat derivative | Rn |
| Penetralia family | ||
| p-Antp | Antermapedia homeodomain | RQIKIWFQNRRMKWKK |
| plsl | Igl-1 homeodomain | RVIRVWFQNKRCKDKK |
| Chimeric CPPs | ||
| Transportan | Galanin-mastoparan | GWTLNSAGYLLGKINLKALAALAKKIL |
| MPG peptides | ||
| Pβ | gp41-SV40 | GALFLGFLGAAGSTMGAWSQPKKKRKV |
| Pα | gp41-SV40 | GALFLAFLAAALSLMGLWSQPKKKRRV |
| Pep-1 | Trp-rich motif-SV40 | KETWWETWWTEWSQPKKKRRV |
| Name . | Origin . | Sequence . |
|---|---|---|
| Tat family | ||
| Tat (48-60) | HIV-1 protein | GRKKRRQRRRPPQQ |
| Oligoarginine | Tat derivative | Rn |
| Penetralia family | ||
| p-Antp | Antermapedia homeodomain | RQIKIWFQNRRMKWKK |
| plsl | Igl-1 homeodomain | RVIRVWFQNKRCKDKK |
| Chimeric CPPs | ||
| Transportan | Galanin-mastoparan | GWTLNSAGYLLGKINLKALAALAKKIL |
| MPG peptides | ||
| Pβ | gp41-SV40 | GALFLGFLGAAGSTMGAWSQPKKKRKV |
| Pα | gp41-SV40 | GALFLAFLAAALSLMGLWSQPKKKRRV |
| Pep-1 | Trp-rich motif-SV40 | KETWWETWWTEWSQPKKKRRV |
Source: Taken from Foerg and Merkle.18
| Name . | Origin . | Sequence . |
|---|---|---|
| Tat family | ||
| Tat (48-60) | HIV-1 protein | GRKKRRQRRRPPQQ |
| Oligoarginine | Tat derivative | Rn |
| Penetralia family | ||
| p-Antp | Antermapedia homeodomain | RQIKIWFQNRRMKWKK |
| plsl | Igl-1 homeodomain | RVIRVWFQNKRCKDKK |
| Chimeric CPPs | ||
| Transportan | Galanin-mastoparan | GWTLNSAGYLLGKINLKALAALAKKIL |
| MPG peptides | ||
| Pβ | gp41-SV40 | GALFLGFLGAAGSTMGAWSQPKKKRKV |
| Pα | gp41-SV40 | GALFLAFLAAALSLMGLWSQPKKKRRV |
| Pep-1 | Trp-rich motif-SV40 | KETWWETWWTEWSQPKKKRRV |
| Name . | Origin . | Sequence . |
|---|---|---|
| Tat family | ||
| Tat (48-60) | HIV-1 protein | GRKKRRQRRRPPQQ |
| Oligoarginine | Tat derivative | Rn |
| Penetralia family | ||
| p-Antp | Antermapedia homeodomain | RQIKIWFQNRRMKWKK |
| plsl | Igl-1 homeodomain | RVIRVWFQNKRCKDKK |
| Chimeric CPPs | ||
| Transportan | Galanin-mastoparan | GWTLNSAGYLLGKINLKALAALAKKIL |
| MPG peptides | ||
| Pβ | gp41-SV40 | GALFLGFLGAAGSTMGAWSQPKKKRKV |
| Pα | gp41-SV40 | GALFLAFLAAALSLMGLWSQPKKKRRV |
| Pep-1 | Trp-rich motif-SV40 | KETWWETWWTEWSQPKKKRRV |
Source: Taken from Foerg and Merkle.18
The vehicular potential of CPPs was realized in 1995 when studies on pAntp demonstrated that the peptide could be attached to a bioactive compound (forming a ‘conjugate’) and used to achieve its intracellular delivery.11 Significantly, the attached cargo (a protein kinase C inhibitor) retained its function upon internalization into a live neurone. The potential for CPPs to act as vectors for therapeutically active macromolecules was realized as a result of preliminary in vivo experiments using Tat. Beta-galactosidase, a macromolecule previously considered to be impermeable to cells, was covalently cross-linked to Tat.12 The delivery of beta-galactosidase to the cytoplasm of several tissues was achieved in a cell-type independent manner.
Until these studies, the peptide-mediated cytoplasmic delivery of macromolecules had not been achieved. Tat and pAntp had provided the mechanism with which it was possible. It was a promising prospect that potentially any molecule could be attached to a CPP in order to achieve its successful intracellular delivery. However, there remain gaps in our knowledge regarding the mechanism by which CPPs enter cells and traffic once internalized. This currently limits their clinical use.
Since the discovery of Tat in 1988, the disparity among results from laboratories attempting to elucidate the uptake mechanism has caused controversy. Experimental procedures used commonly throughout the pioneering phase of CPP investigation (namely, the period from their discovery until 2003) provided much of the evidence supporting a non-endocytic, energy-independent direct traversal of the cell membrane by CPPs. The validity of those experimental procedures has since been called into question.13
One example of this is the use of flow cytometry to quantify accurately the cellular uptake of fluorescently labelled CPPs, which was a technique put into doubt in 2003.14 The source of the ambiguity was considered to be the inability of the procedure to discriminate between extracellular and internalized CPPs. Studies carried out after 2004 incorporated external trypsin protease washing (‘trypsinization’) after CPP incubation15 in an attempt better to represent the quantity of CPP determined within the cell.15
The splice correction assay designed in 1998 has further improved the reliability of CPP quantification (Fig. 2).15 This recombinase system only gives a positive signal in the event of successful (and non-toxic) nuclear delivery of bioactive cargo.16 It was successfully employed in 2004 to quantify Tat internalization.15
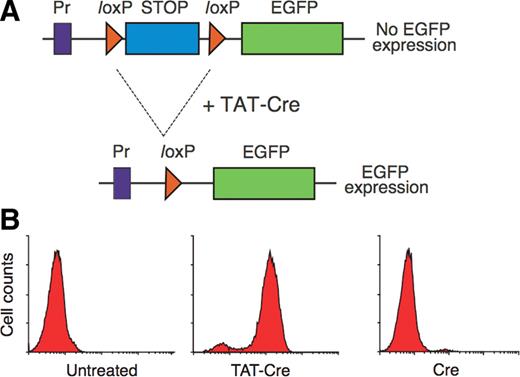
Nuclear delivery of Tat-Cre is required for the expression of enhanced green fluorescent proteins (EGFP) in reporter T cells. (A) The nuclear presence of Tat-Cre mediates the excision of the STOP gene segment, permitting transcription of EGFP. The STOP gene is flanked by loxP recombination sites, which undergoes site-specific recombination mediated by Cre recombinase protein. Pr corresponds to the promoter. (B) The results of flow cytometry quantification of EGFP in untreated cells, cells incubated with 2 µM Tat-Cre and cells incubated with 2 µM control Cre for at least 5 min. All cells were trypsinized post-incubation. Source: Taken from Wadia et al.15
In vivo techniques possible since 1999 have shown that in vitro models may not be accurately representative of in vivo systems.15In vivo studies carried out on the brain have suggested that plasma proteins may bind the conjugated CPP, prohibiting release of the bioactive cargo to the tissues.17
An additional source of unreliability in the pioneering phase was the fixation protocol employed for visualization of CPPs by fluorescence microscopy.18 As early as 1992 it was noted that fluorescently labelled cellular proteins were localized to ‘inappropriate’ locations as a result of cell fixation,13 attributed to the permeating activity of the ethanol and acetic acid on the nuclear membrane.19 Since then CPP studies have avoided nuclear artefacts by using live, unfixed cells to show CPPs colocalized with fluorescent endosomal membrane markers.15 Studies using asolectin liposomes (known as ‘giant unilamellar vesicles’ or ‘GUVs’) have also avoided problems associated with cellular fixation.20
These techniques have been used in attempt to determine, amongst other CPP features, the mechanism of CPP internalization. The current view is that each CPP may be internalized via an idiosyncratic mechanism (Fig. 3),13 the major route being endocytic.21 But despite today's clear evidence for an endosomal route of entry there is also strong evidence for a minor route of cell entry, specifically a ‘direct translocation’ of CPP across the membrane bilayer.21 However, evidence for direct translocation was obtained mostly during the pioneering phase, often via unreliable experimental techniques. The evidence for direct translocation is considered first.
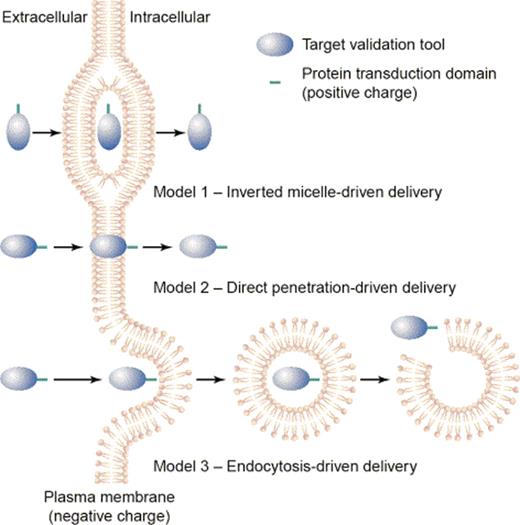
The three proposed routes of CPP entry. Model 1: The inverted micelle model. Model 2: The direct penetration (pore formation) mechanism. Model 3: An endocytic mechanism of uptake. Source: Taken from Trehin and Merkle.13
Since endocytic uptake is energy-dependent, experiments in the pioneering phase were commonly carried out at 4°C and in the absence of ATP22 in attempt to investigate an energy-independent (and therefore non-endocytic) internalization mechanism. Evidence for a direct translocation through the membrane bilayer was accumulated for both Tat and pAntp from studies using the temperature- and ATP-dependent properties of the ATPase required for endocytosis.21, 23 Multiple experiments were carried out in conditions of reduced ATP concentration and low temperatures, which failed to inhibit internalization of Tat23 and pAntp,9 and thus supported a non-endocytic uptake mechanism. In addition, early studies using a caveolae-mediated endocytosis inhibitor also failed to inhibit Tat internalization.23 Furthermore, in 2000, a landmark study found that pAntp was able to move directly into and out of fluorescently labelled GUVs. GUVs do not permit the formation of internal endosomal structures, thus pAntp was shown to have the capacity for non-endosomal entry.20 Investigation using pAntp analogues provided evidence for a stereospecific receptor-independent endocytic route of internalization.1
In 1996, researchers proposed the inverted micelle model in attempt to explain the penetration of the bilayer in terms of receptor-, energy-, and endocytosis-independence.13 The ‘inverted micelle’ was defined as the hydrophilic ‘cavity’ present within the two bilayer leaflets, which accommodates the CPP within the wider hydrophobic environment of the phospholipid tails.1 This micellular phase is transient, and the CPP is subsequently released, directly into the cytosol and independent of any vesicular body, as the micelle passes to the cytosolic side of the bilayer.13 An alternative model of direct translocation of the membrane was proposed with respect to conjugate complexes known as ‘nanoparticles’.24 This model proposes that the nanoparticle induces the formation of a transient β-barrel pore in the cell membrane to allow the passage of the nanoparticle into the cell.25
The evidence for endocytic routes of entry, which mounted after 2002 with the increased use of reliable experimental techniques, is now considered. This evidence was in direct contradiction to that supporting a direct translocation mechanism, gathered during the pioneering phase.
Supporting an endocytic route of entry for pAntp, fluid-phase endocytosis inhibitors were used successfully to prevent pAntp internalization.3 In 2004 evidence for a macropinocytotic mechanism of Tat uptake in live mouse cells was published. These studies showed colocalization of an endosome marker with Tat, suggesting that CPPs were localized to endosomes (Fig. 4).15 Studies carried out in 2006 demonstrated that endosome lysis resulted in an increase in splice correction efficacy,26 suggesting that Tat was localized to vesicles, possibly macropinosomes. Indeed, endosomal disruption agents (such as the viral haemagglutinin protein ‘HA2’) were shown to cause the artificial release of Tat-Cre from the endosome.15
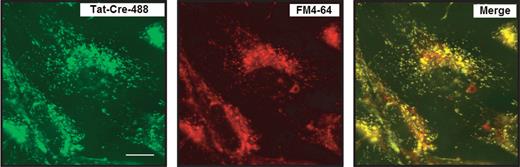
Confocal images showing the colocalization of Tat-Cre-488 with FM4-64 in live 3T3 cells. From left to right: Tat-Cre-488 alone, the fluorescent general marker of endosomes FM4-64 alone and the colocalization of Tat-Cre-488 with FM4-64. Incubation was carried out with 2 µM Tat-Cre-488 and 4 µM of FM4-64. After 8 h cells were washed and images acquired. The fluorescent label used for Tat-Cre is referred as −488. Source: Taken from Wadia et al.15
Today, it is thought that each CPP expresses its own preferred mechanism of uptake.13 Although studies using the most recent, reliable techniques have provided evidence for endocytic internalization, studies such as the experiment using GUVs (the data from which supported a direct bilayer translocation) are yet to be refuted.
One detail of CPP internalization, the interaction with membrane components, has remained a common feature of both major and minor routes of CPP internalization mechanisms. With the arrival of evidence that pAntp could not cross pure phospholipid bilayers in 2001, it was proposed that pAntp may require electrostatic interaction with components other than membrane phospholipids for cell penetration.27 Evidence for such interaction with respect to Tat was published in 2004.15 The involvement of extracellular matrix components, in particular heparin sulphate (HS) and chondroitin sulphate (CS), was considered in the study in order to elucidate the ionic interaction between Tat and the cell surface.15 Pre-incubation of Tat-Cre with soluble HS and CS prevented the uptake of Tat-Cre when the conjugate mixture was subsequently incubated with cells for uptake. It was therefore suggested that the electrostatic interaction (prior to internalization) of positively charged Tat with negatively charged cell-surface glycoproteins was necessary for successful translocation.15, 28
In addition to interaction with the membrane, methods of cargo attachment and cargo type are also factors that have been shown to affect the uptake mechanism.29–31 Lipid raft-mediated CPP uptake has even been implicated.15, 32
Regarding the route of intracellular trafficking, evidence exists for both nuclear and cytoplasmic delivery of CPP-internalized cargo.7, 33 However, nuclear delivery is considered to occur as a result of cytoplasmic delivery.14 There is much contradictory evidence with regards to endosomal accumulation and release, retrograde trafficking and lysosomal degradation.34, 35
Evidence for cytosolic delivery after endosomal localization was published in 2005.33 Colocalization studies of CPP conjugates with HeLa cells showed only a transient localization to early endosomes, prior to conjugate release to the cytoplasm. It has been proposed that the excessive accumulation of CPPs may result in endosomal destabilization and conjugate release.31
Endosome-localized CPPs have been shown to undergo retrograde delivery, through the Golgi apparatus and endoplasmic reticulum (ER), prior to their cytosolic release.34 This study pointed out similarities to the trafficking of the ricin and Shigella toxins, which are known to bind to the host cell surface, internalize by endocytosis, traffic to the Golgi and ER via retrograde transport and then release into the cytosol. There also exists evidence for the trafficking of endosome-localized Tat to late endosomes and lysosomal bodies.35 This has negative therapeutic implications.
Early studies on Tat verified its nuclear translocation using fluorescence microscopy and a positive transcription assay.7 Later studies using the splice correction assay also provided evidence for CPP-mediated nuclear delivery.15 No study has yet established the detailed mechanism of nuclear entry or localization.
In spite of the lack of a complete understanding, CPPs are currently being manufactured for use as transduction tools in laboratory studies.21 A selected example is that of Chariot™, a peptide delivery vector manufactured by Panomics, advertised as the ‘Revolutionary new transfection agent’.36 There are numerous advantages conferred by CPPs that define them as a more appropriate means to intracellular delivery than the principle methods available for intracellular cargo delivery today (which include viruses and liposomes in addition to electroporation and microinjection techniques).37, 38
Unlike liposomes, CPPs have exhibited successful delivery to numerous cell types, including human Jurkat and HeLa cell lines and those notoriously impermeable to retroviral vectors such as osteoclasts.2 Unusually, CPPs exhibit remarkably low toxicity and have an extensive range of possible cargo types.21, 17 Unlike viral vectors, CPPs do not have the capacity to integrate the genetic material they deliver, and therefore there has been no evidence for CPP use resulting in oncogene activation.39 The immunogenicity raised in response to conjugated CPPs alone is said to be very unlikely.2, 39 There is also evidence of (the highly desirable) sustained presence and functionality of CPP-delivered protein cargo.18, 38, 40 In contrast to the membrane rupturing techniques of electroporation and microinjection there is no evidence for membrane perturbation caused by CPP cell entry.20, 38
CPPs have enormous potential for novel therapeutics.18 The potential therapeutic benefits of intracellular delivery of macromolecules such as protein and nucleic acids in vivo have not been realized due to the ineffective uptake of such large, hydrophobic compounds. However, in 1999 biologically active cargo was successfully internalized for the first time, mediated by Tat.41 Tat was used to deliver active protein 116 KDa beta-galactosidase (β-Gal) to all tissues in a mouse model in vivo, including the brain (Fig. 5).
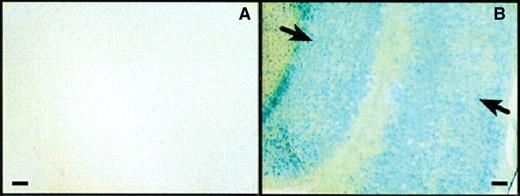
Active Tat-β-Gal was successfully transduced across the blood–brain barrier. The X-Gal staining of brain sections from mice 8 h after intraperitoneal injection with (A) control β-Gal or (B) Tat-β-Gal. β-Gal activity was localized to the cell nuclei indicated by the arrows. Source: Taken from Schwarze et al.41
This set a precedent. In 2004, a CPP was used for the delivery of an siRNA in order to down-regulate a reporter gene representative of a therapeutically detrimental gene.42 The siRNA, complementary to reporter protein ‘firefly luciferase’, was covalently attached to pAntp. The CPP-mediated delivery of anti-luciferase siRNA was successfully achieved. Decreased luciferase activity was observed after CPP-siRNA incubation with the luciferase-expressing cells. CPP-mediated siRNA delivery achieved both increased efficiency of delivery and of sustained expression of the cargo, when compared with liposomal delivery of the same cargo.
In another study, the virally derived CPP ‘VP22’ was successfully used to deliver the transcription factor Gata4 into cardiac cells in vivo.38 Gata4 controls the stress responsiveness of the heart.43 It is a transcription factor naturally expressed in cardiomyocytes in order to upregulate genes of therapeutic benefit in the conditions of increased stress or pressure.44 Ischaemic cardiomyopathy is one such condition, in which decreased blood supply to the heart results in damage to cardiomyocytes. If these conditions are prolonged, cells undergo necrosis and the function of the heart is compromised. Low oxygen levels induce a stress response, one of which is the upregulation of the transcription activator Gata4 by local cells.45 Gata4 acts on multiple routes within the cell to cause hypertrophy.43 Increased dimensions of functional, hypertrophic myocytes enhance their performance and thus compensate for those that have reduced function due to damage.38, 46 This study demonstrated the therapeutic benefits of Gata4 over-expression on a murine model of ischaemic cardiomyopathy.38 Fibroblasts, transformed with expression plasmids constructed to express Gata4-VP22 fusion proteins, were injected into the site of induced cardiac damage.38 Improved cardiac function, increased cardiomyocyte diameter and other favourable effects on tissue repair could be seen after 4 weeks. A survival rate of 6 weeks beyond the time frame required for mounting an antibody response was observed. This provides evidence that protein-based gene therapy could permit sustained release of a functional therapeutic protein, without causing toxicity. In the context of today's increased prevalence of cardiomyopathy, this study has particular significance.
CPPs also have potential in the treatment of strokes. In 2002 Tat was conjugated to an anti-apoptotic protein in order to inhibit the apoptosis of an ischaemic neuronal cell in the brain of a stroke model.47 Cerebral ischaemia is the result of a blood vessel blockage in the brain. The resulting loss of oxygen supply to the surrounding neurones is known to result in the stimulation of their apoptotic pathways. This is mediated by the release of pro-apoptotic proteins. Anti-apoptotic proteins such as Bcl-xL counteract the pro-apoptotic process. Bcl-xL is a mitochondrial integral membrane protein, which acts to inhibit the release of cytochrome C and other apoptotic factors from the mitochondrial intermembrane space. Bcl-xL thus has the potential to prevent neuronal apoptosis, after an ischaemic episode. However, failure to deliver Bcl-xL proteins to cells successfully, let alone to the brain, has always stifled such protein therapy. Tat was selected for the cerebral delivery of Bcl-xL because of its ability to traverse the blood–brain barrier (BBB) upon systemic injection.41
The conjugate-treated brains exhibited anti-apoptotic effects. They showed a 60% reduction in farction volume, even when administered up to 45 min after the end of an ischaemic episode. Of greatest clinical significance, administration of Tat-Bcl-xL has since been shown to reduce neuronal apoptosis and infarct volume in a murine stroke model when administered, both before and after a focal ischaemic episode, intravenously.48
CPPs have also been shown to have potential uses in cancer therapy. The cell-specific in vivo delivery of Caspase 3 was achieved in 2002 using an oxygen-dependent degradation (ODD) domain as a means to artificially stimulate the apoptotic process in cancer cells (Fig. 6).49 Such an approach has long been identified as a potential means to cancer therapy.39 However, bioactive agents causing apoptosis are required to be tumour-cell specific. Through the use of a cell-specific tag, the function of which was based on the differential oxygen concentration of cancer cells, Tat was used to carry out tumour-specific delivery.41
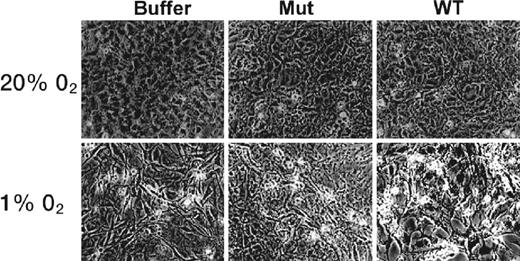
CPP-ODD mediated delivery of pro-apoptotic protein Caspase 3 in normal (20% O2) and hypoxic (1% O2) conditions. Mouse 3T3 cells were treated with buffer, mutant Tat-ODD-Caspase 3 or wild-type (functional) Tat-ODD-Caspase 3 for 24 h. Tat-ODD-Caspase 3 was administered as a fusion protein by intraperitoneal injection. Apoptotic features are clearly visible only in hypoxic cells after treatment with functional Tat-ODD-Caspase 3. Source: Taken from Harada et al.49
Cancer cell proliferation is mediated by transcription factors, some of whose activities are regulated by hypoxia.49 Hypoxia-Inducible Factor-1a (HIF-1a) is one such transcription factor. The stability of HIF-1a is dependent on its ODD domain. When too much oxygen is present, the ODD domain is activated and HIF-1a becomes instable. Thus, hypoxic conditions are required to maintain its stability. Solid tumour cells are characteristically hypoxic. Harada et al.49 proposed that linking an ODD domain to a CPP-Caspase 3 conjugate would stabilise the Caspase cargo in an hypoxic environment only.49 This could achieve hypoxic-cell specific delivery of Caspase 3.
The Caspase 3-mediated stimulation of apoptosis resulted in tumour mass reduction.49 No evidence of toxic side effects on surrounding tissues was observed. The evidence suggested that the Tat-ODD-Caspase 3 conjugate accessed all cells in the tissue but, since it was degraded in normoxic cells by the ODD domain, only retained its stability in hypoxic cells. A reduction in tumour mass was demonstrated.
With regards to cerebral therapy, access of pharmaceutical agents to the brain is notoriously difficult to achieve. As a result of P-glycoprotein expression (P-gp) at the BBB and its over-expression in tumour cells, the therapeutic potential of cerebral anti-cancer agents has been strictly limited.17 The brain is said to exhibit ‘multidrug resistance’ and most methods that achieve brain uptake are invasive, causing high toxicity.17
The potential of CPPs to act as peptide vectors able to penetrate the BBB in a non-toxic fashion was demonstrated in 1999 when a Tat fusion protein was shown to internalize throughout the brain, 8 h after intraperitoneal administration.41 Investigation in 2000 demonstrated that pAntp and SynB vectors were able to deliver the anticancer agent Doxorubicin to the brain upon intravenous administration.17 A study carried out by the same laboratory in 2003 was able to confirm that anticancer agents delivered by SynB retained their pharmacological effects.50
As well as exploring their current applications, researchers have been looking for ways to optimize CPPs in order to tailor them to suit the needs of each therapeutic application. The release from endosomes was considered to be the rate-limiting step in the CPP-mediated delivery of Tat-Cre.15 Exploiting the mechanism of endosomal escape employed by the HIV viral protein haemagglutinin (HA2) has provided a novel mechanism of endosomal escape for CPPs (Fig. 7).15 Cells were co-incubated with Tat-HA2 and Tat-Cre in order to enhance the efficacy of Tat-mediated transduction and cargo release.
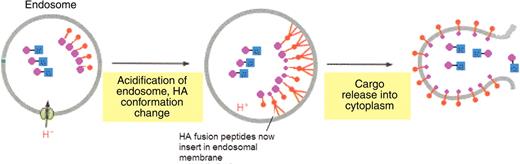
A model for the facilitated release of Tat-Cre from an endosome using Tat-conjugated haemagglutinin (Tat-HA2). Co-treatment with Tat-HA2 and Tat-Cre results in Tat-Cre release from the endosome. Key: Blue, Cre cargo; Pink, Tat CPP; Red, HA2. Source: Adapted from Alberts et al.58
Some CPPs have shown high affinity for specific cell types or intracellular destinations. A recently discovered CPP known as ‘Crotamine’ has shown unusually high affinity for actively proliferating cells.51 Another example lies with MPG, a synthetic CPP derived from the SV40 virus. Originally designed for nuclear delivery of siRNA, it has recently been altered to target the cytoplasm.24, 25 By tampering with the nuclear localization sequence present within its hydrophobic region, the destination of the cargo release can be selected. MPG has been successfully used for the targeted delivery of siRNA to mouse blastocytes in vivo.25, 52
CPPs also have to resist degradation once internalized. It has been documented that peptide delivery to neurones induces the activity of endogenous neuronal proteases.53 However, D-peptide forms of pAntp confer resistance to the neuronal proteolytic activity.53 D-isomers generally confer the loss of biological activity; however, the synthesis of pAntp in reverse order using D-amino acids maintains its biological activity.
Furthermore, the form in which the cargo is presented to the cell has implications for its stability within the cellular environment. Peptide nucleic acids (PNA) are more effective than oligonucleotides because only the latter are subject to nuclease degradation in the cytosol.54 The PNA is comprised of a polyamide backbone, which replaces the phosphodiester backbone of nucleic acids.
The ability of CPPs to cross membranes has been attributed to their high content of positive residues such as arginine and lysine.27 Studies carried out on the Tat sequence necessary for internalization have suggested that the feature required for efficient uptake may not be the cationic nature of the CPP, but rather the guanidinium head group of the arginine residues contained within it.55 Polyarginine uptake was comparable with that of a synthesized polymer of the concerned guanidinium head group of arginine, known as a ‘peptoid’. A peptoid is made up of a polyglycine backbone, which incorporates one guanidium group at every fourth position on the backbone. The high degree of peptoid uptake was attributed to the guanidine head group of the arginine residue.
Polyguanines are promising drug candidates. Designed for the maximum efficiency of uptake, these peptoids are of high stability and resistant to proteolysis.55 Their preparation is achieved with more ease than Tat, with favourable economic implications. Racemization is straightforward in peptoid synthesis and therefore peptoid analogues suitable for individual applications could be simply produced.
The repertoire of possible drug structures has been greatly expanded by the advent of peptide vectors. Macromolecules can be transported into cells using these molecular vehicles, which offer a highly efficient, non-toxic and non-immunogenic route for cell entry. Currently, their only limiting feature is their lack of cell-type specificity, although this has been overcome.49 Advantageously, no cell is known to be excluded from the possibility of CPP internalization. The mechanism of entry, trafficking route and degradation pathway of CPPs remains elusive, though technological advances promise to shed more light on what is presently a rather mysterious field.
Current clinical trials of CPP-based drugs are, however, transforming the conceptual ideas into rather more practical ones. One trial concerns the drug cyclosporine A (CsA) used in ointment for the topical treatment of psoriasis.56 Previous formulations were ineffective due to the impermeable nature of the skin. However, a CPP-based formulation permits skin penetration. A polyarginine heptamer with a pH-sensitive linker allows the release of CsA at functional pH within the skin. Phase 1 trials were successful. An unconfirmed Internet source has stated that the phase 2 trials of ‘Psorban’ are still underway.57 The limited information available on this drug prevents further comment.
Despite the enormous gaps in our understanding, experiments continue to demonstrate the highly desirable features of CPPs. Existing restrictions on the structures of therapeutic agents could be lifted as a consequence of CPP-mediated delivery. The range of potential therapeutic targets could greatly expand as a result. A similar effect was seen in the wake of commercial prodrug manufacture. Until the complete picture is revealed however, the therapeutic potential of CPPs cannot be realized, only predicted.
References
Author notes
Supervisor: Dr J.H. Walker, Faculty of Biological Sciences, University of Leeds, Leeds, UK.


