-
PDF
- Split View
-
Views
-
Cite
Cite
Israt Jahan, Shintaro Munemasa, Toshiyuki Nakamura, Yoshimasa Nakamura, Yoshiyuki Murata, Negative regulation of chitosan-induced stomatal closure by glutathione in Arabidopsis thaliana, Bioscience, Biotechnology, and Biochemistry, Volume 88, Issue 8, August 2024, Pages 918–922, https://doi.org/10.1093/bbb/zbae065
Close - Share Icon Share
ABSTRACT
Chitosan (CHT) is a deacylated derivative of chitin and improves growth and yield performance, activates defensive genes, and also induces stomatal closure in plants. Glutathione (GSH) has significant functions in the growth, development, defense systems, signaling, and gene expression. GSH negatively regulates abscisic acid-, methyl jasmonate-, and salicylic acid-induced stomatal closure. However, the negative regulation by GSH of CHT-induced stomatal closure is still unknown. Regulation of CHT-induced stomatal closure by GSH in guard cells was investigated using two GSH-deficient mutants, cad2-1 and chlorina 1-1 (ch1-1), and a GSH-decreasing chemical, 1-chloro-2,4-dinitrobenzene (CDNB). The cad2-1 and ch1-1 mutations and CDNB treatment enhanced CHT-induced stomatal closure. Treatment with glutathione monoethyl ester restored the GSH level in the guard cells of cad2-1 and ch1-1 and complemented the stomatal phenotype of the mutants. These results indicate that GSH negatively regulates CHT-induced stomatal closure in Arabidopsis thaliana.
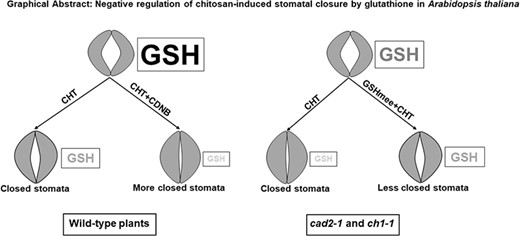
Negative regulation of chitosan-induced stomatal closure by glutathione in Arabidopsis thaliana
The epidermis of leaves is characterized by specialized guard cells, which form stomatal aperture to regulate gas exchange and transpiration. Guard cells regulate stomatal pore apertures via integration of both endogenous hormonal stimuli and environmental signals. Under stress conditions, plants synthesize hormones like abscisic acid (ABA), methyl jasmonate (MeJA), and salicylic acid (SA), which mediate stomatal closure. Stomatal closure is induced not only by phytohormones but also by elicitors (Klüsener et al. 2002). Elicitors are chemical or biological molecules from various sources that mimic microbial attack and induce marked physiological changes in plants (Zhao, Davis and Verpoorte 2005). An elicitor, chitosan (CHT), is a deacylated derivative of chitin, which is a major component of fungal cell walls, arthropods exoskeletons, and crustacean shells. Chitosan has been reported to have antifungal and antimicrobial activities (Kumar et al. 2005; Xing et al. 2005). Chitosan improves growth and yield performance (Khan, Prithiviraj and Smith 2002; Gornik, Grzesik and Duda 2008) and induces the expression of a variety of genes involved in plant defense responses (Loschke et al. 1983; Doares et al. 1995). Chitosan also induces stomatal closure in Solanum lycopersicum and Commelina communis (Lee et al. 1999), Pisum sativum (Srivastava et al. 2009), and Arabidopsis thaliana (Khokon et al. 2010; Salam et al. 2012).
Reactive oxygen species (ROS) act as second messengers in hormone and elicitor signaling in guard cells. Chitosan as well as ABA, MeJA, and SA induces ROS production in guard cells (Pei et al. 2000; Mori et al. 2001; Murata et al. 2001; Suhita et al. 2004; Munemasa et al. 2007; Islam et al. 2010; Khokon et al. 2010, 2011; Wang et al. 2020). Production of ROS is mediated by plasma membrane NAD(P)H oxidases in ABA signaling and MeJA signaling (Pei et al. 2000; Kwak et al. 2003; Munemasa et al. 2007) and by salicylhydroxamic acid-sensitive peroxidases in SA signaling and CHT signaling (Mori et al. 2001; Khokon et al. 2010, 2011; Salam et al. 2012).
Glutathione (GSH) is the most abundant non-protein thiol compound in plants and regulates many physiological functions via the redox state of GSH pools in plants (Noctor and Foyer 1998). It has been reported that GSH is involved in ABA-, MeJA-, and SA-induced stomatal closure (Okuma et al. 2011; Akter et al. 2013; Akter et al. 2022). Abscisic acid-, MeJA-, and SA-induced stomatal closure are enhanced in the Arabidopsis GSH-deficient mutant, cad2-1, which accumulates GSH at lower levels than the wild type does (Howden et al. 1995) because of lower activity of first GSH biosynthesis enzyme, γ-glutamylcysteine synthetase (Cobbett et al. 1998). Another Arabidopsis GSH-deficient mutant, chlorina 1-1 (ch1-1), accumulates less GSH due to impairment of the light harvesting protein in photosystem II, which also enhanced stomatal closure induced by ABA and MeJA (Jahan et al. 2008; Akter et al. 2010). One-chloro-2,4 dinitrobenzene (CDNB) functions as a substrate of GSH S-transferase to form a conjugate with GSH, resulting in decrease in GSH levels (Cummins, Cole and Edwards 1999). Pretreatment with CDNB enhanced ABA- and SA-induced stomatal closure in wild-type plants (Okuma et al. 2011; Akter et al. 2022). Glutathione monoethyl ester (GSHmee) can permeate cell membranes and release free intracellular GSH through hydrolysis by cytosolic esterases (Puri and Meister 1983). Treatment with GSHmee restored the GSH level in the guard cells of GSH-deficient mutant plants and complemented the stomatal phenotype of the mutants (Jahan et al. 2008; Akter et al. 2010; Okuma et al. 2011; Akter et al. 2022). These results indicate that intracellular GSH negatively regulates ABA, MeJA, and SA signaling in guard cells. However, it remains to be clarified whether GSH negatively regulates CHT-induced stomatal closure.
In this study, we clarified the negative regulation of CHT-induced stomatal closure by intracellular GSH in A. thaliana using GSH-deficient mutants, cad2-1 and ch1-1, a GSH-decreasing chemical, CDNB, and a GSH-increasing agent, GSHmee.
Materials and methods
Plant materials and growth conditions
Arabidopsis wild-type (A. thaliana, ecotype Columbia), cad2-1 (Columbia accession, At4g23100), and ch1-1 (Columbia accession, At1g44446) plants were used in this study and were grown as described previously (Munemasa et al. 2007). These plants were grown in square plastic pots filled with a soil mixture containing 70% (v/v) vermiculite (Asahi-Kogyo, Okayama, Japan) and 30% (v/v) Kureha soil (Kureha Chemical, Tokyo, Japan) in a growth chamber under a 16-h light/8-h dark regime with a photon flux density of 80 µmol m−2 s−1. Temperature and relative humidity were maintained at 22 °C ± 2 °C and 60% ± 10%. Water containing a commercial nutrient solution (0.1% Hyponex, Japan) was applied 2-3 times a week.
Measurement of stomatal aperture
Stomatal apertures were measured as described previously (Munemasa et al. 2007). Randomly selected rosette leaves from 5- to 6-week-old plants were excised and floated on a solution containing 5 mm KCl, 50 µm CaCl2, and 10 mm MES-Tris (pH 5.6). The leaves were incubated under light (80 µmol m−2 s−1) for 2 h to induce stomatal opening. Then, CHT was added, and the leaves were kept in the light for 2 h. GSH-decreasing chemical CDNB and GSH-increasing chemical GSHmee were applied 30 min prior to CHT application. After 2 h of incubation in the presence of CHT under light, the leaves were shredded in a blender and the epidermal tissues were collected using nylon mesh. The epidermal tissues were placed on a slide glass and images were captured under an inverted microscope (Olympus IX71; Tokyo, Japan). The width of 20 stomatal apertures was measured using WinRoof 3.0 software (Mitani Corporation, Fukui and Tokyo, Japan) for each experiment. The experiment was replicated three times following a similar procedure.
Measurement of GSH content in guard cells
The contents of GSH in guard cells were examined using monochlorobimane (MCB) as previously described (Akter et al. 2022). Epidermal tissues were collected from Arabidopsis rosette leaves and incubated under light (80 µmol m−2 s−1) for 2 h. Then, 100 µm of MCB and chemicals were added and incubated again for 2 h in the light. Monochlorobimane reacts with intracellular GSH to form fluorescent GSH S-bimane (GSB) in guard cells. The fluorescence intensity of GSB in guard cells was observed under a fluorescent microscope (Biozero BZ-8000, Keyence, Osaka, Japan) with a filter: OP-66 834 BZ filter DAPI-BP (excitation wavelength 360/40 nm, absorption wavelength 460/50 nm, and dichroic mirror wavelength 400 nm). The fluorescence intensity of guard cells was quantified using ImageJ 1.42q software (NIH, Bethesda, MD, USA).
Statistical analysis
The significance of differences between the mean values of data sets was assessed by Tukey's test. Differences at P < 0.05 were considered significant.
Results
Effects of CHT on stomatal apertures and GSH levels in guard cells
We investigated CHT-induced stomatal closure and GSH content in Arabidopsis wild-type (Col-0) plants. The exogenous application of 10 µg/mL and 50 µg/mL CHT significantly narrowed stomatal apertures by 17% (P < 0.05) and 29% (P < 0.05) in Arabidopsis wild-type plants (Figure 1a). Application of CHT at 10 µg/mL and 50 µg/mL decreased the GSH level in the wild-type guard cells by 18% and by 40%, respectively (Figure 1b). These results indicate that CHT-induced stomatal closure is accompanied by depletion of GSH in guard cells.
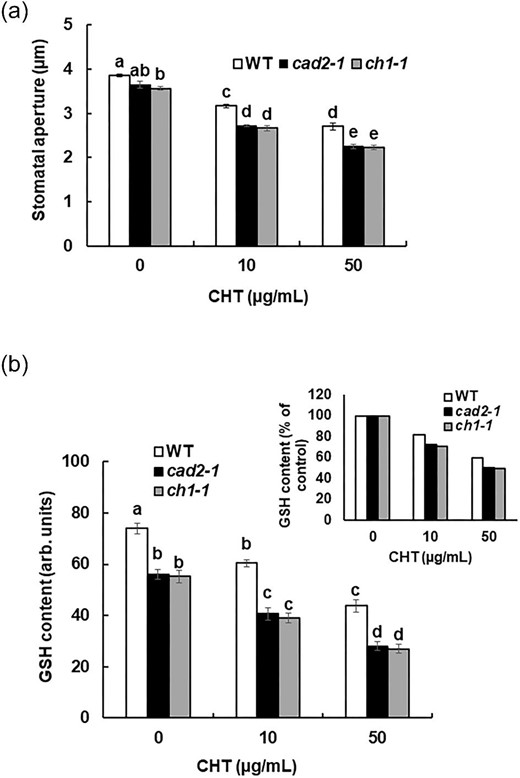
Effects of genetic depletion of GSH in guard cells on CHT-induced stomatal closure. (a) Chitosan-induced stomatal closure in cad2-1 and ch1-1 mutant plants compared with wild-type plants. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent standard errors. (b) Glutathione content in guard cells of cad2-1 and ch1-1 mutant plants, and wild-type plants, measured using MCB. Glutathione content is expressed as GSB fluorescence intensity. Error bars represent standard errors (n = 3). Bars showing the different letter are significantly different at P < 0.05.
Effects of both mutations of GSH on CHT-induced stomatal closure
To investigate the involvement of GSH on CHT-induced stomatal closure, we examined the stomatal responses to CHT of GSH-deficient mutants, cad2-1 and ch1-1. Application of 10 µg/mL and 50 µg/mL CHT significantly decreased stomatal apertures by 25% and 38% (P < 0.05) in the cad2-1 plants and by 25% and 37% (P < 0.05) in the ch1-1 plants (Figure 1a). Stomatal apertures of cad2-1 and ch1-1 plants were significantly narrower than those of wild-type plants when the epidermal tissues of rosette leaves were treated with 10 µg/mL and 50 µg/mL CHT for 2 h. Monochlorobimane staining showed significantly lower levels of GSH in the cad2-1 and ch1-1 mutants than in the wild-type (P < 0.05) (Figure 1b). Application of CHT at 10 µg/mL and 50 µg/mL decreased the GSH level in the cad2-1 and ch1-1 guard cells by 27% and 49%, and by 29% and 51%, respectively (Figure 1b). The GSH level in the guard cells of cad2-1 and ch1-1 mutants was significantly lower than that in the wild-type guard cells when leaves were treated with 10 µg/mL (P < 0.05) and 50 µg/mL CHT (P < 0.05) (Figure 1b). These results suggest that the genetic depletion of GSH in guard cells enhances CHT-induced stomatal closure.
Effects of CDNB on CHT-induced stomatal closure
We investigated the effect of a GSH-decreasing chemical, CDNB, on CHT-induced stomatal closure in Arabidopsis wild-type plants to confirm that the chemical depletion of GSH in guard cells enhances CHT-induced stomatal closure. When leaves were treated with 10 µm CDNB, GSH levels in the Arabidopsis guard cells were significantly decreased. Pretreatment of leaves with 10 µm CDNB significantly lowered the GSH level in the CHT-treated guard cells (P < 0.05) (Figure 2a). Pretreatment of leaves with 10 µm CDNB significantly enhanced CHT-induced stomatal closure by both 10 and 50 µg/mL CHT (P < 0.05) (Figure 2b). These results indicate that chemical depletion of GSH enhances CHT-induced stomatal closure.
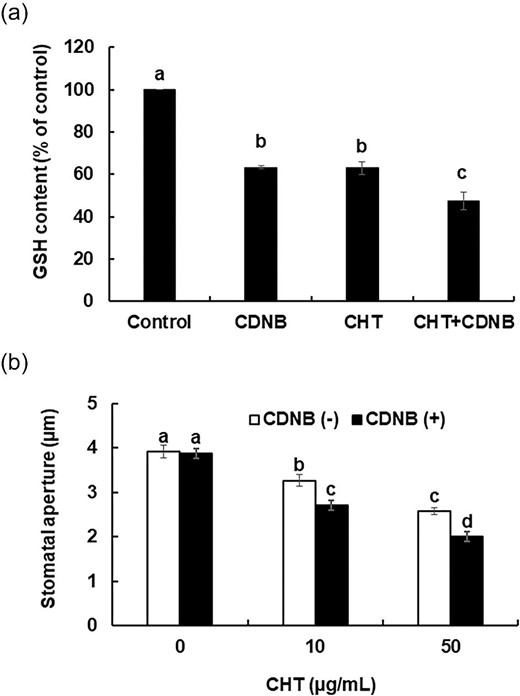
Effects of chemical depletion of GSH in guard cells on CHT-induced stomatal closure in wild-type plants. (a) Glutathione content in guard cells of wild type plants pretreated with CDNB. Glutathione content is expressed as GSB fluorescence intensity. Error bars represent standard errors (n = 3). (b) Chitosan-induced stomatal closure in CDNB-untreated and CDNB-treated wild-type plants. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent standard errors. The leaves that were treated with both CDNB and CHT, were first pretreated with 10 µm CDNB for 30 min and then treated with CHT (10 µg/mL or 50 µg/mL) for 2 h. Bars showing the different letter are significantly different at P < .05.
Effects of GSHmee on CHT-induced stomatal closure in wild type and the mutants
To confirm that the genetic depletion of GSH enhances the CHT-induced stomatal closure in the cad2-1 and ch1-1 mutants, we investigated whether the stomatal phenotype of both mutants is complemented with cytosolic GSH supplement by GSHmee. Stomatal apertures of the cad2-1 and ch1-1 plants treated with 50 µg/mL CHT were significantly widened by the pretreatment with 10 µm GSHmee (P < 0.05) (Figure 3a) suggesting that the increment of intracellular GSH suppresses CHT-induced stomatal closure. The stomatal apertures of the 50 µg/mL CHT-treated cad2-1 and ch1-1 plants pretreated with 10 µm GSHmee were not significantly different from those of the wild-type plants unpretreated with GSHmee (P > 0.05) (Figure 3a). The pretreatment with 10 µm GSHmee significantly increased intracellular GSH level in the guard cells of cad2-1 and ch1-1 (P < 0.05) regardless of CHT treatment (Figure 3b). The pretreatment with 10 µm GSHmee significantly increased the GSH level in the CHT-treated guard cells of cad2-1 and ch1-1 (Figure 3b). These results indicate that cytosolic GSH negatively regulates CHT-induced stomatal closure.
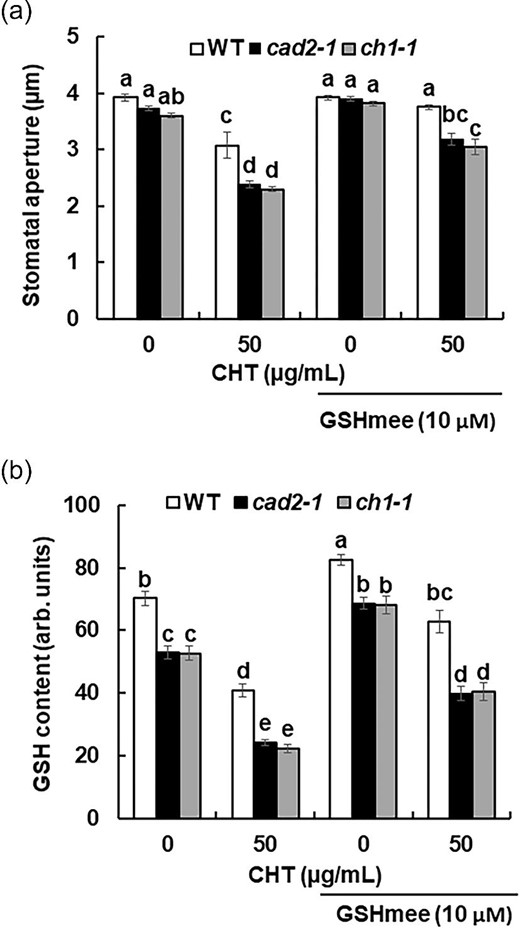
Effects of GSHmee on CHT-induced stomatal closure and GSH content in cad2-1 and ch1-1 mutant plants. (a) Chitosan-induced stomatal closure in wild-type plants and cad2-1 and ch1-1 mutant plants pretreated with 10 µm GSHmee. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent standard errors. (b) Glutathione content in guard cells of wild-type plants and cad2-1 and ch1-1 mutant plants treated with 10 µm GSHmee and 50 µg/mL CHT. Glutathione content is expressed as GSB fluorescence intensity. Error bars represent standard errors (n = 3). Bars showing the different letter are significantly different at P < 0.05.
Discussion
Glutathione modulates ABA, MeJA, and SA signaling, leading to stomatal closure. In our study, we investigated the involvement of GSH in CHT-induced stomatal closure. We found that CHT significantly decreased intracellular GSH levels in the guard cells (Figure 1b). Previous studies reported that ABA, MeJA, and SA decreased intracellular GSH contents in guard cells (Jahan et al. 2008; Akter et al. 2010; Akter et al. 2022). These results suggest that CHT-induced stomatal closure is accompanied by the depletion of intracellular GSH in guard cells. However, the mechanism of decreasing GSH in guard cells in response to CHT remains to be clarified. The CHT-induced stomatal closure is accompanied by ROS production (Lee et al. 1999; Khokon et al. 2010; Salam et al. 2012). Hence, the decrease of GSH can be attributed to scavenging ROS and/or to enzymatical or non-enzymatical conjugation of electrophiles. This mechanism can be commonly shared by ABA, MeJA, and SA signal pathways. However, there was not a higher correlation between ROS production and GSH content in guard cells treated with ITCs (Afrin et al. 2020), suggesting that GSH is not so closely related to ROS scavenging.
In this study, genetic depletion of GSH by the cad2-1 and ch1-1 mutations, and chemical depletion of GSH by the pretreatment of CDNB significantly enhanced CHT-induced stomatal closure (Figures 1b and 2a). It has been reported that depletion of intracellular GSH by GSH-deficient mutants and by CDNB enhanced ABA-, MeJA-, and SA-induced stomatal closure (Jahan et al. 2008; Akter et al. 2010; Akter et al. 2022). Our results show that supplementation of intracellular GSH by GSH-increasing chemical GSHmee attenuated CHT-induced stomatal closure in the mutants (Figure 3a). Previous studies reported that increasing intracellular GSH complements the stomatal phenotype of the cad2-1 plants in response to ABA, MeJA, and SA (Okuma et al. 2011; Akter et al. 2013; Akter et al. 2022). Increasing intracellular GSH by GSHmee also restored the stomatal phenotype of the ch1-1 plants treated-with ABA and MeJA (Jahan et al. 2008; Akter et al. 2010). Taken together, these findings suggest that intracellular GSH functions as a negative regulator of CHT signaling in guard cells. Previous reports suggested that GSH is a common negative signaling factor among ABA signaling, MeJA signaling, and SA signaling (Jahan et al. 2008; Akter et al. 2010; Okuma et al. 2011; Akter et al. 2022). Taken together, the negative regulation by GSH is likely to be shared among these plant hormone signaling and CHT signaling.
It is well-known that GSH has multifaceted physiological functions such as redox regulation and conjugation formation. Reactive carbonyl species (RCS) function as a signal molecule in ABA signaling and MeJA signaling in guard cells (Islam et al. 2016, 2019, 2020) and can react with GSH to form GSH-RCS adducts (Esterbauer, Zollner and Scholz 1975; Davoine et al. 2006). Hence, it can be speculated that GSH negatively regulate CHT signaling through GSH-RCS adduct formation in guard cells.
Conclusion
From our findings, it can be concluded that CHT-induced stomatal closure is also accompanied by depletion of intracellular GSH in guard cells and that GSH negatively regulates CHT-induced stomatal closure in A. thaliana.
Data availability
The analyzed data sets generated during the study are available from the corresponding author upon reasonable request.
Author contribution
I.J., S.M., and Y.M. designed the research. I.J. performed all experiments with support of S.M. and T.N. I.J. analyzed data. T.N. and Y.N. provided suggestions. I.J. and Y.M. wrote the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant number 22H02303 (to Y.M., S.M., and T.N.)] and JSPS Bilateral Collaborations [Grant number JPJSBP120219925 (to I.J., Y.M., S.M., and T.N.)].
Disclosure statement
No potential conflict of interest was reported by the authors.



