-
Views
-
Cite
Cite
Michio Sato, Structural diversity of decalin forming Diels–Alderase, Bioscience, Biotechnology, and Biochemistry, Volume 88, Issue 7, July 2024, Pages 719–726, https://doi.org/10.1093/bbb/zbae040
Close - Share Icon Share
ABSTRACT
The Diels–Alder (DA) reaction, specifically referring to the [4 + 2] cycloaddition reaction in pericyclic reactions, is a process that forms two carbon-carbon covalent bonds in a single step via an electron ring transition state. Among the secondary metabolites produced by microorganisms, numerous compounds are biosynthesized through DA reactions, most of which are enzymatic. Our research group has discovered an enzyme named Diels–Alderase (DAase) that catalyzes the DA reaction in filamentous fungi, and we have been investigating its catalytic mechanism. This review describes the reported microbial DAase enzymes, with a particular focus on those involved in the construction of the decalin ring.
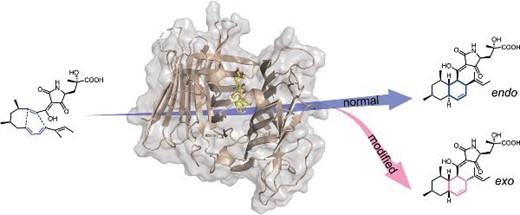
The cyclization of the chain substrate is endo-selective for the wild-type enzyme and exo-selective for the mutant enzyme.





