-
Views
-
Cite
Cite
Mao Hayashi, Yoshinari Wada, Akira Yamamura, Hideki Inoue, Naoya Yamashita, Shigetoshi Ichimura, Yasuhiro Iida, Evaluation of the enzymatic properties of DNA (cytosine-5)-methyltransferase M.ApeKI from archaea in the presence of metal ions, Bioscience, Biotechnology, and Biochemistry, Volume 88, Issue 10, October 2024, Pages 1155–1163, https://doi.org/10.1093/bbb/zbae106
Close - Share Icon Share
ABSTRACT
We previously identified M.ApeKI from Aeropyum pernix K1 as a highly thermostable DNA (cytosine-5)-methyltransferase. M.ApeKI uses the type II restriction-modification system (R-M system), among the best-studied R-M systems. Although endonucleases generally utilize Mg (II) as a cofactor, several reports have shown that MTases exhibit different reactions in the presence of metal ions. This study aim was to evaluate the enzymatic properties of DNA (cytosine-5)-methyltransferase M.ApeKI from archaea in the presence of metal ions. We evaluated the influence of metal ions on the catalytic activity and DNA binding of M.ApeKI. The catalytic activity was inhibited by Cu (II), Mg (II), Mn (II), and Zn (II), each at 5 m m. DNA binding was more strongly inhibited by 5 m m Cu (II) and 10 m m Zn (II). To our knowledge, this is the first report showing that DNA binding of type II MTase is inhibited by metal ions.
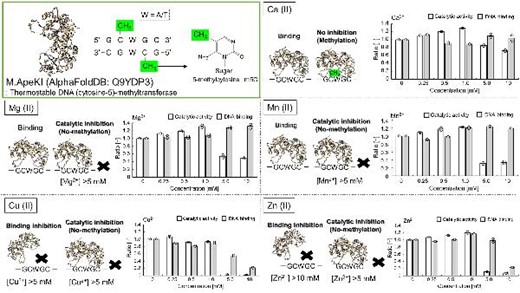
Evaluation of the enzymatic properties of DNA (cytosine-5)-methyltransferase M.ApeKI from archaea in the presence of metal ions.





