-
PDF
- Split View
-
Views
-
Cite
Cite
Brian D. Peer, Spencer G. Sealy, Fate of Grackle (Quiscalus SPP.) Defenses in the Absence of Brood Parasitism: Implications for Long-Term Parasite-Host Coevolution, The Auk, Volume 121, Issue 4, 1 October 2004, Pages 1172–1186, https://doi.org/10.1093/auk/121.4.1172
Close - Share Icon Share
Abstract
We tested grackles (Quiscalus spp.) to determine whether they retain egg rejection behavior in the absence of the selection pressure of brood parasitism. Neither Bronzed Cowbird (Molothrus aeneus) nor Brown-headed Cowbird (M. ater) parasitism was recorded in 797 Great-tailed Grackle (Q. mexicanus) nests. Cross-fostered Bronzed Cowbird nestlings, but not Brown-headed Cowbird nestlings, fledged from Great-tailed Grackle nests, indicating that Brown-headed Cowbird parasitism does not select for rejection in these grackles. Great-tailed Grackle populations sympatric and allopatric with Bronzed Cowbirds rejected 100% of model cowbird eggs. An allopatric population of Boat-tailed Grackle (Q. major), a sister species of the Great-tailed Grackle, also rejected 100% of model eggs. Egg rejection in the Boat-tailed Grackle has apparently been retained in the absence of parasitism for as long as 800,000 years since it split from the Great-tailed Grackle. The Common Grackle (Q. quiscula), which lays the most variable eggs among the grackles, also has the lowest level of egg rejection—which is consistent with the argument that it may have lost most of its rejection behavior in the absence of parasitism. With extreme intraclutch egg-variation, Common Grackles may be more likely to reject their own oddly colored eggs, which would select against rejection behavior in the absence of parasitism. Those results have significant implications for long-term parasite-host coevolution, because they suggest that egg rejection has been retained in most species of Quiscalus in the absence of parasitism. If typical of the world's avifauna, such retention may force brood parasites to specialize on a few host species and to rarely return to using old hosts, which would readily reject their eggs.
Resumen
Evaluamos aves del género Quiscalus para determinar si éstas retienen el comportamiento de rechazo de huevos en la ausencia de la presión selectiva de parasitismo de nido. No se registró parasitismo por parte de Molothrus aeneus ni por parte de M. ater en ninguno de los 797 nidos de Q. mexicanus examinados. Polluelos adoptados de M. aeneus llegaron a volantones en nidos de Q. mexicanus, pero nó polluelos de M. ater, indicando que el parasitismo producido por M. ater no es una fuente de presión selectiva para provocar rechazo en Q. mexicanus. Las poblaciones de Q. mexicanus simpátricas y alopátricas con M. aeneus rechazaron el 100% de los huevos artificiales de Molotrus. Una población alopátrica de Q. major, una especie hermana de Q. mexicanus, también rechazó el 100% de los huevos artificiales. El rechazo de huevos en Q. major aparentemente se ha mantenido en ausencia de parasitismo por más de 800,000 años desde que se separó de Q. mexicanus. La especie Q. quiscula, que exhibe huevos con la mayor variabilidad entre las especies de Quiscalus, también tiene los niveles más bajos de rechazo, lo cual es consistente con el argumento de que probablemente esta especie perdió gran parte de su comportamiento de rechazo en la ausencia de parasitismo. Debido a que la variación intra-nidada de los huevos es enorme, Q. quiscula puede tener una mayor probabilidad de rechazar sus huevos más extraños, lo cual seleccionaría en contra del comportamiento de rechazo en ausencia de parasitismo. Estos resultados tienen implicaciones significativas para los ciclos coevolutivos hospedero-parásito, debido a que sugieren que el rechazo de huevos se ha mantenido en la mayoría de las especies de Quiscalus en la ausencia de parasitismo. Si esto es típico en la avifauna mundial, dicha retención podría forzar a los parásitos de nido a desarrollar adaptaciones especializadas para unas pocas especies hospederas y a que raramente vuelvan a usar viejos hospederos, quienes rechazarían sus huevos sin problema.
Darwin (1859),recognized that traits of an organism that have no current utility probably served some useful function in the past. If a trait does not decrease the fitness of an individual, and there is no significant genetic drift, the trait may be maintained in the absence of its selection pressure. That has been demonstrated in several recent studies. Byers (1997) argued that the American pronghorn (Antilocapra americana) is “ridiculously” too fast for any of its modern predators and that its running adaptations must have resulted from selection by Pleistocene predators in North America that became extinct during the last ice age. Some populations of California ground squirrels (Spermophilus beecheyi) exhibit defensive behaviors used against snakes (Crotalus spp., Pituophis spp.) despite being separated from them for 70,000– 300,000 years (Coss 1999). Likewise, two Arctic moth species (Gynaephora groenlandica, G. rossi) have retained defensive behaviors despite being isolated from bats (Rydell et al. 2000). However, as Byers (1997) has pointed out, researchers still tend to resist the notion that traits are a consequence of past selection pressures, and instead focus on the current adaptiveness of traits (see also Gould and Lewontin 1979). But organisms are a product of both past and current selection pressures; ignoring an organism's phylogenetic history could lead to a misunderstanding of its adaptations (Harvey and Pagel 1991).
One system well suited for ascertaining the fate of adaptations in the absence of selection pressures is avian brood parasitism. The dynamic nature of brood parasite and host interactions, in which parasites switch hosts because of changes in distribution or in evolution of host defenses (e.g. Nakamura 1990, Nakamura et al. 1998), results in relaxation of selection pressures for antiparasite adaptations. The most obvious and perhaps most effective antiparasite adaptation is rejection of a brood parasite's eggs (Peer and Sealy 2004).
The grackles (Quiscalus spp.) are ideal subjects for such a study. They are largely unparasitized by cowbirds and (like all other colonial icterines) do not practice conspecific brood parasitism (CBP; Rohwer and Freeman 1989, Post et al. 1996, Peer and Bollinger 1997b, Peer and Sealy 2000), which occasionally selects for egg rejection behavior. Nonetheless, high frequencies of rejection have been reported for three of the four grackle species tested (Rothstein 1975; Cruz et al. 1985, 1995; Carter 1986; Post et al. 1990; Peer and Bollinger 1997a). In different parts of their range, Great-tailed Grackles (Q. mexicanus) are sympatric with Brown-headed Cowbirds (Molothrus ater), Shiny Cowbirds (M. bonariensis), Bronzed Cowbirds (M. aeneus), and Giant Cowbirds (M. oryzivora). Great-tailed Grackles reject a low frequency of conspecific eggs (Peer and Sealy 2000), and Carter (1986) found that they rejected Bronzed Cowbird eggs from four experimentally parasitized nests. However, no brood parasitism in Great-tailed Grackles has been reported (Friedmann and Kiff 1985, Carter 1986, Rohwer and Freeman 1989). In a previous study, we found that CBP does not select for rejection in Great-tailed Grackles (Peer and Sealy 2000; see below). Boat-tailed Grackles (Q. major) are under no evident selection pressure to maintain egg rejection, in that they are not sympatric with any suitable brood parasites and there are no records of cowbird parasitism or CBP in the species (Friedmann and Kiff 1985, Post et al. 1996). Given the general lack of egg recognition in birds not exposed to interspecific brood parasitism, rejection of foreign eggs by those birds is a reasonable indication of past parasitism (Davies and Brooke 1989b; Rothstein 1990, Rothstein 2001; Nakamura et al. 1998; Bolen et al. 2000).
Only four circumstances are known to select for egg rejection behavior in birds. Interspecific brood parasitism is the most common; hence, when brood parasites stop parasitizing a host, selection for rejection usually stops. Conspecific brood parasitism almost never selects for egg rejection, because conspecific eggs are usually difficult and sometimes impossible to distinguish from one another. Indeed, CBP is known to have selected for rejection in only one passerine group, the weaverbirds (Ploceus spp.; Jackson 1998; but see Rothstein and Robinson 1998). Birds that nest in dense colonies on the ground or on cliffs recognize their eggs to avoid confusing them with those of conspecifics (Tschanz 1959). Finally, one species that lays its eggs in nests it has usurped, the Mourning Dove (Zenaida macroura), rejects the eggs of the nest's former owner (Peer and Bollinger 1998). Birds do not reject foreign eggs from their nests unless they have been exposed to one of those four selection pressures; otherwise, the only eggs rejected would be their own. That is why brood parasites such as cowbirds are so successful. Of the 227 hosts of the Brown-headed Cowbird, only 10% reject cowbird eggs (Peer and Sealy 2004). Many of those hosts, especially those in eastern North America, have only recently become exposed to widespread parasitism and have not had time to evolve egg rejection behavior. That the behavior is almost always evolved in response to parasitism is also evident in species unsuitable as hosts because of diet incompatibilities or inaccessible nest sites. Such birds are avoided by parasites; thus, there is no selection for them to reject parasite eggs, and indeed they do not (Rothstein 1975, Davies and Brooke 1989a).
The fate of host defenses, such as egg rejection, in the absence of brood parasitism has significant implications for parasite-host coevolution. Once rejection behavior becomes fixed in a host population, it is to the advantage of the parasite to switch to a new host that accepts its eggs or to evolve mimetic eggs to circumvent rejection (Davies and Brooke 1989b, Nakamura 1990, Peer et al. 2000). If the frequency of rejection declines or is lost in the initial host population in the absence of parasitism, the parasite may re-exploit the initial host or switch to another host once the second evolves a high frequency of rejection (Davies and Brooke 1989b). If rejection is lost, the parasite-host association may persist through a cyclical process of parasitism and avoidance, or what has been termed the “coevolutionary cycles” model of brood parasite-host coevolution (Rothstein 2001; see also Thompson 1994). Rejection may be lost in the absence of parasitism through genetic drift or if it leads a host to reject its own oddly colored eggs (Lotem et al. 1995, Peer and Bollinger 1997a). If, however, rejection is not costly and is maintained in the absence of parasitism, interactions between brood parasites and their hosts may follow a single-trajectory model. There is usually no reason for birds to express egg rejection behavior in the absence of parasitism, and it may become a neutral trait because brood parasitism is the only context in which birds are regularly subjected to foreign eggs in their nests (Rothstein 1990, Rothstein 2001). In such a case, parasites will rarely go back to using the old hosts because the latter retain defenses; that will lead to parasitism of fewer species over time and evolution of specific adaptations for one or a few host species (Rothstein 2001).
In the present study, we examined whether grackles retain egg rejection behavior in the absence of brood parasitism and the implications for long-term parasite-host coevolution. We had three objectives. The first was to determine whether Great-tailed Grackles reject cowbird parasitism and whether current selection pressure exists that would maintain the behavior in populations sympatric and allopatric with appropriate cowbird species. Second, we determined whether Boat-tailed Grackles reject cowbird eggs. Third, we quantified variation in appearance of eggs in grackle clutches and compared that to the level of egg rejection in grackle species. Such variation may result in birds rejecting their own oddly colored eggs by mistake, which would select against the maintenance of rejection. We predicted that high frequencies of rejection in the absence of parasitism should be correlated with low levels of intraclutch egg-variation.
Methods
Study Area
Most of the study was conducted from 1994 to 1996 at the Welder Wildlife Refuge in San Patricio County, Texas (28°0’N, 97°5’W), where Great-tailed Grackles breed in sympatry with Bronzed and Brownheaded cowbirds (Fig. 1; after Lowther 1995, Post et al. 1996, Wehtje 2003). The emphasis of that portion of the study was on interactions between Bronzed Cowbirds and Great-tailed Grackles, because Brownheaded Cowbirds do not likely parasitize this grackle, given the grackle's much larger size (see below). Nevertheless, experiments were conducted with Brown-headed Cowbirds to examine the possibility that parasitism by them has influenced egg rejection in the Great-tailed Grackle. Up to 500 Great-tailed Grackles nested annually at the refuge, mostly at Big Lake and Pollita Lake. A few grackles nested at smaller bodies of water throughout the refuge. Most nests were in bulrush (Scirpus californicus).
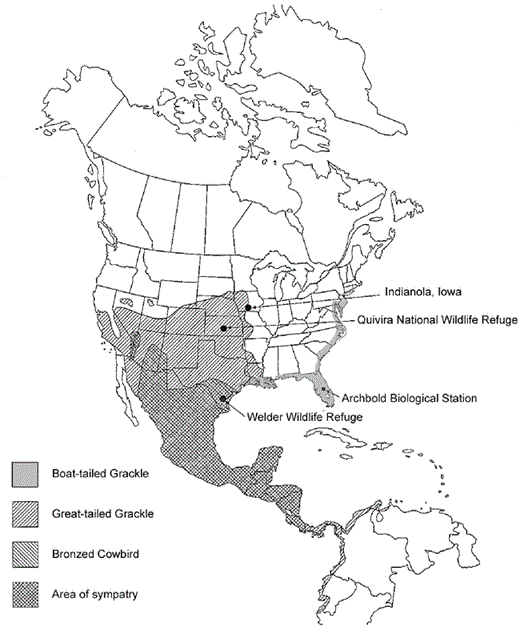
Breeding ranges of the Great-tailed Grackle, Boat-tailed Grackle, and Bronzed Cowbird, and locations of the Welder Wildlife Refuge, Quivira National Wildlife Refuge, Archbold Biological Station, and Indianola, Iowa.
Nest Inspections
We monitored grackle nests daily, beginning 30 min prior to sunrise and completing nest checks within an hour after sunrise. Bronzed and Brown-headed cowbirds lay eggs just prior to sunrise (Scott 1991, Peer and Sealy 1999a), and many hosts often reject cowbird eggs immediately (Sealy 1996; see below). Thus, early watches allowed us to more accurately determine the frequency of parasitism. Nests of other species in the refuge were monitored less regularly for parasitism.
Cross-fostering Experiments
Bronzed and Brown-headed cowbird nestlings were placed into grackle nests to determine whether they could fledge from nests of the larger grackles. If the nestlings did not fledge from grackle nests, it would suggest that grackles are inappropriate hosts that would likely be avoided, and that cowbird parasitism was not the selection pressure for rejection in the Great-tailed Grackle. Cowbird eggs were incubated artificially; as nestlings hatched, they were placed into Great-tailed Grackle nests between 0 and 3 days prior to any grackles hatching. Cowbird nestlings were placed into nests prior to the hatching of host nestlings to simulate the shorter incubation periods characteristic of parasitic species. Incubation period of Great-tailed Grackles is 13–14 days (Johnson and Peer 2001), whereas that of the two cowbird species is 11–12 days (Lowther 1993, Lowther 1995). Three nests were parasitized with two cowbirds each, the reason being that cowbirds multiply parasitize hosts, especially large ones (Carter 1986, Mason 1986, Trine 2000); thus, we attempted to create conditions that simulate natural parasitism. Nestling masses were measured to the nearest 0.01 g with a portable electronic scale every 1–2 days. Length and width of eggs of Great-tailed Grackles, and Bronzed and Brown-headed cowbirds were measured to the nearest 0.1 mm with calipers.
Experimental Cowbird Parasitism
Great-tailed Grackles; Texas.
Great-tailed Grackle nests were experimentally parasitized with real and artificial Bronzed and Brown-headed cowbird eggs. Artificial eggs were wooden, painted with water-based acrylic paints and coated with an acrylic sealer to simulate genuine cowbird eggs. Bronzed Cowbird eggs are immaculate pale blue; Brownheaded Cowbird eggs are white, densely spotted with brown and gray. Wooden artificial eggs measured 22.7 (± 0.1) × 16.7 (± 0.1) mm. Real Bronzed Cowbird eggs measured 24.0 (± 0.2) × 18.9 (± 0.2) mm (n = 16), and real Brown-headed Cowbird eggs measured 21.4 mm (± 0.2) × 16.1 (± 0.2) mm (n = 13). Artificial eggs effectively simulate cowbird eggs; hosts respond to the eggs in the same manner as they do to real parasite eggs, and hosts accept artificial host-eggs constructed in the same fashion (Rothstein 1975; Peer and Bollinger 1997a, Peer 1998; Peer et al. 2000; see below). Only one model egg (Bronzed or Brown-headed cowbird) was added to each nest, either after 1–2 host eggs were present during laying or up to the third day of incubation. Cowbird eggs were added to grackle nests between 0500 and 1200 hours. No grackle eggs were removed from experimentally parasitized nests. Nests were checked daily and, in some cases, 1 and 9 h after being parasitized. Cowbird eggs were considered rejected if they disappeared from an active nest, and accepted if they remained for at least five days. Rejection rates of all Great-tailed and Boat-tailed grackle populations were compared for five days and 24 h, because the latter species is informative in terms of egg recognition mechanisms (Rothstein 1982, Peer and Sealy 2001). Host eggs were numbered, and eggs damaged or missing after ejections were noted. Eggs in control nests were numbered, and the nest contents checked daily, but no parasitic eggs were added.
Great-tailed Grackles; Kansas and Iowa.
Two Greattailed Grackle populations allopatric with Bronzed Cowbirds were tested for rejection to determine whether the grackles reject at a lower frequency than populations sympatric with Bronzed Cowbirds (Davies and Brooke 1989a, Davies b; Briskie et al. 1992). Experiments were conducted in June 1995 at the Quivira National Wildlife Refuge in Stafford County, Kansas (38°2’N, 98°5’W), where grackles were parasitized with wooden Bronzed Cowbird eggs; and in June and July 2003 and May and June 2004 in Warren County, Iowa, just north of Indianola (41°4’N, 93°6’W), where grackles were parasitized with plaster Brownheaded Cowbird eggs that measured 20.9 (± 0.04) × 16.0 (± 0.05) mm (Fig. 1). Brown-headed Cowbirds are present in Kansas and Iowa (Lowther 1993), but there is no evidence that they can successfully parasitize hosts as large as the Great-tailed Grackle (see below).
Boat-tailed Grackles; Florida.
A Boat-tailed Grackle population was tested for rejection with wooden Bronzed Cowbird eggs in Highlands County, Florida (27°4’N, 81°4’W), near the Archbold Biological Station, in March and April 1996 (Fig. 1). Experiments were conducted there because the area is mostly free of cowbirds. Florida is being colonized by three species of cowbirds (Cruz et al. 2000), but only the Brownheaded Cowbird had been known to parasitize hosts in Florida at the time of our study. Boat-tailed and Great-tailed grackles are similar in size (Dunning 1993); thus, Boat-tailed Grackles also are unlikely hosts of the Brown-headed Cowbird.
Analysis of Intraclutch Egg-variation
Differences in appearance of eggs in Boat-tailed, Carib (Q. lugubris), Common (Q. quiscula), and Greattailed grackle clutches were scored by B.D.P. Greattailed Grackle eggs were scored in the field in Texas and from the egg collection at the Field Museum of Natural History; eggs of the other species were scored using museum specimens. Clutches were scored from 1 to 4 using a method similar to that of Møller and Petrie (1991): (1) all eggs within a clutch appeared the same, (2) at least one egg was moderately different from the other eggs in a clutch but the difference was small to moderate, (3) at least one egg was dramatically different and was readily distinguished from the other eggs in the clutch, and (4) all eggs differed. A set of clutches was also scored by a volunteer, and those scores were compared to B.D.P.'s scores for consistency. The volunteer was shown randomly selected slides of clutches of all the grackle species scored. That individual had no knowledge of the rationale for the study.
Statistical Analyses
A Fisher's exact test was used to compare the proportions of Great-tailed and Boat-tailed grackles that rejected. An approximate t-test was used to test for differences between cowbird and grackle nestling masses at hatching. That t-test does not assume equality of variances; degrees of freedom are approximated and are conservative in comparison with t-tests, in which equal variances are assumed (Zar 1996). The KruskalWallis test was used to analyze variation in appearance of eggs within clutches, and nonparametric multiple comparisons for unequal samples corrected for ties were used to test for differences in the species (Zar 1996). Scores for intraclutch egg-variation were tested for consistency using a goodness-of-fit test. Standard errors were used as measures of variance, and P < 0.05 was the accepted level of significance. All tests were two-tailed, unless otherwise indicated.
Results
Frequency of Cowbird Parasitism
None of the 797 Great-tailed Grackle nests monitored at Welder Wildlife Refuge was parasitized by either cowbird species, but seven other species were parasitized by at least one cowbird species (Peer and Sealy 1999b). There was also no parasitism recorded on 13 nests of Great-tailed Grackles in Kansas, 17 nests in Iowa, or 19 nests of Boat-tailed Grackles in Florida.
Cross-fostering Experiments
Ten Bronzed Cowbird nestlings were introduced into seven Great-tailed Grackle nests. Three were depredated, and two of the remaining seven survived. At hatching, Great-tailed Grackle nestlings (n = 27) weighed more than Bronzed Cowbird nestlings (6.21 ± 0.13 g vs. 3.54 ± 0.11 g; t = −16.15, df = 30, P < 0.001). A Bronzed Cowbird nestling introduced one day prior to the hatching of its only grackle nestmate survived along with the nestmate. The Bronzed Cowbird weighed 46.2 g on day 12, when it fledged. At a second nest, a Bronzed Cowbird nestling was introduced 2.5 days before the first grackle nestling hatched and 4.5 days before the second hatched. The second grackle nestling was gone two days later. The remaining grackle and Bronzed Cowbird nestlings fledged; that cowbird weighed 37.6 g on day 10, after which it fledged. Five Bronzed Cowbird nestlings died at a mean age of 3.4 ± 0.7 days. Two died before any grackles hatched, and the other three that died were placed into grackle nests between 0 and 3 days before any grackles hatched.
The six Brown-headed Cowbird nestlings that were introduced into Great-tailed Grackle nests died at a mean age of 3.7 ± 1.5 days. Greattailed Grackle nestlings (see above) weighed more at hatching than Brown-headed Cowbirds (2.2 ± 0.13 g; t = −21.89, df = 15, P < 0.001). Two cowbirds died before any grackles hatched, and the remaining four were placed into nests between 0 and 2 days before any grackles hatched. Grackles fledged from at least four of the six foster nests.
Responses to Experimental Cowbird Parasitism
Great-tailed Grackles; Texas.
Great-tailed Grackles ejected 100% of artificial and real Bronzed Cowbird eggs (n = 77 and 3 nests, respectively) and Brown-headed Cowbird eggs (n = 74 and 6 nests, respectively). One grackle egg was missing after an ejection of a cowbird egg. Of 89 nests checked 1 and 9 h after being parasitized (and every 24 h thereafter), 77.5% were rejected within 1 h of parasitism, 88.9% (n = 89) within 9 h, 95% (n = 160) within 24 h, 98.1% (n = 160) within 48 h, and 100% (n = 160) within 72 h. Thirty-four ejections, all performed by females, were witnessed by B.D.P. Each female typically looked into the nest, grasped the foreign egg between her mandibles, flew away, and placed the egg into the water or on a lily pad. Once, a male returned to a nest before the female and repeatedly stuck his head into the nest, but did not eject the egg. Upon returning, the female immediately ejected the egg.
Great-tailed Grackles; Kansas and Iowa.
All artificial Bronzed Cowbird eggs added to nests in Kansas (n = 13) and Iowa (n = 17) were ejected within 24 h and no grackle eggs were missing or damaged after ejections.
Boat-tailed Grackles; Florida.
All artificial Bronzed Cowbird eggs (n = 19) were ejected within 48 h (16 within 24 h, 3 within 48 h), and no grackle eggs were missing or damaged after ejections. One artificial cowbird egg was present at one nest at the 24-h check, but the nest was depredated before the 48-h check. Great-tailed Grackles ejected more eggs within 24 h (96% of 190) than Boat-tailed Grackles (84% of 19), and the difference approached significance (Fisher's exact test, P = 0.07).
Intraclutch Egg-variation
Common Grackles demonstrated the greatest amount of intraclutch egg-variation, followed by Great-tailed, Carib, and Boat-tailed grackles (Kruskal-Wallis one-way ANOVA, H = 13.0, df = 3, P = 0.005; Fig. 2). Common Grackle clutches had significantly more variation (multiple comparisons) than Boat-tailed (P < 0.005) and Great-tailed (P < 0.05) grackles, but the sample was too small to demonstrate differences with Carib Grackles (P > 0.05). There were no significant differences between the other grackles. More than 50% of Common Grackle clutches demonstrated some variation (i.e. score of 2–4; Fig. 2); whereas only 34% of Great-tailed clutches, 23% of Boat-tailed clutches, and 4% of Carib clutches demonstrated any variation. All eggs differed (i.e. score of 4) in twice as many Common Grackle clutches as Great-tailed Grackle clutches, and no Carib or Boat-tailed grackle clutches received scores of 4 (Fig. 2). The volunteer's scores were consistent with B.D.P.'s; 20 of 29 clutches were scored the same (Goodness-of-fit-test, G = 24.53, df = 1, P < 0.001). The likelihood of the volunteer's and B.D.P.'s scores matching for 20 of 29 clutches, given a choice of four ranks per clutch, is less than one in a million trials. It is clear that our scoring of intraclutch egg-variation is repeatable and compelling.
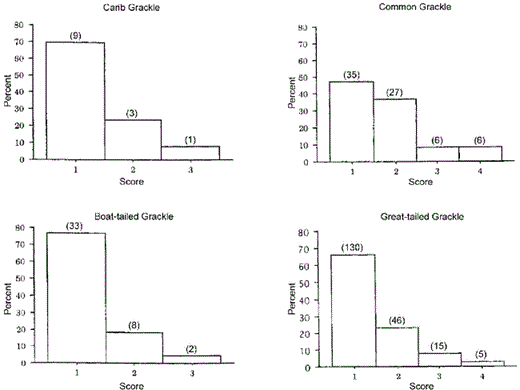
Intraclutch variation in eggs of grackles. Scores were: (1) all eggs within a clutch appeared the same, (2) at least one egg was moderately different from the other eggs, (3) at least one egg was dramatically different from the others in the clutch, and (4) all eggs were different. Number of clutches that received a particular score is given above the bars.
Discussion
Suitability of Great-tailed Grackles as Cowbird Hosts, Frequency of Parasitism, and Retention of Egg Rejection Behavior
Of the seven Bronzed Cowbird nestlings that were not depredated, 29% fledged. That is not surprising, because Bronzed Cowbirds parasitize larger hosts more frequently than Brownheaded Cowbirds presently do, including hosts larger than the Great-tailed Grackle (Lowther 1995, Sealy et al. 1997). Those results indicate that the Great-tailed Grackle is a suitable host for the Bronzed Cowbird. As predicted, no Brown-headed Cowbird nestlings fledged from Great-tailed Grackle nests. The largest known host to raise a Brown-headed Cowbird is the Common Grackle. Brown-headed Cowbird nestlings are 45% of the mass of Common Grackle nestlings at hatching, compared with only 35% of the mass of Great-tailed Grackle nestlings, and Brown-headed Cowbirds have difficulty fledging from nests of Common Grackles. Only 20% of 15 cross-fostered Brown-headed Cowbird nestlings fledged from Common Grackle nests (Peer and Bollinger 1997a). By contrast, the larger Bronzed Cowbird nestlings weighed 57% of the mass of Greattailed Grackles at hatching.
Great-tailed Grackles at Welder Refuge were rarely, if ever, parasitized by either Bronzed or Brown-headed cowbirds. We found no cowbird eggs in grackle nests, despite checking nests immediately after the time of day at which cowbirds lay. Using the same method, Scott (1977) found that 44% of Gray Catbird (Dumetella carolinensis) nests were parasitized, compared with only 11.4% when nests were checked later in the day. We saw cowbirds at grackle colonies only when the cowbirds roosted. Approximately 300 cowbirds roosted each evening at Big Lake, in the bulrushes near grackle nests. They left each morning around sunrise. During the remainder of the day, cowbirds foraged and searched for nests elsewhere, and nests in those areas were parasitized (Peer and Sealy 1999b). Bronzed Cowbirds also punctured host eggs in association with parasitism (Peer and Sealy 1999b). All hosts that were parasitized by Bronzed Cowbirds, or are known hosts of that cowbird, suffered egg puncture; whereas species that were not parasitized had no eggs punctured, including the Great-tailed Grackle (Peer and Sealy 1999b). Despite no evident selection pressures to account for egg rejection behavior, Great-tailed Grackles have maintained rejection at 100% in the population sympatric with Bronzed Cowbirds, as well as in the two allopatric populations in Kansas and Iowa. Great-tailed Grackles did not commit recognition errors that would cause rejection to be selected against; none of their own oddly colored eggs was rejected from control nests that we scored for intraclutch egg-variation during the study. Also, only one grackle egg was missing after 190 ejections, indicating a low frequency of rejection errors.
Rejection may be maintained through gene flow from populations parasitized by Giant Cowbirds. Great-tailed Grackles are sympatric with Giant Cowbirds from southern Mexico to northern South America, and Giant Cowbirds almost exclusively parasitize large colonial icterines (Ortega 1998). Great-tailed Grackles have been moving north in historical times (Johnson and Peer 2001, Wehtje 2003); thus, it is reasonable that populations in the United States are derived from those sympatric with Giant Cowbirds. Giant Cowbirds are also slightly larger than Great-tailed Grackles (Dunning 1993) and would have greater reproductive success than Bronzed Cowbirds did with that host. Giant Cowbird parasitism on Great-tailed Grackles has not been recorded (Friedmann 1963), but little is known of Great-tailed Grackles in the area of sympatry. Interestingly, Giant Cowbird eggs closely resemble Great-tailed and Boat-tailed grackle eggs (Fig. 3). Kuschel (1896) noted that the eggs of the Giant Cowbird resembled those of grackles more than the eggs of the other cowbird species, and the Giant Cowbird was once known as the “Rice Grackle" (Friedmann 1929). The similarity between Giant Cowbird eggs and Great-tailed Grackle eggs could explain the extreme intolerance of Greattailed Grackles toward foreign eggs. Not only do Great-tailed Grackles reject 100% of cowbird eggs, but they also eject 8% of conspecific eggs (Peer and Sealy 2000). However, CBP does not appear to select for rejection, because we found no evidence of CBP in 797 Great-tailed Grackle nests, and Tutor (1962) did not find any in 584 nests monitored at the same location. We also removed 13 nests during laying, which typically induces conspecific parasites to lay eggs parasitically (Rothstein 1993, Peer and Sealy 2000), but Great-tailed Grackles did not. A recent genetic analysis also found no evidence of that behavior in 54 nests (Johnson et al. 2000, Johnson and Peer 2001). Parasitism by Giant Cowbirds may have selected for the refined recognition ability exhibited by Great-tailed Grackles. This is similar to rejection of conspecific eggs by other species despite infrequent occurrence of CBP (Moksnes and Roskaft 1992); those species have apparently evolved the ability because they are parasitized by Common Cuckoos (Cuculus canorus) that lay mimetic eggs.
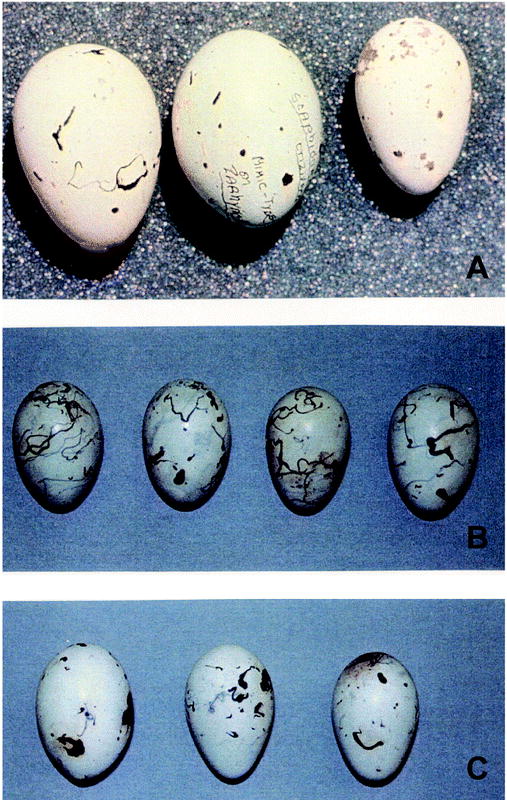
Eggs of (A) Giant Cowbird, (B) Great-tailed Grackle, and (C) Boat-tailed Grackle. Measurements of Giant Cowbird eggs were 32.5 × 26.3 mm (Haverschmidt 1967); Great-tailed Grackle eggs, 31.8 (± 0.3) × 22.4 (± 0.2) mm (n = 52; B. D. Peer unpubl. data); and Boat-tailed Grackle eggs, 31.9 (± 0.06) × 21.8 (± 0.03) mm (Post et al. 1996).
Retention of Egg Rejection Behavior in Boat-tailed Grackles
In contrast to Great-tailed Grackles, Boattailed Grackles—which also reject 100% of cowbird eggs, albeit more slowly—are not sympatric with any suitable brood parasites. Because Boat-tailed Grackles are not exposed to current cowbird parasitism, and none of the other three selection pressures applies to this species, rejection is almost undoubtedly a result of past brood parasitism. The most parsimonious explanation is that Boat-tailed Grackles inherited rejection from a common ancestor with the Great-tailed Grackle. Boattailed and Great-tailed grackles are sister species, once considered the same species (Selander and Giller, 1961; see also Johnson and Lanyon 1999). Selander and Giller (1961) suggested that the Boat-tailed and Great-tailed grackles were separated by glacial events during the Pleistocene. The ancestral form of the Boat-tailed Grackle was confined to the coastal areas of the southeastern United States, and the ancestral Great-tailed Grackle retreated into Mexico. Mitochondrial DNA used to date their split suggests that it occurred 800,000 years ago (Klicka and Zink 1997, Klicka 1998; see also Arbogast and Slowinski 1998). If rejection evolved in an ancestor of those species prior to their split, it has been maintained by the Boat-tailed Grackle for 800,000 years. It is possible that Boat-tailed Grackles were parasitized by a brood parasite that went extinct, or they may have been sympatric with one of the larger extant cowbird species whose range may have subsequently changed. If so, many hosts in eastern North America should also reject foreign eggs as a result of past parasitism. However, only ∼10% of hosts of the Brown-headed Cowbird are rejecters, which indicates that the North American avifauna, especially that of the eastern half of the continent, has probably not come in contact with any brood parasites before the Brownheaded Cowbird.
Retention and Loss of Egg Rejection Behavior in the Other Grackle Species
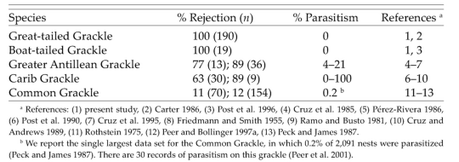
Two Caribbean grackle species—Greater Antillean (Q. niger) and Carib grackles—also exhibit high levels of egg rejection (Cruz et al. 1985, Post et al. 1990). Carib Grackles in Venezuela are heavily parasitized by Shiny Cowbirds (M. bonariensis; Table 1), and gene flow from those populations could account for the rejection frequencies in the Caribbean population. But rejection in the Greater Antillean Grackle cannot be a result of gene flow, because it is confined to the Greater Antilles. The Common Grackle is the one species in which rejection appears to have been selected against in the absence of parasitism, because of recognition errors. Common Grackles are unique in their expression of a low level of egg rejection behavior and a high frequency of intraclutch eggvariation (Table 1 and Fig. 2; Peer and Bollinger 1997a, Peer et al. 2001). Peer and Bollinger (1997a) argued that the Common Grackle once possessed a higher level of egg rejection, but lost most of it in the absence of parasitism because of the high level of intraclutch egg-variation in the species, which would have caused them to reject their own oddly colored eggs (Davies and Brooke 1989b, Marchetti 1992, Lotem et al. 1995). Our finding that Common Grackles demonstrated the highest level of egg-variation among the grackles (Fig. 2)—along with the work of Møller and Petrie (1991), who found that Common Grackle eggs were more variable than Great-tailed Grackle eggs—supports Peer and Bollinger's (1997a) argument. The high intraclutch egg-variation in Common Grackles results from the lighter-colored last-laid eggs that occur in 20–30% of clutches (S. I. Rothstein unpubl. data; see Fig. 4). Because rejecters imprint on the first eggs they lay (Rothstein 1978), Common Grackles may reject the oddly colored last-laid egg. In the absence of parasitism, such behavior would be selected against, which may explain why Common Grackles have the lowest level of egg rejection among the grackles (Rothstein 1975, Peer and Bollinger 1997a). Recognition errors will be difficult to detect, given their rarity, but one might suspect errors if partial egg loss occurred regularly in a species that practices egg recognition. The simple loss of eggs from nests is not sufficient evidence that they were mistakenly rejected, because birds, including accepters, regularly lose some but not all of their eggs (Rothstein 2001).
Rejection and parasitism frequencies of five of the six extant grackle species.
Rejection and parasitism frequencies of five of the six extant grackle species.


Variation in a clutch of Common Grackle eggs from Coles County, Illinois.
Evidence of Loss and Retention of Rejection from Other Studies and Implications for Long-term Parasite—Host Coevolution
Four of the grackle species have retained egg rejection of between 63% and 100%, largely in the absence of parasitism (Table 1 and Fig. 5). Perhaps egg rejection evolved once in the grackles and then became enhanced as needed (e.g. in Great-tailed Grackles); or it may have evolved multiple times. Given the rarity of the behavior in North American passerines, the former explanation may be more likely (Peer and Bollinger 1997a). This suggests that rejection behavior is nearly cost-free and is neutral in the absence of parasitism in most species (Rothstein 1990, Rothstein 2001). Egg rejection should not be expressed in the absence of parasitism, because interspecific brood parasitism is the only circumstance in which birds are typically exposed to foreign eggs in their nests (Rothstein 1990). Like other organisms that retain behaviors resulting from past selection pressures (e.g. Byers 1997, Coss 1999, Rydell et al. 2000), these grackles—especially the Boat-tailed Grackle—have retained adaptations against brood parasitism even though they do not appear to be parasitized by cowbirds.
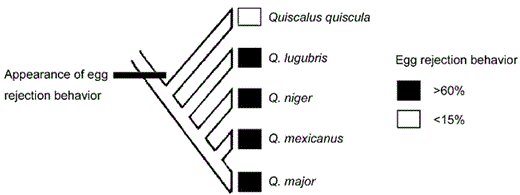
Phylogeny (from Johnson and Lanyon 1999) and rejection frequencies of five of the six extant grackle species. Egg rejection has apparently decreased in the Common Grackle in the absence of parasitism.
Previously, the consensus was that egg rejection behavior is costly to maintain in the absence of brood parasitism and is lost (Davies and Brooke 1989b, Marchetti 1992). But the validity of one of the most widely cited examples of the loss of rejection behavior (Cruz and Wiley 1989) has been questioned. The Village Weaver (Ploceus cucullatus) purportedly lost most of its rejection behavior within ∼200 years after it was introduced to Hispaniola from its native Africa, where it demonstrated a high frequency of egg rejection in response to Diederick Cuckoo (Chrysococcyx caprius) parasitism (Victoria 1972, Cruz and Wiley 1989). However, the two populations tested may have been from different stocks, the eggs used were not consistent between the populations (Payne 1997, Rothstein 2001), and the birds may exhibit phenotypic plasticity in response to foreign eggs (Robert and Sorci 1999).
Recent studies have found that other hosts maintain egg rejection behavior despite the fact that they are no longer parasitized (Baltz and Burhans 1998, Nakamura et al. 1998, Bolen et al. 2000, Rothstein 2001). Thus, as Rothstein (2001) pointed out, with the exception of species like Common Grackles that have extreme levels of intraclutch egg-variation, the existing data indicate that retention of egg rejection is at least as common as, and perhaps more common than, the loss of rejection, which supports the singletrajectory model of brood parasite-host coevolution. If retention of rejection predominates, it will force brood parasites to specialize on relatively few hosts. Parasites will be unable to alternate from well-defended hosts to those that have lost rejection. Instead, once most cowbird hosts become rejecters, cowbirds may be forced to evolve mimetic eggs for only one or a few hosts with similar eggs, as in Common Cuckoo gentes (Brooke and Davies 1988).
Acknowledgments
We thank members of the Welder Wildlife Refuge, Quivira National Wildlife Refuge, and Archbold Biological Station for facilitating our research. N. Smith allowed us to include his photo of Giant Cowbird eggs. The following individuals assisted with intraclutch egg analysis: D. Willard, Field Museum; S. Sumida, Western Foundation of Vertebrate Zoology; A. Andors, American Museum of Natural History; G. Hess, Delaware Museum of Natural History; J. Dean, National Museum of Natural History; and C. Cicero, Museum of Vertebrate Zoology. A. Patterson helped draft Figure 1. Two anonymous reviewers provided helpful comments on the manuscript. The research was supported by an NSERC (Natural Sciences and Engineering Reasearch Council of Canada) research grant to S. G. S. and a Lubinsky Memorial Scholarship from the Department of Zoology, University of Manitoba, to B.D.P.
Literature Cited



