-
PDF
- Split View
-
Views
-
Cite
Cite
Sean Kwang Howe Leow, Robert John William Knight, Contemporary Trends in Antiseptic Pocket Rinse in Primary Breast Implant Surgery, Aesthetic Surgery Journal, Volume 44, Issue 8, August 2024, Pages 809–817, https://doi.org/10.1093/asj/sjad351
Close - Share Icon Share
Abstract
Broad evidence supports the use of antiseptic pocket rinse in breast implant surgery to minimize the risk of capsular contracture or other complications. However, there is limited consensus or standardization of antiseptic rinse in practice.
In this preliminary study, we sought to determine contemporary trends in antiseptic rinse use in primary breast implant surgery based on Australian Breast Device Registry (ABDR) data, and whether these trends align with the suggestions of the 14-point plan. This further served as a feasibility study for subsequent comparison of antiseptic rinse effects on clinical outcomes.
Institutional ethics approval was obtained and national ABDR data for primary breast implant surgery from 2015 to 2020 were analyzed for the use and type of antiseptic rinse. The surgeon-reported data were homogenized with regard to terminology and categorized by major trends, and the literature was reviewed.
We analyzed data for 37,143 patients, totaling 73,935 primary implants. Antiseptic rinse included povidone-iodine (PVP-I) in 35,859 (48.5%), no antiseptic use in 24,216 (32.8%), other concentrations of PVP-I in 4200 (5.7%), and Betadine triple antibiotic in 1831 implants (2.5%). Multiple other antiseptic permutations were noted in 7004 implants (9.5%).
The majority (56.7%) of Australian practitioners utilize previously described antiseptic pocket irrigation solutions that align with the 14-point plan. A third (32.8%), however, do not record any antiseptic pocket irrigation. These findings will permit a subsequent (ongoing) study of outcomes comparing PVP-I pocket rinse to no antiseptic pocket rinse, which will likely constitute the largest study of its kind.
See the Commentary on this article here.
Breast implant surgery has seen a significant growth in numbers in recent decades, remaining one of the most common cosmetic procedures performed.1,2 It has been estimated that there are around 60 to 70 million breast implants in over 35 million patients worldwide.3
The most common complication requiring further surgical intervention after primary breast implant surgery is capsular contracture.4-6 Revisional surgeries are also commonly necessitated by implant rupture, hematoma, and wound infection.4
Biofilm has been increasingly recognized as a leading cause of capsular contracture.7,8 Subclinical infection was first pinpointed as the etiological factor behind capsular contracture by Burkhardt et al.9 The authors cultured open capsulotomies for capsular contracture, with 71% returning positive cultures; this yielded a pattern of Staphylococcus epidermidis in 90% and multiorganism cultures in the remainder.9S. epidermidis, the dominant organism, is a known commensal of nipple secretions as well as deeper glandular tissue of the breast.9,10
Since then, multiple studies have either replicated these findings with modern culture techniques or reaffirmed this causative relationship between subclinical infection, biofilm, and capsular contracture.11-15 Inoculation of miniature implants in a rabbit model with varying concentrations of S. epidermidis resulted in Baker grade III or IV capsular contracture as opposed to lower grades in contralateral control implants.12 A similar phenomenon with S. aureus in guinea pigs was examined with electron microscopy, with histological findings supportive of immune mediation of capsular contracture.13 A further multicenter study at which bacteria from explanted implants were cultured with the use of sonication showed a strong correlation of bacterial presence with grades of capsular contracture, and found Propionibacterium acnes and coagulase-negative staphylococci species to be most frequently cultured.14 Finally, in a porcine model, a causal link was shown between S. epidermidis inoculation of implant pockets, biofilm development, and subsequent capsular contracture.15
Breast implant–associated anaplastic large cell lymphoma (BIA ALCL), first described in 1997, is a rare lymphoproliferative disorder, with an estimated risk between 1 in 3000 to 1 in 60,000 in the Australian and New Zealand population.16 It presents, on average, 7 to 8 years following implant insertion.16 In recent years, increasing evidence has allowed the proposition of a unifying pathophysiological hypothesis that is gaining acceptance—that BIA ALCL is a disorder arising when bacterial contamination of textured implants in a genetically susceptible individual results in chronic inflammation and eventual T-cell transformation over time.16-,18
Reminiscent of the pivotal studies showing high rates of subclinical infection in capsular contracture, Hu et al (2016) utilized modern immunological and molecular techniques including fluorescent in situ hybridization (FISH), real-time quantitative polymerase chain reaction, next-generation sequencing, and scanning electron microscopy (SEM) to evaluate the microbiome of BIA ALCL samples and compare them to nontumor samples.19 This pivotal study showed not only that a high bacterial load was present, but that the microbiome of BIA ALCL showed a significant shift toward a gram-negative component, particularly Ralstonia spp., as opposed to the Staphylococcus spp.–dominant microbiome of nontumor capsules. Biofilm was visualized on both FISH and SEM, further supporting the pathophysiological contribution of subclinical infection.19
Deva et al (2013) highlighted the evolving evidence base for subclinical infection playing a key role in the development of capsular contracture, and accurately alluded to the possibility it would play in BIA ALCL.7 The authors proposed a series of 14 recommendations to minimize the risk of device-associated infections through fastidious avoidance of intraoperative contamination, which they termed the “14-point plan.” Key among these recommendations was performing pocket irrigation with triple antibiotic solution or Betadine (Sanofi-Aventis, NSW, Australia).7 This study relates to the adoption and contemporary practice with respect to pocket irrigation as described in point 7 of the 14-point plan, but also as described in the literature.20-25
The origins of “Betadine triple,” also known anecdotally as “Adams’ solution” within the Australian Breast Device Registry (ABDR) database, stem from a practice-changing in vitro study by Adams et al (2000).23 Frustrated at the lack of evidence behind antiseptic pocket rinse practice then, the authors conducted in vitro testing of various combinations of antiseptic/antibiotic solutions against cultures of common breast implant organisms in sequential phases, determining the minimum concentrations required for effectiveness against the pathogens.
As a result of their findings as well as concern about the cytotoxic effect Betadine had been noted to have on fibroblasts in vitro, the authors proposed a Betadine-cefazolin-gentamicin combination in prescribed proportions, referred to as Betadine triple, to achieve optimal antibacterial coverage.23,26
Following that pivotal in vitro study, the FDA issued instructions stating that contact between Betadine and implants was contraindicated. This has since been rescinded because the concerns of the time were disproved.27 Nonetheless, Adams et al (2001) followed the FDA instructions at the time with updated studies showing that a bacitracin, cefazolin, and gentamicin combination provided a suitable alternative to Betadine-containing irrigation solutions.28 Bacitracin as used in this irrigation solution is not available in Australia and so did not show up in our analysis.
A combination of factors make Betadine (povidone-iodine, 10% PVP-I) a reliable and easy choice for antiseptic pocket irrigation, including its ease of use without premixing, ready availability, low cost, and rarity of true allergy.29,30 With multiple gram-positive bacteria associated with capsular contracture, and gram-negative Ralstonia spp. (which are resistant to aminoglycosides but susceptible to PVP-I of no more than a 50% dilution, or 5% PVP-I) associated with BIA-ALCL, the broad microbicidal spectrum of PVP-I provides a reliable irrigation option.27,31 Burkhardt's studies pioneered PVP-I pocket irrigation and showed a reduction in the early capsular contracture rates, but comprised small patient populations and involved different implant types of earlier generations, a factor known to influence contracture rates.32-34 The last of the 3 randomized controlled trials (RCTs) Burkhardt reported showed a significant reduction in capsular contracture rates over a 4-year period in then-textured as opposed to smooth implants, with 5% PVP-I pocket irrigation as opposed to saline irrigation.34
Multiple studies have since supported antibiotic pocket irrigation in augmentation surgery, including strong basic science supporting the antimicrobial spectrum of Betadine or Betadine triple antibiotic against the breast capsule microbiome.22,23,25,27,28,35,36 The prominence of subclinical infection as a major (if not key) factor in influencing risks of infection, capsular contracture, and BIA ALCL in the literature, as mentioned above, has led prominent authors of the field to suggest that a double-blinded, prospective, randomized trial would not be an ethically acceptable option.24 Coupled with costs and limitations inherent to surgical studies, the evidence base has an understandable preponderance of retrospective comparative analyses and both prospective and retrospective case series, amounting to National Health and Medical Research Council (NHMRC) level III-2 evidence.24,29, 37-44 As a result, systematic reviews on the topic of antibiotic or PVP-I pocket irrigation and related clinical outcomes consistently support the use of pocket irrigation, but are also understandably cautious in their recommendations, citing the relatively large proportion of retrospective studies, a lack of RCTs, and the heterogeneity of antibiotic regimes and study design.20-22,35,36 The Australian Senate inquiry that followed soon after the events of the Poly Implant Prothèse (PIP; PACA, France) crisis recommended the establishment of an “opt-out Breast Implant Registry as a priority.”45 This new “Breast Device Registry” (BDR) pilot formally initiated its national implementation in 2015 and is now known as the Australian Breast Device Registry (ABDR).46 Early in the ABDR's implementation, the opt-out rate was 1.75%, and by August 2016, less than 1% of participants had opted out of the ABDR, equating to a capture rate of over 99% of all patients undergoing breast device surgery in participating centers.46,47 It is the first registry in the world to be supported by all groups involved in breast device surgery in the country, namely, plastic and reconstructive surgeons, breast surgeons, and cosmetic doctors.48
Our study objective was to determine the contemporary antiseptic (pocket) rinse trends in Australia for primary breast implant surgery with national level data from ABDR, and whether the major practice trends align with the evidence in the literature and the 14-point plan recommendations.
METHODS
An observational study with retrospective analysis of prospectively collected data from the ABDR was undertaken. Existing ethics approvals for the ABDR processes, summarized on the registry's website, are overseen by the Monash University Human Research Ethics Committees (HREC).48 Project and ethics approval was further obtained from the ABDR national steering committee and the University of Sydney HREC.
An ABDR data collection form (DCF), available on the ABDR website, is completed by contributing surgeons immediately following surgery. The DCF is a 1-page, double-sided validated collection form with a “stick and tick” approach, containing the minimum data set that the ABDR utilizes to generate reports. The DCF section on “Intraoperative Techniques” is 1 of few segments permitting free text by the operator.48
Data for all patients aged 18 years old and above at the time of surgery, for whom surgery occurred between January 1, 2015, and December 31, 2020, inclusive, was extracted from the ABDR database. This timeline coincided with the implementation of the current form of the DCF (unpublished data from ABDR).
Inclusion and exclusion criteria are summarized in Table 1.
| Data collection form fields . | ||
|---|---|---|
| Form fields . | Included . | Excluded . |
| Demographics | ||
| 18 years old and above at time of surgery | N/A | |
| Surgery between January 1, 2015 and December 31, 2020, inclusive | ||
| Device type | ||
| Breast implant | Breast tissue expander | |
| Not known/unrecorded | ||
| Operation category and type | ||
First implant insertion for all indications:
| Tissue expander insertion | |
| Tissue expander removal and implant insertion | ||
| Revision of in situ devices | ||
| Operation Elements | ||
| N/A | Concurrent mastectomy | |
| Axillary surgery including sentinel node biopsy | ||
| Concurrent flap cover | ||
| Data collection form fields . | ||
|---|---|---|
| Form fields . | Included . | Excluded . |
| Demographics | ||
| 18 years old and above at time of surgery | N/A | |
| Surgery between January 1, 2015 and December 31, 2020, inclusive | ||
| Device type | ||
| Breast implant | Breast tissue expander | |
| Not known/unrecorded | ||
| Operation category and type | ||
First implant insertion for all indications:
| Tissue expander insertion | |
| Tissue expander removal and implant insertion | ||
| Revision of in situ devices | ||
| Operation Elements | ||
| N/A | Concurrent mastectomy | |
| Axillary surgery including sentinel node biopsy | ||
| Concurrent flap cover | ||
| Data collection form fields . | ||
|---|---|---|
| Form fields . | Included . | Excluded . |
| Demographics | ||
| 18 years old and above at time of surgery | N/A | |
| Surgery between January 1, 2015 and December 31, 2020, inclusive | ||
| Device type | ||
| Breast implant | Breast tissue expander | |
| Not known/unrecorded | ||
| Operation category and type | ||
First implant insertion for all indications:
| Tissue expander insertion | |
| Tissue expander removal and implant insertion | ||
| Revision of in situ devices | ||
| Operation Elements | ||
| N/A | Concurrent mastectomy | |
| Axillary surgery including sentinel node biopsy | ||
| Concurrent flap cover | ||
| Data collection form fields . | ||
|---|---|---|
| Form fields . | Included . | Excluded . |
| Demographics | ||
| 18 years old and above at time of surgery | N/A | |
| Surgery between January 1, 2015 and December 31, 2020, inclusive | ||
| Device type | ||
| Breast implant | Breast tissue expander | |
| Not known/unrecorded | ||
| Operation category and type | ||
First implant insertion for all indications:
| Tissue expander insertion | |
| Tissue expander removal and implant insertion | ||
| Revision of in situ devices | ||
| Operation Elements | ||
| N/A | Concurrent mastectomy | |
| Axillary surgery including sentinel node biopsy | ||
| Concurrent flap cover | ||
All free text values for antiseptic rinse were sequentially and methodically categorized in the following sequence:
All free text values were first checked for spelling. All meaningless entries were categorized as “illegible” and excluded.
All alternative nomenclature was homogenized (eg, povidone, Betadine, povidone-iodine, and other misspellings recategorized as Betadine).
All brand names were converted to generic nomenclature and homogenized. The exception to this was Betadine, the generic name of which is povidone-iodine (PVP-I), due to the use of the brand name in the description of recognized irrigation solutions such as Betadine triple by Adams and colleagues.23,24,26,28
Narrowing of categorization by antibiotic class, when appropriate.
Narrowing of categorization by common antibiotic substitutions for allergy, when appropriate.
Finally, the “cleaned” data was reviewed for any major trends, and allocated to the major categories.
RESULTS
Patient Demographics
Over 6 years, from 2015 to 2020 inclusive, a total of 37,178 patients underwent primary breast implant surgery, falling within our inclusion criteria. This translated to a total of 73,935 implants from 421 unilateral and 36,757 bilateral surgeries.
The age of the patients at the time of surgery ranged from 18 to 86. The average age was 32.7, median age was 31, and the mode was 25 years of age.
Intraoperative “Antiseptic Rinse”
ABDR-transcribed data for “Antiseptic Rinse” were classified as either:
“Betadine,” when the check box was ticked, and the free text indicated the use of Betadine (total 34,970 implants);
“Null,” when the check box was left unticked (total 24,216 implants); or
“Other,” when the check box was ticked, and the free text stated something other than Betadine (total 14,749 implants).
Entries classified as “Other” gave rise to a total of 1311 distinct free text descriptive values. As described in the “Methods” section, initial categorization of the 1311 distinct free text values resulted in 48 broad categories. This was collapsed into 6 meaningful trend categories in antiseptic pocket rinse, with the distribution described in Table 2 and the Figure 1.
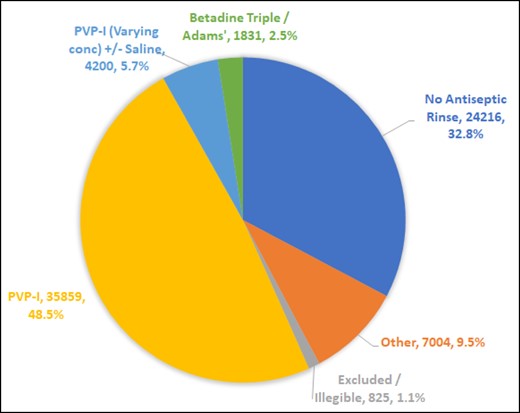
Intraoperative pocket antiseptic rinse practices in Australia from 2015 to 2020, inclusive. PVP-I, povidone-iodine or Betadine.
Illegible and meaningless entries constituted 1.1% of the data and had to be excluded. These included such entries as blank entries (check box ticked but no description written), stated volumes (“100 mL”), illegible and unreadable entries, or meaningless entries (for example, “XXX hospital protocol,” “AL,” “Aqueous”).
Antiseptic (Pocket) Rinse Trends Broken Down by Number of Implants and Patients
| Antiseptic pocket rinse . | No. of implants . | Percentage . | No. of patients . | Percentage . |
|---|---|---|---|---|
| Povidone-iodine (PVP-I) | 35,859 | 48.5% | 18,029 | 48.5% |
| No antiseptic rinse | 24,216 | 32.8% | 12,216 | 32.9% |
| Other | 7004 | 9.5% | 3512 | 9.4% |
| PVP-I (varying concentration) ± saline | 4200 | 5.7% | 2103 | 5.7% |
| Betadine triple/Adams’ | 1831 | 2.5% | 924 | 2.5% |
| Excluded/illegible | 825 | 1.1% | 416 | 1.1% |
| Total | 73,935 | 37,178 |
| Antiseptic pocket rinse . | No. of implants . | Percentage . | No. of patients . | Percentage . |
|---|---|---|---|---|
| Povidone-iodine (PVP-I) | 35,859 | 48.5% | 18,029 | 48.5% |
| No antiseptic rinse | 24,216 | 32.8% | 12,216 | 32.9% |
| Other | 7004 | 9.5% | 3512 | 9.4% |
| PVP-I (varying concentration) ± saline | 4200 | 5.7% | 2103 | 5.7% |
| Betadine triple/Adams’ | 1831 | 2.5% | 924 | 2.5% |
| Excluded/illegible | 825 | 1.1% | 416 | 1.1% |
| Total | 73,935 | 37,178 |
Antiseptic (Pocket) Rinse Trends Broken Down by Number of Implants and Patients
| Antiseptic pocket rinse . | No. of implants . | Percentage . | No. of patients . | Percentage . |
|---|---|---|---|---|
| Povidone-iodine (PVP-I) | 35,859 | 48.5% | 18,029 | 48.5% |
| No antiseptic rinse | 24,216 | 32.8% | 12,216 | 32.9% |
| Other | 7004 | 9.5% | 3512 | 9.4% |
| PVP-I (varying concentration) ± saline | 4200 | 5.7% | 2103 | 5.7% |
| Betadine triple/Adams’ | 1831 | 2.5% | 924 | 2.5% |
| Excluded/illegible | 825 | 1.1% | 416 | 1.1% |
| Total | 73,935 | 37,178 |
| Antiseptic pocket rinse . | No. of implants . | Percentage . | No. of patients . | Percentage . |
|---|---|---|---|---|
| Povidone-iodine (PVP-I) | 35,859 | 48.5% | 18,029 | 48.5% |
| No antiseptic rinse | 24,216 | 32.8% | 12,216 | 32.9% |
| Other | 7004 | 9.5% | 3512 | 9.4% |
| PVP-I (varying concentration) ± saline | 4200 | 5.7% | 2103 | 5.7% |
| Betadine triple/Adams’ | 1831 | 2.5% | 924 | 2.5% |
| Excluded/illegible | 825 | 1.1% | 416 | 1.1% |
| Total | 73,935 | 37,178 |
DISCUSSION
The 2 largest groups and dominant trends in antiseptic pocket rinse included stock povidone-iodine and no antiseptic rinse at all. Nearly a third of all patients and implants (12,216 and 24,216, 32.9% and 32.8%, respectively) did not receive any antiseptic rinse. Stock povidone-iodine was provided as antiseptic rinse in nearly half (48.5%) of all patients and implants, amounting to 18,029 patients and 35,859 implants.
Our results show that prominent practice trends correspond with the suggestions of the 14-point plan, with a total of 56.7% of implants (Figure) placed with a pocket rinse comprising either povidone-iodine (and varying concentrations of the same, with or without additional saline rinse), or Betadine triple antibiotic, as described by Adams et al (2000).23
A survey of 253 members of the American Society of Plastic Surgery in April 2015 showed a similar dominant pocket irrigation practice: that of Betadine in 44%, but a smaller proportion with non-antiseptic irrigation (13% normal saline, and 14% sterile water) or no irrigation (2%).49
While the 14-point plan has gained prominence, it is also clear that it is not completely or universally accepted in practice; the authors of the 14-point plan recognize the discretion of surgeons to implement a proportion, if not all, of the individual recommendations.50 The lack of universal assent to the 14-point plan was also reflected in discussions at the national Plastic Surgery Congress 2022, at which some of these data were presented. This mixed opinion on antiseptic pocket rinse is also likely to be a reflection of the restrained recommendations on its use, as mentioned in our introduction.21,22,35,36
The choice of a third of reporting physicians not to use antiseptic pocket irrigation while utilizing other preventative measures provides a feasible control group for an ongoing follow-up study that will allow for a large cohort analysis. In order to homogenize the long-term risk of subclinical infection in this primary study population, concurrent mastectomies, axillary surgery including sentinel node biopsy, and concurrent flap cover surgeries were excluded from our sample population.4 Due to the strict requirements for deidentification of patient data, data categories upon which inclusion and exclusion decisions could be imposed were strictly limited to the fields of the DCF made available to the research team. There was no access to information about comorbidities or patient factors (such as immunocompromise or the presence of diabetes) that might have influenced antiseptic rinse choice and longer term (individual) risk of complications.
Sample Losses and Exclusions
The patient opt-out rate from the ABDR remains small, at 1%, as noted in 2016 and 2020.46,51 Within these data, exclusion due to illegible, missing, or nonsensical free text input into the ABDR DCF “Antiseptic Rinse” field constituted a further 1.1% (Figure). This translates to an overall capture rate of antiseptic pocket rinse practice from ABDR submissions of 97.9%. In the initial implementation of the ABDR DCF in its current form, a small but notable number of DCFs were returned where the “Antiseptic Rinse” checkbox was ticked, but no reagent of choice was specified. This practice of omission has decreased with time. Our data excludes such entries under the category of “Excluded/Illegible,” making up the 1.1% stated above.
Data Representation by Eligible Centers and Surgeons
The overall ABDR source data has a high rate of national patient capture, with 88% of both eligible centers and eligible surgeons participating in the ABDR as of December 31, 2020.51 Due to deidentification requirements, we were not privy to the exact breakdown of our specific data by eligible sites or surgeons. However, the complete ABDR capture rates are a highly reliable estimation of our data (as a subset of the national database) capture rate because eligible centers (Table 3) and surgeons (Table 4) engaged by the ABDR report on all breast device surgeries through the same submission processes.48
| State . | Closed sites . | In progress sites . | Eligible private sites . | Participating private sites . | Engagement of eligible private sites . | Eligible public sites . | Participating public sites . | Engagement of eligible public sites . |
|---|---|---|---|---|---|---|---|---|
| NSW | 6 | 12 | 70 | 63 | 90% | 32 | 22 | 69% |
| VIC | 4 | 3 | 51 | 48 | 94% | 26 | 21 | 81% |
| QLD | 6 | 3 | 48 | 48 | 100% | 16 | 13 | 81% |
| WA | 1 | 5 | 22 | 21 | 95% | 5 | 0 | 0% |
| SA | 3 | 1 | 18 | 18 | 100% | 7 | 6 | 86% |
| ACT | 0 | 1 | 6 | 5 | 83% | 2 | 1 | 50% |
| TAS | 0 | 0 | 4 | 4 | 100% | 2 | 2 | 100% |
| NT | 0 | 0 | 2 | 2 | 100% | 1 | 1 | 100% |
| Total | 20 | 25 | 221 | 209 | 95% | 91 | 66 | 73% |
| State . | Closed sites . | In progress sites . | Eligible private sites . | Participating private sites . | Engagement of eligible private sites . | Eligible public sites . | Participating public sites . | Engagement of eligible public sites . |
|---|---|---|---|---|---|---|---|---|
| NSW | 6 | 12 | 70 | 63 | 90% | 32 | 22 | 69% |
| VIC | 4 | 3 | 51 | 48 | 94% | 26 | 21 | 81% |
| QLD | 6 | 3 | 48 | 48 | 100% | 16 | 13 | 81% |
| WA | 1 | 5 | 22 | 21 | 95% | 5 | 0 | 0% |
| SA | 3 | 1 | 18 | 18 | 100% | 7 | 6 | 86% |
| ACT | 0 | 1 | 6 | 5 | 83% | 2 | 1 | 50% |
| TAS | 0 | 0 | 4 | 4 | 100% | 2 | 2 | 100% |
| NT | 0 | 0 | 2 | 2 | 100% | 1 | 1 | 100% |
| Total | 20 | 25 | 221 | 209 | 95% | 91 | 66 | 73% |
Note: As of December 31, 2020, 88% of total eligible sites were participating in the ABDR. Reproduced with permission from the ABDR. ABDR, Australian Breast Device Registry.
| State . | Closed sites . | In progress sites . | Eligible private sites . | Participating private sites . | Engagement of eligible private sites . | Eligible public sites . | Participating public sites . | Engagement of eligible public sites . |
|---|---|---|---|---|---|---|---|---|
| NSW | 6 | 12 | 70 | 63 | 90% | 32 | 22 | 69% |
| VIC | 4 | 3 | 51 | 48 | 94% | 26 | 21 | 81% |
| QLD | 6 | 3 | 48 | 48 | 100% | 16 | 13 | 81% |
| WA | 1 | 5 | 22 | 21 | 95% | 5 | 0 | 0% |
| SA | 3 | 1 | 18 | 18 | 100% | 7 | 6 | 86% |
| ACT | 0 | 1 | 6 | 5 | 83% | 2 | 1 | 50% |
| TAS | 0 | 0 | 4 | 4 | 100% | 2 | 2 | 100% |
| NT | 0 | 0 | 2 | 2 | 100% | 1 | 1 | 100% |
| Total | 20 | 25 | 221 | 209 | 95% | 91 | 66 | 73% |
| State . | Closed sites . | In progress sites . | Eligible private sites . | Participating private sites . | Engagement of eligible private sites . | Eligible public sites . | Participating public sites . | Engagement of eligible public sites . |
|---|---|---|---|---|---|---|---|---|
| NSW | 6 | 12 | 70 | 63 | 90% | 32 | 22 | 69% |
| VIC | 4 | 3 | 51 | 48 | 94% | 26 | 21 | 81% |
| QLD | 6 | 3 | 48 | 48 | 100% | 16 | 13 | 81% |
| WA | 1 | 5 | 22 | 21 | 95% | 5 | 0 | 0% |
| SA | 3 | 1 | 18 | 18 | 100% | 7 | 6 | 86% |
| ACT | 0 | 1 | 6 | 5 | 83% | 2 | 1 | 50% |
| TAS | 0 | 0 | 4 | 4 | 100% | 2 | 2 | 100% |
| NT | 0 | 0 | 2 | 2 | 100% | 1 | 1 | 100% |
| Total | 20 | 25 | 221 | 209 | 95% | 91 | 66 | 73% |
Note: As of December 31, 2020, 88% of total eligible sites were participating in the ABDR. Reproduced with permission from the ABDR. ABDR, Australian Breast Device Registry.
| State . | Eligible plastic surgeons . | Participating plastic surgeons . | Plastic surgeons engagement . | Eligible general/breast surgeons . | Participating general/breast surgeons . | General/breast surgeons engagement . | Eligible cosmetic physicians . | Participating cosmetic physicians . | Cosmetic physicians engagement . |
|---|---|---|---|---|---|---|---|---|---|
| NSW | 100 | 91 | 91% | 64 | 57 | 89% | 24 | 20 | 83% |
| VIC | 108 | 101 | 94% | 39 | 25 | 64% | 8 | 8 | 100% |
| QLD | 71 | 67 | 94% | 47 | 39 | 83% | 16 | 13 | 81% |
| WA | 41 | 37 | 90% | 19 | 13 | 68% | 4 | 4 | 100% |
| SA | 31 | 29 | 94% | 13 | 10 | 77% | 2 | 2 | 100% |
| TAS | 12 | 12 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| ACT | 3 | 3 | 100% | 4 | 4 | 100% | 0 | 0 | 0% |
| NT | 2 | 2 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| Total | 368 | 342 | 93% | 192 | 154 | 80% | 54 | 47 | 87% |
| State . | Eligible plastic surgeons . | Participating plastic surgeons . | Plastic surgeons engagement . | Eligible general/breast surgeons . | Participating general/breast surgeons . | General/breast surgeons engagement . | Eligible cosmetic physicians . | Participating cosmetic physicians . | Cosmetic physicians engagement . |
|---|---|---|---|---|---|---|---|---|---|
| NSW | 100 | 91 | 91% | 64 | 57 | 89% | 24 | 20 | 83% |
| VIC | 108 | 101 | 94% | 39 | 25 | 64% | 8 | 8 | 100% |
| QLD | 71 | 67 | 94% | 47 | 39 | 83% | 16 | 13 | 81% |
| WA | 41 | 37 | 90% | 19 | 13 | 68% | 4 | 4 | 100% |
| SA | 31 | 29 | 94% | 13 | 10 | 77% | 2 | 2 | 100% |
| TAS | 12 | 12 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| ACT | 3 | 3 | 100% | 4 | 4 | 100% | 0 | 0 | 0% |
| NT | 2 | 2 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| Total | 368 | 342 | 93% | 192 | 154 | 80% | 54 | 47 | 87% |
Note: As of December 31, 2020, 88% of total eligible physicians were participating in the ABDR. Reproduced with permission from the ABDR. ABDR, Australian Breast Device Registry.
| State . | Eligible plastic surgeons . | Participating plastic surgeons . | Plastic surgeons engagement . | Eligible general/breast surgeons . | Participating general/breast surgeons . | General/breast surgeons engagement . | Eligible cosmetic physicians . | Participating cosmetic physicians . | Cosmetic physicians engagement . |
|---|---|---|---|---|---|---|---|---|---|
| NSW | 100 | 91 | 91% | 64 | 57 | 89% | 24 | 20 | 83% |
| VIC | 108 | 101 | 94% | 39 | 25 | 64% | 8 | 8 | 100% |
| QLD | 71 | 67 | 94% | 47 | 39 | 83% | 16 | 13 | 81% |
| WA | 41 | 37 | 90% | 19 | 13 | 68% | 4 | 4 | 100% |
| SA | 31 | 29 | 94% | 13 | 10 | 77% | 2 | 2 | 100% |
| TAS | 12 | 12 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| ACT | 3 | 3 | 100% | 4 | 4 | 100% | 0 | 0 | 0% |
| NT | 2 | 2 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| Total | 368 | 342 | 93% | 192 | 154 | 80% | 54 | 47 | 87% |
| State . | Eligible plastic surgeons . | Participating plastic surgeons . | Plastic surgeons engagement . | Eligible general/breast surgeons . | Participating general/breast surgeons . | General/breast surgeons engagement . | Eligible cosmetic physicians . | Participating cosmetic physicians . | Cosmetic physicians engagement . |
|---|---|---|---|---|---|---|---|---|---|
| NSW | 100 | 91 | 91% | 64 | 57 | 89% | 24 | 20 | 83% |
| VIC | 108 | 101 | 94% | 39 | 25 | 64% | 8 | 8 | 100% |
| QLD | 71 | 67 | 94% | 47 | 39 | 83% | 16 | 13 | 81% |
| WA | 41 | 37 | 90% | 19 | 13 | 68% | 4 | 4 | 100% |
| SA | 31 | 29 | 94% | 13 | 10 | 77% | 2 | 2 | 100% |
| TAS | 12 | 12 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| ACT | 3 | 3 | 100% | 4 | 4 | 100% | 0 | 0 | 0% |
| NT | 2 | 2 | 100% | 3 | 3 | 100% | 0 | 0 | 0% |
| Total | 368 | 342 | 93% | 192 | 154 | 80% | 54 | 47 | 87% |
Note: As of December 31, 2020, 88% of total eligible physicians were participating in the ABDR. Reproduced with permission from the ABDR. ABDR, Australian Breast Device Registry.
The most glaring omission of representation is that of West Australian public hospitals (Table 3), which are presently prevented from participating by state legislation.51 Indeed, the lack of an Australian ethics approval process with nationwide recognition is a concurrent problem, alongside state legislation that hinders the efficient implementation of clinical registries such as the ABDR, in contrast to countries such as the Netherlands and Sweden.47,52
Overall Capture Rate
Based on the information above supporting a low opt-out rate of 1%, and a percentage capture of eligible site and surgeon data of 88%, the data we have for our patient population from the ABDR represents an estimated capture of 87% of true nationwide practice.
Hypothetically, the most reliable, encompassing method to obtain nationwide data is to obtain product numbers from all breast device manufacturers, because this would include all patients outside of the ABDR's purview.16 However, the raw data would lack intraoperative context and data, and such a study would require retrospective collection of nationwide operative data, an untenable undertaking with process and recall biases of its own.
Data Integrity, Strengths, and Weaknesses
Data from the ABDR are prospectively collected at the time of surgery, avoiding recall bias. The national breadth of data collection also minimizes any center-specific biases. It is a possible weakness that reporting bias or mistakes can be introduced when surgeons delegate the completion of the DCF to a registrar (resident) or surgical assistant. However, just as that supposition is anecdotal, the authors’ combined practice across 5 states leads us to note that, at least in the plastic surgery physician group, data submission to the ABDR is ingrained in the practice of our peers and colleagues. In addition, the “tick-and-stick” approach of the ABDR DCF should minimize such mistakes.
Betadine Concentration and Uniformity of Free-Text Descriptors
A correctable weakness of our data was the lack of uniformity in the free-text descriptions and nomenclature of antiseptic rinse choices, for example, inconsistent nomenclature such as “Betadine triple,” “Adam's [sic] solution” (and variants), and nonstandard abbreviations (“B/C/G”, etc). These were standardized and categorized across the vast amount of data. A related inherent weakness is the lack of detail of specific reagents utilized, including the concentration of Betadine. The exact dilutions or concentration of reagents and the constituents of eponymous antiseptic combinations were often omitted. This prevented the reporting of minor trends. When undiluted betadine (10% PVP-I) was employed, the surgeon would report “Betadine,” “PVPI,” etc. The lack of detail about concentration or constitution of antiseptic rinse arose whenever the surgeon stated “Betadine and saline,” “dilute Betadine,” “Adams’ solution,” “Betadine triple antibiotic,” etc, as their irrigation of choice without further details. In the subsequent study, these latter categories are being excluded completely.
Contact Time of Irrigation Solution (and Other Surgical Mea Culpas)
It is a general critique of any large database that finer detail will often be lacking, including that of contact time of irrigation. Adams and Calobrace et al have noted that it takes less than 30 seconds to fully irrigate both breast pockets, and that antibiotic irrigation remains present in the implant pocket for at least 18 hours.25,26 It is reasonable to expect that the self-reported Betadine pocket irrigation surgeon cohort would have experienced this.
We recognize that with any multicenter case-control study a potential list of missteps or practice inconsistencies may extend beyond this due to human factors. There are several established ways to address this: a randomized controlled trial; optimizing, improving, and standardizing collection of data; and analysis of the data recognizing that these factors may overestimate or underestimate the effect of the intervention.
RCT: Following the pivotal RCTs conducted by Burkhardt and colleagues, there has been a relative lack of RCTs addressing this specific question. Rightly so, because the evidence base for antibiotic irrigation constitutes a strong argument that conducting further RCTs with a “no irrigation” control group is ethically unacceptable.24
Optimizing collection of data: In collaboration with the ABDR, we will suggest possible updates to the DCF. We also recognize that a significant amount of work has already been invested by our colleagues in the ABDR and the International Collaboration of Breast Registry Activities (ICOBRA) global consortium in working out common data elements and definitions for breast implant registries that take multiple elements into consideration, with global patient safety at the forefront.53 We continue to appreciate the data provided by respondents, even as the ABDR ensures that the data collection forms do not become onerous to the point of encouraging “omissions” in reporting intraoperative detail.
Analysis: As mentioned above, Adams and Calobrace et al note that it takes less than 30 seconds to fully irrigate both breast pockets, and we note this in our practice as well. The esteemed authors also noted that antibiotic irrigation remains present in the implant pocket for at least 18 hours.25,26 It is reasonable to expect that the surgeon cohort with Betadine pocket irrigation would experience this as well.
In our next study, analysis of our data (as is) will evaluate intraoperative interventions because they are actually provided by physicians at a national level and would be representative of real-world outcomes. The comprehensive record of other intraoperative factors will also allow multivariate analysis.
If we assume suboptimal implementation of proper Betadine pocket irrigation by broad numbers of physicians, we could expect an underestimation of the beneficial effects of Betadine pocket irrigation in analysis. However, the corollary to this is that any protective effect seen in our eventual statistical analysis of the upcoming study will add to the clinical significance of any such positive finding.
CONCLUSIONS
This study notes that a simple majority (56.7%) of Australian practitioners utilize previously described antiseptic pocket irrigation solutions that align with the 14-point plan.7
As described in our introduction, ethical and practical considerations limit the contemporary evidence base to studies of up to NHMRC level III-2 evidence.44 Our present findings will allow us to proceed with the largest retrospective cohort analysis of prospectively collected “real-world” data (data collection ongoing), which we hope will provide NHMRC level III-2 evidence addressing the question of whether PVP-I pocket irrigation improves clinical outcomes, and strengthen this evidence base.44
In collaboration with the ABDR, we will suggest DCF amendments for pocket irrigation based on the contemporary trends in our results, pave the way for a prospective cohort study (NHMRC level II), and work toward greater patient safety by advocating for the ABDR in current regions of reporting deficit.
Acknowledgments
All data were courtesy of the Australian Breast Device Registry (ABDR). The authors would like to recognize the assistance of Susannah Ahern and the steering committee and staff of the ABDR, in particular, Pragya Gartoulla, ABDR research manager, and Judy Hankin, research officer, as well as Rodney Cooter for his advice and insights on the ABDR and the consultants of the WA State Acute Burns Unit, especially Dr Anna Goodwin-Walters and Dr Sandeep B, for reviewing our previous presentation of these data at the national congress.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Integrated Specialist Healthcare Education and Research Foundation Safer Breast Implants. ISHCERF. Published 2022. Accessed 01 June, 2022. https://saferbreastimplants.org/doctors/your-pledge/
Author notes
Dr Leow is a plastic and reconstructive surgery service registrar, Department of Plastic and Reconstructive Surgery, Fiona Stanley Hospital, Perth, Australia.
Dr Knight is a consultant plastic, reconstructive, and aesthetic surgeon, Department of Plastic and Reconstructive Surgery, Wollongong Hospital, Wollongong, Australia.



