-
PDF
- Split View
-
Views
-
Cite
Cite
Amanda N Awad, Adee J Heiman, Ashit Patel, Implants and Breast Pocket Irrigation: Outcomes of Antibiotic, Antiseptic, and Saline Irrigation, Aesthetic Surgery Journal, Volume 42, Issue 2, February 2022, Pages NP102–NP111, https://doi.org/10.1093/asj/sjab181
Close - Share Icon Share
Abstract
Breast implant–associated infection and capsular contracture are challenging complications that can result in poor outcomes following implant-based breast surgery. Antimicrobial irrigation of the breast pocket or implant is a widely accepted strategy to prevent these complications, but the literature lacks an evidence-based consensus on the optimal irrigation solution.
The objective of this systematic review was to compare clinical outcomes, specifically capsular contracture, infection, and reoperation rates, associated with the use of antibiotic, antiseptic, and saline irrigation.
A systematic review was performed in March 2020 based on the following search terms: “breast implant,” “irrigation,” “antibiotic,” “bacitracin,” “antiseptic,” “povidone iodine,” “betadine,” “low concentration chlorhexidine,” and “hypochlorous acid.” Capsular contracture, infection, and reoperation rates were compared by analysis of forest plots.
Out of the 104 articles screened, 14 met the inclusion criteria. There was no significant difference in capsular contracture rates between antibiotic and povidone-iodine irrigation, although the data comparing these 2 groups were limited and confounded by the concurrent use of steroids. Antibiotic irrigation showed a significantly lower rate of capsular contracture compared with saline irrigation and a lower rate of capsular contracture and reoperation compared with no irrigation at all. Povidone-iodine was associated with lower rates of capsular contracture and reoperation compared with saline irrigation but there were no data on infection rates specific to povidone-iodine irrigation.
Our study supports the use of antibiotics or povidone-iodine for breast implant irrigation. Further research is required to better determine which of these 2 irrigation types is superior.

Implant-based reconstructive and aesthetic breast surgeries are among the most common procedures performed by plastic surgeons worldwide. Implant-based reconstruction represents the most prevalent form of breast reconstruction in the United States, and breast augmentation remains the most performed aesthetic operative procedure.1 The increasing use of implantable devices in breast surgeries emphasizes the need for evidence-based approaches when performing all surgeries of this type to optimize patient outcomes.2 Implant-associated infections and capsular contracture are 2 challenging complications that can result in poor outcomes, including patient discomfort, poor aesthetics, premature explantation, and reoperation.3 Prosthesis-based surgeries are at a higher risk of infection because of the incorporation of a foreign body. Additionally, there is increasing evidence supporting the importance of infections as a cause of poor outcomes in plastic surgery.4,5
Capsular contracture is frequently cited as the most common reason for reoperation following implant placement, in addition to implant rupture and malposition, leading to an increased morbidity for patients.6-8 Although the etiology of capsular contracture has not been fully elucidated, it is likely a multifactorial process and is thought to serve as a surrogate for subclinical infection, an immunologic response, and chronic inflammation.9 There is a well-established correlation between capsular contracture and bacterial infection.6 Nonetheless, capsular contracture remains a feared complication in both reconstructive and cosmetic breast surgery, for both patients and their surgeons.
In an effort to reduce these complications, breast pocket and implant antimicrobial irrigation before implant placement is a widely accepted strategy that has been advocated for to prevent infection and capsular contracture. First described by Burkhardt, pocket irrigation with povidone-iodine was used to reduce the level of bacterial contact with implants prior to their insertion to minimize the risks of contracture and periprocedural infections.4,10,11 The initial techniques and antimicrobial solutions utilized by Burkhardt have since evolved. In 2000, the FDA restricted the use of povidone-iodine for pocket irrigation, which prompted a shift towards the use of alternative antimicrobial solutions.12 In 2017, however, povidone-iodine was reapproved by the FDA and its use reemerged, further contributing to the heterogeneity of antimicrobial choice.13
Breast implant and pocket irrigation before implant placement is a widely accepted practice, supported by over 21 studies,14 but the current literature lacks an evidence-based consensus on the optimal irrigant solution. Results of a survey completed by 2488 American Society of Plastic Surgery members to assess pocket irrigation technique during implant-based reconstructions reported over 30 distinct irrigation solutions, including normal saline, various triple- and single-antibiotic solutions, povidone-iodine at varying concentrations, and alternative antiseptics such as chlorhexidine and hypochlorous acid–containing products.15 Numerous studies have compared the effects of various topical antibiotics, povidone-iodine, and saline irrigation on breast implant complications, but the literature lacks a consensus on an appropriate standardized irrigant. Prior meta-analyses have attempted to evaluate the efficacy of the various irrigation techniques and solutions; however, these studies have been limited in scope, focused primarily on capsular contracture, and did not draw any conclusions between the superiority of antibiotic irrigation vs povidone-iodine.9,16,17 The purpose of this systematic review was to more broadly evaluate the literature regarding antibiotic, antiseptic, and saline irrigation and their relation to a broader range of complications to determine which is associated with a decrease in capsular contracture, infections, and reoperation rates.
METHODS
Literature Search and Selection Criteria
A systematic review of the literature was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 A PubMed (US National Library of Medicine, Bethesda, MD) database search was performed in March 2020 based on 2 separate search queries:
(1) an antiseptic search, for the terms: “breast implant” AND “irrigation” AND (“antiseptic” OR “povidone iodine” OR “betadine” OR “low concentration chlorhexidine” OR “hypochlorous acid”)
(2) an antibiotic search, for the terms: “breast implant” AND “irrigation” AND (“antibiotic” OR “bacitracin”).
Two reviewers (A.A. and A.H.) independently screened articles in 2 steps: (1) titles and abstracts and (2) full-text articles. Inclusion criteria included studies that reported complication rates of implant-based breast surgery (both aesthetic and reconstructive) after the use of antibiotic and/or antiseptic breast pocket irrigation. Exclusion criteria included review articles, systematic reviews, in vitro investigations, studies without full text availability, surveys, case reports, technique descriptions without data, publications irrelevant to breast pocket irrigation, studies that did not specify irrigation type, publications that did not discuss clinical outcomes, studies with pre-existing implant infection, and non–English language publications. Where disagreement occurred regarding inclusion, consensus was reached after discussion with the senior author (A.P.)
Assessment of Methodologic Quality
The methodologic quality of the included studies was assessed by A.A. The methodologic index for nonrandomized studies (MINORS) scale was used to assess observational nonrandomized studies.19 The 12 items of this scale are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate) with an ideal score of 16 for noncomparative studies and 24 for comparative studies. Ideal scores indicate a high-quality study. The methodologic quality of the included randomized controlled trails was assessed with the Jadad scale, which categorizes the quality of reporting as poor (score <3) or robust (≥3).20
Data Extraction
The following data were extracted from each article by 2 reviewers (A.A. and A.H.) via a Microsoft Excel (Microsoft Corporation, Redmond, WA) spreadsheet and used for comparison: publication details (author, year of publication, study type), number of patients, number of breasts, irrigation solution, procedure type (cosmetic or reconstructive), tissue expander/implant type, location of implant placement, use of a dermal matrix, and complications (capsular contracture, infections, and unplanned reoperations, defined as premature tissue expander removal, implant explantation, reoperation for capsular contracture, or reoperations for any unspecified complication). Capsular contracture was defined as Baker grade III or IV contractures, with the exception of 1 paper that grouped grade II to IV contractures together.21
Capsular contracture rates were compared with forest plot analysis. Microsoft Excel and Select Statistical Service’s odds ratio (OR) and CI calculator (Select Statistical Services Ltd, Exeter, UK) were used for statistical analysis. Statistical significance was defined as α < 0.05.
RESULTS
A total of 104 publications were identified based on our database search criteria; after duplicate article removal, 77 articles remained. Two reviewers (A.A. and A.H.) then independently extracted the data in 2 steps: (1) 77 records were screened based on titles and abstracts; (2) after exclusion of 54 articles, the remaining 23 full-text articles were assessed for eligibility. Ultimately, 14 were included for qualitative and quantitative analysis in this study (Figure 1).10,12,21-32
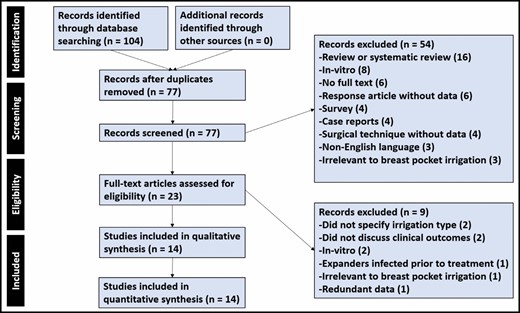
Study Characteristics
The 14 studies that met inclusion criteria comprised 8 retrospective cohort studies,21-28 3 randomized control trials,10,29,30 2 prospective cohort studies,12,31 and 1 retrospective chart review.32 Ten studies focused on aesthetic augmentations or mastopexy-augmentations,10,21-24,26,29-32 3 focused on breast reconstruction,25,27,28 and 1 paper included patients who underwent both aesthetic and reconstructive implant/tissue expander placement.12 Four studies focused on textured implants,12,22,24,28 2 focused on smooth implants,10,21 5 studies used both textured and smooth implants,27,19-32 and 3 studies did not specify. Some type of antibiotic irrigation was used in 11 studies.10,12,22-28,31,32 Four studies included patients who underwent pocket irrigation with antiseptic solution without antibiotics.10,21,23,29-31 Six studies included patients who underwent pocket irrigation with saline alone.10,21,22,26,29,30 Two studies included patients who underwent no form of pocket irrigation at all.23,24 One study included patients who underwent antibiotic irrigation, povidone-iodine irrigation, or neither. For the purposes of data analysis, it was assumed that antibiotic irrigation and povidone-iodine were mutually exclusive although this was never directly stated in the study31 (Table 1).
| Author . | Year . | Study type . | Cosmetic vs reconstructive . | Smooth vs textured . | Irrigation types . | Number of patients . | Jadad/ MINORS score . |
|---|---|---|---|---|---|---|---|
| Adams et al12 | 2006 | Prospective cohort | Both | Textured | Antibiotic with or without antiseptic | 250 | 13 (M) |
| Blount et al23 | 2013 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, antiseptic, none | 856 | 14 (M) |
| Burkhardt and Demas29 | 1994 | Randomized control trial | Cosmetic | Smooth | Antiseptic, saline | 45 | 4 (J) |
| Burkhardt and Eades30 | 1995 | Randomized control trial | Cosmetic | Both | Antiseptic, saline | 52 | 4 (J) |
| Burkhardt, et al10 | 1986 | Randomized control trial | Cosmetic | Both | Antibiotic, antiseptic, saline | 92 | 2 (J) |
| Calobrace et al31 | 2018 | Prospective cohort | Cosmetic | Both | Antibiotic, antiseptic, neither | 2565 | 8 (M) |
| Drinane et al26 | 2016 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, saline | 55 | 18 (M) |
| Giordano et al24 | 2013 | Retrospective cohort | Cosmetic | Textured | Antibiotic with antiseptic, none | 330 | 16 (M) |
| Hunsicker et al27 | 2017 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 276 | 16 (M) |
| Kenna et al28 | 2018 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 74 | 15 (M) |
| Kim32 | 2015 | Retrospective chart review | Cosmetic | Both | Antibiotic | 162 | 10 (M) |
| Pfeiffer et al22 | 2009 | Retrospective cohort | Cosmetic | Textured | Antibiotic, saline | 414 | 14 (M) |
| Tutela et al25 | 2015 | Retrospective cohort | Reconstructive | Not Reported | Antibiotic | 79 | 19 (M) |
| Wiener21 | 2007 | Retrospective cohort | Cosmetic | smooth | Antiseptic, saline | 1244 | 12 (M) |
| Author . | Year . | Study type . | Cosmetic vs reconstructive . | Smooth vs textured . | Irrigation types . | Number of patients . | Jadad/ MINORS score . |
|---|---|---|---|---|---|---|---|
| Adams et al12 | 2006 | Prospective cohort | Both | Textured | Antibiotic with or without antiseptic | 250 | 13 (M) |
| Blount et al23 | 2013 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, antiseptic, none | 856 | 14 (M) |
| Burkhardt and Demas29 | 1994 | Randomized control trial | Cosmetic | Smooth | Antiseptic, saline | 45 | 4 (J) |
| Burkhardt and Eades30 | 1995 | Randomized control trial | Cosmetic | Both | Antiseptic, saline | 52 | 4 (J) |
| Burkhardt, et al10 | 1986 | Randomized control trial | Cosmetic | Both | Antibiotic, antiseptic, saline | 92 | 2 (J) |
| Calobrace et al31 | 2018 | Prospective cohort | Cosmetic | Both | Antibiotic, antiseptic, neither | 2565 | 8 (M) |
| Drinane et al26 | 2016 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, saline | 55 | 18 (M) |
| Giordano et al24 | 2013 | Retrospective cohort | Cosmetic | Textured | Antibiotic with antiseptic, none | 330 | 16 (M) |
| Hunsicker et al27 | 2017 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 276 | 16 (M) |
| Kenna et al28 | 2018 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 74 | 15 (M) |
| Kim32 | 2015 | Retrospective chart review | Cosmetic | Both | Antibiotic | 162 | 10 (M) |
| Pfeiffer et al22 | 2009 | Retrospective cohort | Cosmetic | Textured | Antibiotic, saline | 414 | 14 (M) |
| Tutela et al25 | 2015 | Retrospective cohort | Reconstructive | Not Reported | Antibiotic | 79 | 19 (M) |
| Wiener21 | 2007 | Retrospective cohort | Cosmetic | smooth | Antiseptic, saline | 1244 | 12 (M) |
| Author . | Year . | Study type . | Cosmetic vs reconstructive . | Smooth vs textured . | Irrigation types . | Number of patients . | Jadad/ MINORS score . |
|---|---|---|---|---|---|---|---|
| Adams et al12 | 2006 | Prospective cohort | Both | Textured | Antibiotic with or without antiseptic | 250 | 13 (M) |
| Blount et al23 | 2013 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, antiseptic, none | 856 | 14 (M) |
| Burkhardt and Demas29 | 1994 | Randomized control trial | Cosmetic | Smooth | Antiseptic, saline | 45 | 4 (J) |
| Burkhardt and Eades30 | 1995 | Randomized control trial | Cosmetic | Both | Antiseptic, saline | 52 | 4 (J) |
| Burkhardt, et al10 | 1986 | Randomized control trial | Cosmetic | Both | Antibiotic, antiseptic, saline | 92 | 2 (J) |
| Calobrace et al31 | 2018 | Prospective cohort | Cosmetic | Both | Antibiotic, antiseptic, neither | 2565 | 8 (M) |
| Drinane et al26 | 2016 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, saline | 55 | 18 (M) |
| Giordano et al24 | 2013 | Retrospective cohort | Cosmetic | Textured | Antibiotic with antiseptic, none | 330 | 16 (M) |
| Hunsicker et al27 | 2017 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 276 | 16 (M) |
| Kenna et al28 | 2018 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 74 | 15 (M) |
| Kim32 | 2015 | Retrospective chart review | Cosmetic | Both | Antibiotic | 162 | 10 (M) |
| Pfeiffer et al22 | 2009 | Retrospective cohort | Cosmetic | Textured | Antibiotic, saline | 414 | 14 (M) |
| Tutela et al25 | 2015 | Retrospective cohort | Reconstructive | Not Reported | Antibiotic | 79 | 19 (M) |
| Wiener21 | 2007 | Retrospective cohort | Cosmetic | smooth | Antiseptic, saline | 1244 | 12 (M) |
| Author . | Year . | Study type . | Cosmetic vs reconstructive . | Smooth vs textured . | Irrigation types . | Number of patients . | Jadad/ MINORS score . |
|---|---|---|---|---|---|---|---|
| Adams et al12 | 2006 | Prospective cohort | Both | Textured | Antibiotic with or without antiseptic | 250 | 13 (M) |
| Blount et al23 | 2013 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, antiseptic, none | 856 | 14 (M) |
| Burkhardt and Demas29 | 1994 | Randomized control trial | Cosmetic | Smooth | Antiseptic, saline | 45 | 4 (J) |
| Burkhardt and Eades30 | 1995 | Randomized control trial | Cosmetic | Both | Antiseptic, saline | 52 | 4 (J) |
| Burkhardt, et al10 | 1986 | Randomized control trial | Cosmetic | Both | Antibiotic, antiseptic, saline | 92 | 2 (J) |
| Calobrace et al31 | 2018 | Prospective cohort | Cosmetic | Both | Antibiotic, antiseptic, neither | 2565 | 8 (M) |
| Drinane et al26 | 2016 | Retrospective cohort | Cosmetic | Not reported | Antibiotic, saline | 55 | 18 (M) |
| Giordano et al24 | 2013 | Retrospective cohort | Cosmetic | Textured | Antibiotic with antiseptic, none | 330 | 16 (M) |
| Hunsicker et al27 | 2017 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 276 | 16 (M) |
| Kenna et al28 | 2018 | Retrospective cohort | Reconstructive | Textured | Antibiotic | 74 | 15 (M) |
| Kim32 | 2015 | Retrospective chart review | Cosmetic | Both | Antibiotic | 162 | 10 (M) |
| Pfeiffer et al22 | 2009 | Retrospective cohort | Cosmetic | Textured | Antibiotic, saline | 414 | 14 (M) |
| Tutela et al25 | 2015 | Retrospective cohort | Reconstructive | Not Reported | Antibiotic | 79 | 19 (M) |
| Wiener21 | 2007 | Retrospective cohort | Cosmetic | smooth | Antiseptic, saline | 1244 | 12 (M) |
The majority of studies used triple-antibiotic solutions (7 studies). Bacitracin and gentamicin were the most commonly used agents (8 studies each), followed by cefazolin (7 studies). Specialized antibiotic irrigation regimens included 2 regimens involving antibiotics combined with steroids,10,31 2 regimens involving postoperative continuous antibiotic irrigation,25,27 and 1 regimen of antibiotic irrigation followed by antibiotic bead implantation.28 Povidone-iodine, with concentrations ranging from 4% to 10%, was the only antiseptic agent used in the included studies (Table 2).
| Author paper . | Irrigation types . | Antibiotic agent . | Antiseptic concentration . | Capsular contracture rate . | Infection rate . | Reoperation rate . |
|---|---|---|---|---|---|---|
| Adams et al12 | Antibiotic with or without antiseptic | Cefazolin, gentamicin, with or without bacitracin | 10% povidone-iodine | 4% | 1% | 6% |
| Blount et al23 | Antibiotic, povidone-iodine, no irrigation | Bacitracin with or without cefazolin and gentamicin | 5% povidone-iodine | 3% | 1% | 18% |
| 0% (antibiotic), 4% (no antibiotics) | 8% (antibiotic), 23% (no antibiotic) | |||||
| Burkhardt and Demas29 | Povidone-iodine, saline | NA | 5% povidone-iodine | 21% | 7% | 2% |
| 18% (povidone-iodine), 24% (saline) | 2% (povidone-iodine), 2% (saline) | |||||
| Burkhardt and Eades30 | Povidone-iodine, saline | NA | 5% povidone-iodine | 18% | 6% | 3% |
| 10% (povidone-iodine), 27% (saline) | ||||||
| Burkhardt et al10 | Antibiotic steroid foam,a povidone-iodine, saline | Bacitracin, oxacillin | 5% povidone-iodine | 23% | 7% | 9% |
| 14% (antibiotic), 18% (povidone-iodine), 33% (saline) | ||||||
| Calobrace et al31 | Antibiotic (with or without steroids),a povidone-iodine | Bacitracin, cefazolin, gentamicin | Not reported | 7% | Not reported | Not reported |
| 8% (antibiotic), 5% (povidone-iodine) | ||||||
| Drinane et al26 | Antibiotic, saline | Bacitracin, cefazolin, gentamicin | NA | 4% 4% (antibiotic), 4% (saline) | Not reported | Not reported |
| Giordano et al24 | Antibiotic with antiseptic, no irrigation | Cefuroxime, gentamicin | 4% povidone-iodine | 3% | 2% | 4% |
| 1% (antibiotic with antiseptic), 6% (no irrigation) | 1% (antibiotic with antiseptic), 2% (no irrigation) | 2% (antibiotic with antiseptic), 7% (no irrigation) | ||||
| Hunsicker et al27 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, gentamicin, vancomycin | NA | Not reported | 7% | Not reported |
| Kenna et al28 | Antibiotic, antibiotic beadsa | Bacitracin, cefazoline, gentamicin | NA | Not reported | 6% | 6% |
| Kim32 | Antibiotic | Cefazolin | NA | 1% | 0% | 2% |
| Pfeiffer et al22 | Antibiotic, saline | Cephalothin | NA | 7% | 10% | 2% |
| 6% (antibiotic), 8% (saline) | 7% (antibiotic), 13% (saline) | 1% (antibiotic), 3% (saline) | ||||
| Tutela et al25 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, cefazolin, gentamicin | NA | 15% | Not reported | 13% |
| Wiener21 | Povidone-iodine, saline | NA | 5% povidone-iodine | 2% | 0% | 1% |
| 1% (povidone-iodine), 4% (saline) | 1% (povidone-iodine), 2% (saline) |
| Author paper . | Irrigation types . | Antibiotic agent . | Antiseptic concentration . | Capsular contracture rate . | Infection rate . | Reoperation rate . |
|---|---|---|---|---|---|---|
| Adams et al12 | Antibiotic with or without antiseptic | Cefazolin, gentamicin, with or without bacitracin | 10% povidone-iodine | 4% | 1% | 6% |
| Blount et al23 | Antibiotic, povidone-iodine, no irrigation | Bacitracin with or without cefazolin and gentamicin | 5% povidone-iodine | 3% | 1% | 18% |
| 0% (antibiotic), 4% (no antibiotics) | 8% (antibiotic), 23% (no antibiotic) | |||||
| Burkhardt and Demas29 | Povidone-iodine, saline | NA | 5% povidone-iodine | 21% | 7% | 2% |
| 18% (povidone-iodine), 24% (saline) | 2% (povidone-iodine), 2% (saline) | |||||
| Burkhardt and Eades30 | Povidone-iodine, saline | NA | 5% povidone-iodine | 18% | 6% | 3% |
| 10% (povidone-iodine), 27% (saline) | ||||||
| Burkhardt et al10 | Antibiotic steroid foam,a povidone-iodine, saline | Bacitracin, oxacillin | 5% povidone-iodine | 23% | 7% | 9% |
| 14% (antibiotic), 18% (povidone-iodine), 33% (saline) | ||||||
| Calobrace et al31 | Antibiotic (with or without steroids),a povidone-iodine | Bacitracin, cefazolin, gentamicin | Not reported | 7% | Not reported | Not reported |
| 8% (antibiotic), 5% (povidone-iodine) | ||||||
| Drinane et al26 | Antibiotic, saline | Bacitracin, cefazolin, gentamicin | NA | 4% 4% (antibiotic), 4% (saline) | Not reported | Not reported |
| Giordano et al24 | Antibiotic with antiseptic, no irrigation | Cefuroxime, gentamicin | 4% povidone-iodine | 3% | 2% | 4% |
| 1% (antibiotic with antiseptic), 6% (no irrigation) | 1% (antibiotic with antiseptic), 2% (no irrigation) | 2% (antibiotic with antiseptic), 7% (no irrigation) | ||||
| Hunsicker et al27 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, gentamicin, vancomycin | NA | Not reported | 7% | Not reported |
| Kenna et al28 | Antibiotic, antibiotic beadsa | Bacitracin, cefazoline, gentamicin | NA | Not reported | 6% | 6% |
| Kim32 | Antibiotic | Cefazolin | NA | 1% | 0% | 2% |
| Pfeiffer et al22 | Antibiotic, saline | Cephalothin | NA | 7% | 10% | 2% |
| 6% (antibiotic), 8% (saline) | 7% (antibiotic), 13% (saline) | 1% (antibiotic), 3% (saline) | ||||
| Tutela et al25 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, cefazolin, gentamicin | NA | 15% | Not reported | 13% |
| Wiener21 | Povidone-iodine, saline | NA | 5% povidone-iodine | 2% | 0% | 1% |
| 1% (povidone-iodine), 4% (saline) | 1% (povidone-iodine), 2% (saline) |
NA, not available. aSpecialized antibiotic regimens.
| Author paper . | Irrigation types . | Antibiotic agent . | Antiseptic concentration . | Capsular contracture rate . | Infection rate . | Reoperation rate . |
|---|---|---|---|---|---|---|
| Adams et al12 | Antibiotic with or without antiseptic | Cefazolin, gentamicin, with or without bacitracin | 10% povidone-iodine | 4% | 1% | 6% |
| Blount et al23 | Antibiotic, povidone-iodine, no irrigation | Bacitracin with or without cefazolin and gentamicin | 5% povidone-iodine | 3% | 1% | 18% |
| 0% (antibiotic), 4% (no antibiotics) | 8% (antibiotic), 23% (no antibiotic) | |||||
| Burkhardt and Demas29 | Povidone-iodine, saline | NA | 5% povidone-iodine | 21% | 7% | 2% |
| 18% (povidone-iodine), 24% (saline) | 2% (povidone-iodine), 2% (saline) | |||||
| Burkhardt and Eades30 | Povidone-iodine, saline | NA | 5% povidone-iodine | 18% | 6% | 3% |
| 10% (povidone-iodine), 27% (saline) | ||||||
| Burkhardt et al10 | Antibiotic steroid foam,a povidone-iodine, saline | Bacitracin, oxacillin | 5% povidone-iodine | 23% | 7% | 9% |
| 14% (antibiotic), 18% (povidone-iodine), 33% (saline) | ||||||
| Calobrace et al31 | Antibiotic (with or without steroids),a povidone-iodine | Bacitracin, cefazolin, gentamicin | Not reported | 7% | Not reported | Not reported |
| 8% (antibiotic), 5% (povidone-iodine) | ||||||
| Drinane et al26 | Antibiotic, saline | Bacitracin, cefazolin, gentamicin | NA | 4% 4% (antibiotic), 4% (saline) | Not reported | Not reported |
| Giordano et al24 | Antibiotic with antiseptic, no irrigation | Cefuroxime, gentamicin | 4% povidone-iodine | 3% | 2% | 4% |
| 1% (antibiotic with antiseptic), 6% (no irrigation) | 1% (antibiotic with antiseptic), 2% (no irrigation) | 2% (antibiotic with antiseptic), 7% (no irrigation) | ||||
| Hunsicker et al27 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, gentamicin, vancomycin | NA | Not reported | 7% | Not reported |
| Kenna et al28 | Antibiotic, antibiotic beadsa | Bacitracin, cefazoline, gentamicin | NA | Not reported | 6% | 6% |
| Kim32 | Antibiotic | Cefazolin | NA | 1% | 0% | 2% |
| Pfeiffer et al22 | Antibiotic, saline | Cephalothin | NA | 7% | 10% | 2% |
| 6% (antibiotic), 8% (saline) | 7% (antibiotic), 13% (saline) | 1% (antibiotic), 3% (saline) | ||||
| Tutela et al25 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, cefazolin, gentamicin | NA | 15% | Not reported | 13% |
| Wiener21 | Povidone-iodine, saline | NA | 5% povidone-iodine | 2% | 0% | 1% |
| 1% (povidone-iodine), 4% (saline) | 1% (povidone-iodine), 2% (saline) |
| Author paper . | Irrigation types . | Antibiotic agent . | Antiseptic concentration . | Capsular contracture rate . | Infection rate . | Reoperation rate . |
|---|---|---|---|---|---|---|
| Adams et al12 | Antibiotic with or without antiseptic | Cefazolin, gentamicin, with or without bacitracin | 10% povidone-iodine | 4% | 1% | 6% |
| Blount et al23 | Antibiotic, povidone-iodine, no irrigation | Bacitracin with or without cefazolin and gentamicin | 5% povidone-iodine | 3% | 1% | 18% |
| 0% (antibiotic), 4% (no antibiotics) | 8% (antibiotic), 23% (no antibiotic) | |||||
| Burkhardt and Demas29 | Povidone-iodine, saline | NA | 5% povidone-iodine | 21% | 7% | 2% |
| 18% (povidone-iodine), 24% (saline) | 2% (povidone-iodine), 2% (saline) | |||||
| Burkhardt and Eades30 | Povidone-iodine, saline | NA | 5% povidone-iodine | 18% | 6% | 3% |
| 10% (povidone-iodine), 27% (saline) | ||||||
| Burkhardt et al10 | Antibiotic steroid foam,a povidone-iodine, saline | Bacitracin, oxacillin | 5% povidone-iodine | 23% | 7% | 9% |
| 14% (antibiotic), 18% (povidone-iodine), 33% (saline) | ||||||
| Calobrace et al31 | Antibiotic (with or without steroids),a povidone-iodine | Bacitracin, cefazolin, gentamicin | Not reported | 7% | Not reported | Not reported |
| 8% (antibiotic), 5% (povidone-iodine) | ||||||
| Drinane et al26 | Antibiotic, saline | Bacitracin, cefazolin, gentamicin | NA | 4% 4% (antibiotic), 4% (saline) | Not reported | Not reported |
| Giordano et al24 | Antibiotic with antiseptic, no irrigation | Cefuroxime, gentamicin | 4% povidone-iodine | 3% | 2% | 4% |
| 1% (antibiotic with antiseptic), 6% (no irrigation) | 1% (antibiotic with antiseptic), 2% (no irrigation) | 2% (antibiotic with antiseptic), 7% (no irrigation) | ||||
| Hunsicker et al27 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, gentamicin, vancomycin | NA | Not reported | 7% | Not reported |
| Kenna et al28 | Antibiotic, antibiotic beadsa | Bacitracin, cefazoline, gentamicin | NA | Not reported | 6% | 6% |
| Kim32 | Antibiotic | Cefazolin | NA | 1% | 0% | 2% |
| Pfeiffer et al22 | Antibiotic, saline | Cephalothin | NA | 7% | 10% | 2% |
| 6% (antibiotic), 8% (saline) | 7% (antibiotic), 13% (saline) | 1% (antibiotic), 3% (saline) | ||||
| Tutela et al25 | Antibiotic, continuous antibiotic irrigationa | Bacitracin, cefazolin, gentamicin | NA | 15% | Not reported | 13% |
| Wiener21 | Povidone-iodine, saline | NA | 5% povidone-iodine | 2% | 0% | 1% |
| 1% (povidone-iodine), 4% (saline) | 1% (povidone-iodine), 2% (saline) |
NA, not available. aSpecialized antibiotic regimens.
Assessment of Methodologic Quality
All 8 included retrospective studies, 2 prospective cohort studies, and 1 retrospective chart review were scored with the MINORS scale to determine their methodologic quality. The studies by Adams, Drinane, Hunsicker, and Tutela were determined to be high quality, with MINORS scores of ≥16. The remaining studies by Blount, Calobrace, Kenna, Kim, Pfeiffer, and Weiner were all low-quality studies with MINORS scores of <16. The most common reasons for the low quality included their retrospective data collections, lack of blinding, lack of appropriate sample size calculation, and failure to report patients lost to follow-up.
The 3 randomized controlled trials were assessing with the Jadad scale to determine their methodologic quality. The 2 studies by Burkhardt and Demas and Burkhardt and Eades were both determined to be of good quality, with scores of 4. The last study by Burkhardt et al was a poor-quality study, with a score of 2, mainly due to an inappropriate method of randomization.
Capsular Contracture
Capsular contracture was the most frequently discussed complication. Overall capsular contracture rates across all 14 studies ranged from 1% to 23%. Capsular contracture rates associated specifically with antibiotic irrigation ranged from 0% to 15% whereas rates associated with povidone-iodine irrigation ranged from 1% to 18%. For comparison, capsular contracture rates associated with saline irrigation ranged from 4% to 33%.
Calobrace et al showed a significantly higher rate of capsular contracture after antibiotic irrigation compared with povidone-iodine irrigation,31 whereas Burkhardt et al did not.10 Overall, there was no significant difference between the 2 (OR, 1.31; CI, 0.92-1.86). However, it is worth noting that steroid irrigation was used in some or all patients in both studies (Figure 2).
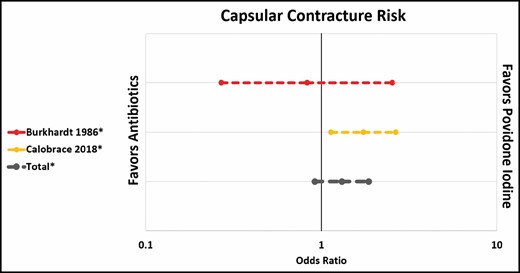
Capsular contracture risk: antibiotic irrigation vs povidone-iodine.
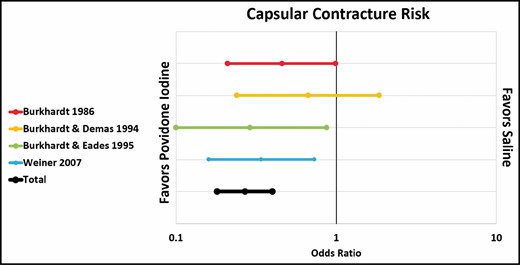
Pfeiffer et al and Drinane et al showed no significant difference in capsular contracture rate between standard antibiotic irrigation and saline irrigation.22,26 However, when 1 paper that combined antibiotics and steroids was included (Burkhardt et al),10 the overall rate of capsular contracture was significantly lower in the antibiotic group (OR, 0.46; CI, 0.26-0.82) (Figure 3). Both Giordano et al and Blount et al showed a significantly lower rate of capsular contracture after antibiotic irrigation compared with no irrigation at all (OR, 0.11; CI, 0.03-0.44), although it is worth noting that Giordano et al’s antibiotic solution also included povidone-iodine (Figure 4).23,24 Three out of 4 papers showed a significantly lower rate of contracture associated with povidone-iodine compared with saline. All 4 studies used 5% povidone-iodine solution.10,21,29,30 Overall, the difference was statistically significant (OR, 0.27; CI, 0.18-0.40) (Figure 5).
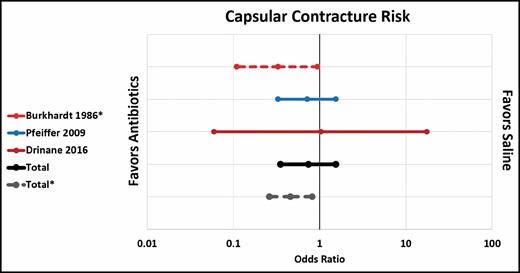
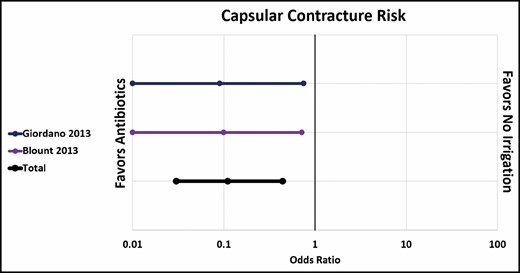
Capsular contracture risk: antibiotic irrigation vs no irrigation.
Infection
Infection rates across all studies ranged from 0% to 10%. The irrigation types associated with these infections was frequently not reported. One study (Pfeiffer et al) showed a significantly lower infection rate after antibiotic irrigation compared with saline irrigation (OR, 0.48; CI, 0.24-0.94).22 Meanwhile, another study (Giordano et al) failed to show a significant difference between antibiotic/povidone-iodine irrigation and no irrigation at all (OR, 0.66; CI, 0.11-4.02).24 However, it is important to note that Giordano et al’s irrigation group received a lower dose of perioperative intravenous antibiotics than the no-irrigation group. None of the included studies compared infection rates after povidone-iodine irrigation with any of the other irrigation types.
Kenna et al reported a statistically significant reduction in infection rates associated with implantation of antibiotic beads in addition to antibiotic irrigation compared with antibiotic irrigation alone (OR, 0.11; CI, 0.01-0.93).28 Two studies looked at the rate of infection associated with the use of continuous antibiotic irrigation. Hunsicker et al found a statistically significant decrease in infection rates compared with standard antibiotic irrigation alone, whereas Tutela et al did not.25,27 The difference was statistically significant overall (OR, 0.25; CI, 0.11-0.57).
Reoperation Rates
Reoperation rates across all 14 studies ranged from 1% to 18%. Reoperations included 44 cases of capsulectomy/capsulotomy/explantation for capsular contracture, 21 explantations for infection, 9 explantations for deflation or rupture, 5 explantations for seroma, 3 reoperations for hematoma, 2 explantations for extrusion, and 1 exploration for malposition. There were also 34 patients for whom the reason for reoperation was not specified.23,25
Pfeiffer et al showed no significant difference in reoperation rates between antibiotic and saline irrigation (OR, 1.7; CI, 0.07-1.70).22 However, both Blount et al and Giordano et al showed a significantly lower rate of reoperation after antibiotic irrigation compared with no irrigation at all (OR, 0.25; CI, 0.16-0.39).23,24
Weiner et al showed a significantly lower rate of reoperation after povidone-iodine irrigation compared with saline,21 whereas Burkhard and Demas did not.29 This difference was significant overall (OR, 0.30; CI, 0.10-0.87).
DISCUSSION
Breast pocket antimicrobial irrigation before implant placement is a widely accepted strategy among plastic surgeons to prevent capsular contracture and infection, 2 of the commonest complications following implant-based breast surgery.3,6-8 Despite the advanced understanding and evolution of best surgical practices for breast augmentation and reconstruction, these procedures are still associated with various postoperative complications that contribute significantly to unplanned reoperations. The increasing number of breast prostheses inserted annually emphasizes the need for a standardized protocol to prevent patient morbidity.2
Initially investigated by Burkhardt, povidone-iodine irrigation gained popularity as the antimicrobial irrigation of choice in reducing capsular contracture, specifically when used in augmentation mammoplasty. Of note, Burkhardt elected to use 5% povidone-iodine because it is “simple, inexpensive, and as effective as any other agent,” which certainly suggests that other suitable agents exist.10 Several years later, Adams et al studied serial dilutions of irrigation solutions to determine the most effective solution and concentration to maximize bacterial control of common breast implant organisms while minimizing the detrimental effects on wound healing. They determined that 10% povidone-iodine/gentamicin/cefazolin solution could be used for effective breast pocket irrigation.33 For many years thereafter, povidone-iodine was used as the “standard” breast pocket irrigant until 2000, when the FDA recommended against direct contact of povidone-iodine solution with implants due to concerns about the solutions causing implant deflation.12 Following these FDA restrictions, Adams et al tested various agents to find an effective alternative to the combination irrigation solution that they had previously demonstrated to be most effective. They observed that triple-antibiotic irrigation (bacitracin, cefazolin, and gentamicin) quantitatively reduced bacterial bioburden in vitro and was an effective alternative to povidone-iodine–containing breast irrigants, although it was not as universally effective against all the bacteria as the original povidone-iodine–containing solution. Similar to the mechanism of action described in the initial study, bacitracin attacks cell membranes directly to allow penetration of the 2 other antibiotics but was not as effective as povidone-iodine. Their clinical practice recommendations for the “post-betadine era” (an alternative to betadine-containing solutions) included 50,000 U of bacitracin, 1 g of cefazolin, 80 mg of gentamicin, and 500 mL of saline, which can be used to soak the implant and irrigate the pocket for 5 minutes prior to implant placement. Alternatively, it was suggested to use 10% betadine, cefazolin, and gentamicin in the breast pocket for 5 minutes, then clean this with sterile saline before implant placement, because the FDA policy specifically restricted direct contact of betadine with implants.11 In their subsequent prospective study, they concluded that irrigation with a triple-antibiotic solution (povidone-iodine, cefazolin, gentamicin; or bacitracin, cefazolin, and gentamicin) had proven efficacy in reducing capsular contracture in both vitro and in vivo clinical studies.12 They later developed a 14-point plan to minimize bacterial load at the time of surgery, which includes specific irrigation solutions and overall surgical technique that many surgeons still utilize.34
Although the povidone-iodine restriction was lifted by the FDA in 2017, multiple novel antibiotic and antiseptic solutions continue to be investigated and utilized without standardized, evidenced-based guidelines.13 Data have shown that breast implant irrigation is beneficial at reducing the bacterial load of an implant, but there is continuous debate regarding which irrigant solution is most effective at preventing implant-based complications without significant side effects. There is significant evidence that the outcome of implant-based procedures is dependent on the quantitative bacterial load at the time of closure, further emphasizing the importance of irrigation.14 With over 30 distinct irrigation solutions currently being utilized by plastic surgeons, there is a wide range of options and efficacies for irrigation, all with the common goal of reducing bacterial contamination around implants to reduce subsequent implant complications.15
It is important to note that the breast gland is not sterile regardless of the sterility of the implant procedure followed. The gland has been shown to harbor significant concentrations of endogenous bacteria.10,35 The addition of a foreign body has the potential to introduce contamination, leading to an increased risk of infection. Previous studies have demonstrated a strong association between bacteria and surgical complications including clinical infections, capsular contracture, hematoma formation, seroma formation, and implant rupture.36 Evidence has suggested that both subclinical infection and biofilm formation play a role in the development of capsular contractures.37 As such, finding and eliminating the source of contamination contributing to infection, and therefore capsular contracture development, may provide an ultimate solution for prevention.
The efficacy of povidone-iodine at reducing capsular contracture rates is well established in the literature. Our study clearly showed a lower rate of capsular contracture and reoperation rates associated with the use of povidone-iodine compared with saline. Concentration rates of povidone-iodine in these studies ranged from 4% to 10%. The optimal concentration of antiseptic required to be effective without causing cytotoxicity to fibroblasts and further tissue damage has yet to be determined. Additionally, many authors use povidone-iodine and betadine interchangeably, although their concentrations are different.38 Previous studies utilize various concentrations, solution exposure times, and solution volume, which contributes to the variety of solutions used and the lack of a standardized protocol. Lastly, several studies do not define their antiseptic concentrations or specific techniques utilized.
The efficacies of other less frequently used antiseptic solutions have also been analyzed in vitro, including chlorhexidine and hypochlorous acid. Culbertson et al reported that chlorhexidine gluconate (50% and 95%) and hypochlorous acid (95%) had equivalent bactericidal effects to povidone-iodine at various concentrations.39 Zhadan and Becker showed that chlorhexidine solution (0.05%) was effective at completely eliminating methicillin-resistant and methicillin-sensitive Staphylococcus aureus, Group A Streptococcus, and Pseudomonas aeruginosa after 15 minutes of irrigation, whereas triple-antibiotic solution and povidone-iodine required 30 minutes of irrigation.38 Meanwhile, Hu et al reported inferior activity of hypochlorous acid against S. aureus compared with 10% povidone-iodine.4 It is speculated that the presence of blood reduces the activity of hypochlorous acid, which would hypothetically make this particular agent ineffective in a true clinical scenario. However, we were unable to identify any clinical data involving the use of these alternative antiseptic solutions, so are unable to make recommendations regarding their use.
One of the major questions this study sought to address was to determine whether antiseptic or antibiotic irrigation was associated with a lower rate of complications. This question has already been addressed in several in vitro models. Ngaage et al tested the relative efficacy of 10% povidone-iodine, Clorpactin, Prontosan, triple-antibiotic solution, and normal saline. They concluded that povidone-iodine was the most efficacious of the irrigants at reducing methicillin-resistant S. aureus and Staphylococcus epidermidis contamination. These results demonstrated that the triple-antibiotic solution initially described by Adams et al reduces the bacterial bioburden of most Staphylococcus strains tested, but bacterial counts were only reduced by a factor of 10 or less, which led the authors to question the clinical efficacy of that solution.40 Culbertson et al drew a similar conclusion in their own in vitro study, where povidone-iodine at multiple different concentrations was found to have lasting bactericidal effects against 5 different commonly encountered organisms (up to 18 hours post-irrigation), with and without the presence of antibiotic solution. This was not the case for antibiotic irrigation without povidone-iodine.39 Both of these in vitro studies suggest that povidone-iodine is superior to antibiotic solution in reducing bioburden contamination.
Whether this conclusion also holds true in a clinical setting is unclear. Calobrace et al showed a significantly lower rate of capsular contracture associated with povidone-iodine irrigation compared with antibiotic irrigation but it is worth noting that this was not a controlled study and that the study does not account for the difference in other variables between the patients in the povidone-iodine group and the antibiotic irrigation group. In fact, the study does not even specify whether there was a clear delineation between patients who underwent antibiotic vs povidone-iodine irrigation, although for the purpose of our systematic review, we assumed that the 2 groups were mutually exclusive.31 Moreover, Calobrace himself has cautioned against the use of this study in a systematic review, given the lack of standardization and control group.14
Burkhardt et al did not show a significant difference in capsular contracture associated with antibiotic and povidone-iodine irrigation, although it is important to note the inclusion of steroids in the antibiotic group which may confound the results.10 Overall, we were unable to draw definitive conclusions about the superiority of antibiotic or antiseptic irrigation.
Studies by Pfeiffer et al and Drinane et al did not show a difference in capsular contracture rate between antibiotic and saline irrigation,22,26 but the addition of Burkhardt et al’s cohort of patients who received antibiotic steroid foam showed a significantly lower capsular contracture rate with antibiotics than with saline.10 This suggests a possible role for steroid inclusion in antimicrobial irrigation techniques. Calobrace et al. reported that on multivariate analysis, steroid irrigation was independently associated with a lower capsular contracture rate, although the limitations of this particular study have already been discussed.31
The only type of irrigation that was clearly shown to be associated with a higher rate of capsular contracture compared with antibiotic irrigation was the use of no irrigation at all. This is consistent with the oft-quoted surgical adage, “The solution to pollution is dilution.”
The rates of reoperation followed a similar pattern to the rates of capsular contracture, with a significant difference seen between povidone-iodine vs saline and antibiotic irrigation vs no irrigation at all, but no significant difference between antibiotic irrigation and saline. This is consistent with the fact that capsular contracture was the most commonly reported indication for reoperation.
There were insufficient data to draw conclusions between the protective effects of antibiotic or antiseptic irrigation on the rate of infectious complications, although the use of specialized antibiotic regimens appears to be superior in this regard than standard antibiotic irrigation alone. One of the limitations we encountered in assessing infection rates was that many of the papers either did not report infectious complications or did not specify which type of irrigation was used in patients who did experience infectious complications.
Ultimately, surgical site irrigation with either antibiotics or antiseptics is commonly used intraoperatively when performing reconstructive and cosmetic breast implant surgery due to their bactericidal mechanisms of action. To reduce bacterial contamination of the breast implant and thus postoperative complications, breast pocket irrigation is employed by the vast majority of plastic surgeons. A survey distributed to members of the American Society of Plastic Surgery reported that 95% of surgeons agree on the importance of antimicrobial pocket irrigation, although the choice of solution varied among respondents.41 Irrigation with triple-antibiotic solution was most popular among respondents although data are lacking on its efficacy compared with other irrigants. A similar survey by Epps et al reported the use of many different solutions and techniques, with 61% of respondents utilizing some variation of the triple-antibiotic solution initially recommended by Adams et al in 2006.12,15 These 2 surveys emphasize the significant variability among antimicrobial breast implant irrigation techniques, further stressing the need for evidence-based guidelines and standardized protocols.
The major limitation of this study that must be acknowledged is that the included data are vastly heterogenous with a wide variety of specific antibiotic choice, antimicrobial concentrations, contact time with the breast pocket, implant type, surgical technique, and follow-up time, making it difficult to directly compare these articles. Some authors used slightly different combinations and concentrations of antimicrobials throughout the course of their studies that were not separated in their final analysis. This makes it difficult to define the exact antimicrobial agent directly responsible for the reported complications. There was also no uniformity in the studies regarding textured vs smooth implants. This is important to note given that some studies have suggested a decreased rate of capsular contracture associated with textured implants, particular in those placed subglandular,42,43 although other studies have challenged this assertion.44 Additionally, the majority of included articles focused on augmentation mammoplasty, with only 3 studies including patients who underwent breast reconstruction; this makes it difficult to generalize these results across all implant-based breast surgeries. A meta-analysis by Lynch et al has previously identified this gap in the literature which is evident from our results as well.45
Most of our data were derived from retrospective studies, several of which showed poor methodologic and reporting quality and have inherent limitations of their own. Therefore, we recommend that more methodologically sound, high-quality randomized controlled trials be performed to determine the optimal irrigation solution.
CONCLUSIONS
Plastic surgeons have employed a variety of practices aimed at preventing complications following implant-based breast augmentations and reconstructions, which continue to evolve. The literature currently lacks evidence-based practice guidelines regarding the optimal antimicrobial solution for implant irrigation. In order to provide the best care for our patients, more standardized research is needed to better determine which irrigation solution is superior.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES



