-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel Knoedler, Martin Kauke-Navarro, Valentin Haug, P Niclas Broer, Bohdan Pomahac, Leonard Knoedler, Adriana C Panayi, Perioperative Outcomes and Risk Profile of 4730 Cosmetic Breast Surgery Cases in Academic Institutions: An ACS-NSQIP Analysis, Aesthetic Surgery Journal, Volume 43, Issue 4, April 2023, Pages 433–451, https://doi.org/10.1093/asj/sjac320
Close - Share Icon Share
Abstract
Cosmetic breast surgery (CBS) can be subdivided into augmentation, mastopexy, reduction, and reconstruction.
The aim of this study was to retrospectively analyze a multi-institutional national database to investigate the outcomes of CBS and identify clinical patterns to optimize care.
The American College of Surgeons National Surgical Quality Improvement Program database (2008-2020) was reviewed to identify female patients who underwent CBS. Postoperative outcomes (30-day surgical and medical complications, reoperation, readmission, and mortality) and risk factors for complications were assessed.
In total, 4733 patients were identified (mean age, 40 [13] years; mean BMI, 24 [4.5] kg/m2) with augmentation accounting for 54% of cases. There were complications in 2.0% of cases. Age >65 years (P = .002), obesity (P < .0001), setting (P < .0001), and diabetes (P = .04) were risk factors for any complication. Age >65 years (P = .02), obesity (P = .03), diabetes (P = .01), history of chronic obstructive pulmonary disease (COPD) (P = .002) and congestive heart failure (P < .0001), smoking in the past year (P = .003), setting (P = .007), and increased American Society of Anesthesiology score (P < .0001) were predictors of surgical complications such as dehiscence and infection. Multivariable analysis confirmed that chronic obstructive pulmonary disease, obesity Class 1 and 3, and inpatient status were independent risk factors for occurrence of any complication (P = .0005, .0003, < .0001 and <.0001, respectively). Additionally, multiple procedures (P = .02) and smoking (P = .005) were found to be risk factors for surgical complications.
This study confirms the positive safety profile of CBS. Healthy BMI is a protective factor, while complications were more likely among inpatient procedures. A correlation between multiple procedures and increased surgical complications was identified. Awareness of these risk factors can assist surgeons to further refine their perioperative protocols.
Özet
Estetik meme cerrahisi (CBS); meme büyütme, mastopeksi (meme dikleştirme), meme küçültme ve rekonstrüksiyon alt bölümlerine ayrılabilir.
Bu çalışmanın amacı, estetik meme cerrahisinin sonuçlarını araştırmak maksadıyla birden fazla kurumun dahil olduğu ulusal veri tabanını geriye dönük olarak incelemek ve hastaların bakımını iyileştirmek için gerekli klinik paternleri tanımlamaktır.
Amerikan Cerrahlar Birliği Ulusal Cerrahi Kalite Geliştirme Programı (The American College of Surgeons National Surgical Quality Improvement Program) veri tabanı (2008-2020), CBS olmuş kadın hastaları belirlemek üzere incelendi. Postoperatif sonuçlar (30 günlük cerrahi ve tıbbi komplikasyonlar, reoperasyon, hastaneye yeniden yatış ve mortalite) ve komplikasyonlar için risk faktörleri değerlendirildi.
Toplamda, vakaların %54’üne karşılık gelen meme büyütme operasyonu geçirmiş 4733 hasta belirlendi (ortalama yaş, 40 [13] ; ortalama VKİ (Vücut Kitle İndeksi), 24 [4,5] kg/m2). Vakaların %2,0'sinde komplikasyon mevcuttu. Her türlü komplikasyon için; yaş >65 (P=0,002), obezite (P<0,0001), ortam (p<0,0001) ve diyabet (P=0,04) risk faktörleri idi. Yaş >65 (P=0,02), obezite (P=0,03), diyabet (P=0,01), kronik obstrüktif akciğer hastalığı (KOAH) öyküsü (P=0,002) ve konjestif kalp yetmezliği (p<0,0001), son bir yıl içinde sigara kullanımı (P=0,003), ortam (P=0,007), ve artan Amerikan Anestezi Derneği (American Society of Anesthesiology) skoru (P<0,0001); dikişlerin açılması ve enfeksiyon gibi cerrahi komplikasyonların ön göstergesi idi. Yapılan çoklu değişkenli analizler neticesinde; kronik obstrüktif akciğer hastalığı, 1. ve 3. Sınıf obezite ve hastanede yatış durumunun her birinin, birbirlerinden ayrı olarak, her türlü komplikasyonu yaratacak bağımsız risk faktörleri olduğu teyit edildi. (sırasıyla P=0,0005, 0,0003, <0,0001 ve <0,0001). Ayrıca, birden fazla prosedürün (P=0,02) ve sigara kullanımının da (P=0,005) cerrahi komplikasyonlar için risk faktörleri olduğu tespit edildi.
Bu çalışma, estetik meme cerrahisinin (CBS) güvenlik profilinin olumlu olduğunu onaylamaktadır. Komplikasyonlar daha büyük oranda yatan hasta prosedürlerinde görülürken, sağlıklı bir VKİ’ye sahip olunması koruyucu bir faktördür. Birden fazla prosedür ve artan cerrahi komplikasyonlar arasında bir korelasyon tanımlanmıştır. Bu risk faktörlerinin farkına varılması, cerrahların perioperatif protokollerini daha da geliştirmelerine yardımcı olabilir.

See the Commentary on this article here.
Breast hypoplasia, hypertrophy, and ptosis are 3 of the most common complaints of patients presenting for cosmetic breast surgery (CBS). Such presentations are often associated with significant psychological, and even physical, implications. The extent of the importance placed on breast cosmesis is reflected in the numbers. CBS is considered a cornerstone of plastic surgery with 193,073 augmentation mammoplasties, 87,051 mastopexies, and 137,808 breast reconstructions performed by US plastic surgeons in 2020 alone.1 The field of CBS can be broadly subdivided into: (1) augmentation mammoplasty, (2) mastopexy, (3) reduction mammoplasty, and in some cases (4) breast reconstruction.2–6
In most cases, CBS is performed in surgeons’ private clinics and outpatient facilities.1 Given this setting distribution pattern, complication rates from these patient cohorts are often not reported in academic publications. Further, outcomes research investigating complication rates and risk factors in CBS is mainly based on retrospective analysis from single-surgeon, single-institution, or technique-specific medical records which can introduce bias and limit the research significance and translatability.7
Analysis of multicenter national databases can help overcome such limitations and limit bias by pooling together patient data with geographic and institutional variation. Use of such multicenter national databases within the context of CBS would allow identification of more robust risk factors and provide a comprehensive overview of the postoperative outcomes of this diverse cohort of patients.
In 2017, for example, Gupta et al investigated the outcomes and complications of CBS by utilizing CosmetAssure (Aesthetic Surgeons’ Financial Group; Birmingham, AL), a large, prospective, multi-institutional insurance-based database.8 They reported overall low complication rates (1.9%) and identified age and BMI as perioperative risk factors. Interestingly, the authors found a higher risk of complications in patients who underwent combined augmentation-mastopexy surgery or concomitant abdominoplasty. Given the insurance-based nature of the CosmetAssure database, the procedures included tend to have been performed in office-based surgical suites or ambulatory surgery centers.
As such, there is a paucity of studies evaluating the outcomes and occurrence of adverse events of CBS procedures performed in larger, academic hospital centers. Such procedures tend to be more complex, including challenging patient cases (eg, advanced breast cancer) or multiple concomitant procedures (eg, combined augmentation-mastopexy surgery).9
The American College of Surgeon's National Surgical Quality Improvement Program (ACS-NSQIP) prospectively collects validated data from more than 700 US hospitals, resulting in an extensive and diverse patient collection. To date, and to the best of our knowledge, the NSQIP data have not been analyzed with the goal of determining CBS outcomes. We aimed to fill this research gap by querying the ACS-NSQIP database and identifying the occurrence of, and risk factors for, complications in this unique, multi-institutional cohort.
METHODS
Data Source and Patient Selection
Data were collected between 2008 and 2020 from the ACS-NSQIP database. The ACS-NSQIP database is a nationwide, multi-institutional, and risk-adjusted catalog of surgical patients developed by the ACS and available to participating institutions. Peer reviews and spot audits ensure the quality, validity, and reliability of the data collected from more than 150 hospitals. The records analyzed did not contain patient-identifying information. Therefore, the study is exempt from IRB approval.
The ACS-NSQIP database was queried between 2008 and 2020 to identify all patients who underwent CBS. To this end, 13 annual data sets were filtered by the codes ICD-9-CM V50.1 (“Other plastic surgery for unacceptable cosmetic appearance”) and ICD-10-CM Z41.1 (“Encounter for cosmetic surgery”). As a second step, we combed this cohort of cosmetic procedures, extracting all cases in which breast surgery was performed exclusively. As a result, the analyzed cohort does not contain any cases in which body regions other than the breast were surgically treated—either as the main surgery or as a concurrent procedure. Further, we excluded male and nonbinary patients to obtain a more homogeneous cohort with only female patients. Finally, the generated patient pool was manually reviewed by 2 investigators (S.K. and A.P.) and the classification as CBS was verified for each individual case. In case of differing assessment, a third investigator (M.K.N.) was consulted.
Variable Extraction
Pre-, peri-, and 30-day postoperative variables were extracted:
1. In terms of preoperative data, we collected patient demographics (sex, age, race, height in inches, and weight in pounds), comorbidities (diabetes mellitus, history of chronic obstructive pulmonary disease [COPD], and congestive heart failure [CHF], active dialysis treatment, hypertension, dyspnea, nicotine abuse in the past year, steroid use/immunosuppressive therapy, weight loss greater than 10% of body weight in the 30 days before surgery, metastatic cancer, wound infection, ventilator dependency, and functional health status), as well as preoperative scores (American Society of Anesthesiology [ASA] physical status classification [score 1-4], and wound classification [score 1-4]. In addition, we calculated the BMI for all patients as (weight [pounds]/height [inches]2 × 703). All preoperative variables extracted are presented in Table 1.
2. As perioperative data we evaluated the setting (inpatient or outpatient), the type of anesthesia (general, monitored anesthesia care, epidural/spinal, local/regional, and other), the surgical specialty (plastic surgery, general surgery, and other), the year of surgery within a 13-year period from 2008 to 2020, and the total operating time in minutes. All perioperative information is shown in Table 2.
| Characteristic . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Demographics | |
| Age (years) | 40 [13] |
| BMI (kg/m2) | 24 [4.5] |
| Race | 12 (0.3) |
| American Indian or Alaskan native | 128 (2.7) |
| Asian | 3 (0.1) |
| Native Hawaiian or Pacific Islander | 200 (4.2) |
| Black or African American | 3876 (82) |
| White | 511 (11.0) |
| Other or unknown | |
| Preoperative health and comorbidities | 67 (1.4) |
| Diabetes | 23 (0.5) |
| Insulin treated diabetes | 9 (0.2) |
| COPD | 480 (10.0) |
| Obesity | 2 (0.0) |
| CHF | 0 (0.0) |
| Dialysis | 327 (6.9) |
| Hypertension | 22 (0.5) |
| Dyspnea | 505 (11.0) |
| Current smoker | 35 (0.7) |
| Corticosteroid use | 1 (0.0) |
| Weight loss >10% | 1 (0.0) |
| Disseminated cancer | 1 (0.0) |
| Wound infection | |
| ASA class | |
| 1. No disturbance | 2321 (49.0) |
| 2. Mild disturbance | 2242 (47.0) |
| 3. Severe disturbance | 160 (3.4) |
| 4. Life threatening | 2 (0.0) |
| Wound class | |
| 1. Clean | 4684 (99) |
| 2. Clean/contaminated | 36 (0.8) |
| 3. Contaminated | 9 (0.2) |
| 4. Dirty/infected | 1 (0.0) |
| Functional status | |
| Independent | 4714 (100.0) |
| Partially or totally dependent | 1 (0.0) |
| Characteristic . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Demographics | |
| Age (years) | 40 [13] |
| BMI (kg/m2) | 24 [4.5] |
| Race | 12 (0.3) |
| American Indian or Alaskan native | 128 (2.7) |
| Asian | 3 (0.1) |
| Native Hawaiian or Pacific Islander | 200 (4.2) |
| Black or African American | 3876 (82) |
| White | 511 (11.0) |
| Other or unknown | |
| Preoperative health and comorbidities | 67 (1.4) |
| Diabetes | 23 (0.5) |
| Insulin treated diabetes | 9 (0.2) |
| COPD | 480 (10.0) |
| Obesity | 2 (0.0) |
| CHF | 0 (0.0) |
| Dialysis | 327 (6.9) |
| Hypertension | 22 (0.5) |
| Dyspnea | 505 (11.0) |
| Current smoker | 35 (0.7) |
| Corticosteroid use | 1 (0.0) |
| Weight loss >10% | 1 (0.0) |
| Disseminated cancer | 1 (0.0) |
| Wound infection | |
| ASA class | |
| 1. No disturbance | 2321 (49.0) |
| 2. Mild disturbance | 2242 (47.0) |
| 3. Severe disturbance | 160 (3.4) |
| 4. Life threatening | 2 (0.0) |
| Wound class | |
| 1. Clean | 4684 (99) |
| 2. Clean/contaminated | 36 (0.8) |
| 3. Contaminated | 9 (0.2) |
| 4. Dirty/infected | 1 (0.0) |
| Functional status | |
| Independent | 4714 (100.0) |
| Partially or totally dependent | 1 (0.0) |
Reported as mean [standard deviation] or n (%). ASA, American Society of Anesthesiology; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
| Characteristic . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Demographics | |
| Age (years) | 40 [13] |
| BMI (kg/m2) | 24 [4.5] |
| Race | 12 (0.3) |
| American Indian or Alaskan native | 128 (2.7) |
| Asian | 3 (0.1) |
| Native Hawaiian or Pacific Islander | 200 (4.2) |
| Black or African American | 3876 (82) |
| White | 511 (11.0) |
| Other or unknown | |
| Preoperative health and comorbidities | 67 (1.4) |
| Diabetes | 23 (0.5) |
| Insulin treated diabetes | 9 (0.2) |
| COPD | 480 (10.0) |
| Obesity | 2 (0.0) |
| CHF | 0 (0.0) |
| Dialysis | 327 (6.9) |
| Hypertension | 22 (0.5) |
| Dyspnea | 505 (11.0) |
| Current smoker | 35 (0.7) |
| Corticosteroid use | 1 (0.0) |
| Weight loss >10% | 1 (0.0) |
| Disseminated cancer | 1 (0.0) |
| Wound infection | |
| ASA class | |
| 1. No disturbance | 2321 (49.0) |
| 2. Mild disturbance | 2242 (47.0) |
| 3. Severe disturbance | 160 (3.4) |
| 4. Life threatening | 2 (0.0) |
| Wound class | |
| 1. Clean | 4684 (99) |
| 2. Clean/contaminated | 36 (0.8) |
| 3. Contaminated | 9 (0.2) |
| 4. Dirty/infected | 1 (0.0) |
| Functional status | |
| Independent | 4714 (100.0) |
| Partially or totally dependent | 1 (0.0) |
| Characteristic . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Demographics | |
| Age (years) | 40 [13] |
| BMI (kg/m2) | 24 [4.5] |
| Race | 12 (0.3) |
| American Indian or Alaskan native | 128 (2.7) |
| Asian | 3 (0.1) |
| Native Hawaiian or Pacific Islander | 200 (4.2) |
| Black or African American | 3876 (82) |
| White | 511 (11.0) |
| Other or unknown | |
| Preoperative health and comorbidities | 67 (1.4) |
| Diabetes | 23 (0.5) |
| Insulin treated diabetes | 9 (0.2) |
| COPD | 480 (10.0) |
| Obesity | 2 (0.0) |
| CHF | 0 (0.0) |
| Dialysis | 327 (6.9) |
| Hypertension | 22 (0.5) |
| Dyspnea | 505 (11.0) |
| Current smoker | 35 (0.7) |
| Corticosteroid use | 1 (0.0) |
| Weight loss >10% | 1 (0.0) |
| Disseminated cancer | 1 (0.0) |
| Wound infection | |
| ASA class | |
| 1. No disturbance | 2321 (49.0) |
| 2. Mild disturbance | 2242 (47.0) |
| 3. Severe disturbance | 160 (3.4) |
| 4. Life threatening | 2 (0.0) |
| Wound class | |
| 1. Clean | 4684 (99) |
| 2. Clean/contaminated | 36 (0.8) |
| 3. Contaminated | 9 (0.2) |
| 4. Dirty/infected | 1 (0.0) |
| Functional status | |
| Independent | 4714 (100.0) |
| Partially or totally dependent | 1 (0.0) |
Reported as mean [standard deviation] or n (%). ASA, American Society of Anesthesiology; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
| Characteristics . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Type of surgery | |
| Breast augmentation, of which: | 2537 (54) |
| With prosthetic implant | 2507 (53) |
| Without prosthetic implant | 24 (0.5) |
| Isolated augmentation | 2386 (50) |
| + Capsule procedure | 45 (1.0) |
| + Implant removal | 59 (1.2) |
| + Capsule procedure + implant removal | 34 (0.7) |
| + Other/unknown procedure | 13 (0.3) |
| Breast augmentation + mastopexy, of which | 241 (5.1) |
| Isolated augmentation + mastopexy | 206 (4.4) |
| + Capsule procedure | 6 (0.1) |
| + Implant removal | 15 (0.3) |
| + Capsule procedure + implant removal | 11 (0.2) |
| + Other/unknown procedure | 3 (0.1) |
| Mastopexy, of which | 232 (4.9) |
| Isolated mastopexy | 175 (3.7) |
| + Capsule procedure | 26 (0.5) |
| + Implant removal | 16 (0.3) |
| + Capsule procedure + implant removal | 8 (0.2) |
| + Other/unknown procedure | 7 (0.1) |
| Breast reduction, of which | 354 (7.5) |
| Isolated reduction | 341 (7.2) |
| + Capsule procedure | 4 (0.1) |
| + Other/unknown procedure | 9 (0.2) |
| Breast reduction + mastopexy, of which | 26 (0.5) |
| Isolated reduction + mastopexy | 25 (0.5) |
| + Capsule procedure | 1 (0.0) |
| Breast augmentation + breast reduction | 6 (0.1) |
| Breast reconstruction, of which | 1140 (24) |
| Immediate implant-based, of which | 707 (20) |
| Isolated immediate implant-based breast reconstruction | 144 (4.0) |
| Immediate implant-based breast reconstruction + Mastopexy | 202 (5.6) |
| Implant replacement | 142 (4.0) |
| Implant replacement + mastopexy | 35 (1.0) |
| Implant replacement + capsulotomy/capsulectomy | 29 (0.8) |
| Capsulotomy/capsulectomy | 111 (3.1) |
| Capsulotomy/capsulectomy + mastopexy | 27 (0.8) |
| Other revision procedures | 17 (0.5) |
| Delayed implant-based, of which | 154 (4.3) |
| Isolated delayed implant-based breast reconstruction | 142 (4.0) |
| Revision of delayed implant-based breast reconstruction | 12 (0.3) |
| Using tissue expander | 37 (1.0) |
| Immediate autologous | 3 (0.1) |
| Delayed autologous | 17 (0.5) |
| Not further specified revision procedures | 115 (3.2) |
| Other/unknown | 107 (3.0) |
| Isolated implant removal | 57 (1.2) |
| Capsulectomy/capsulotomy, of which | 50 (1.1) |
| Isolated capsulectomy/capsulotomy | 46 (1.0) |
| + Other/unknown procedure | 4 (0.1) |
| Other | 62 (1.3) |
| Type of anesthesia | |
| General | 4688 (99) |
| Monitored anesthesia care | 18 (0.4) |
| Epidural/spinal | 7 (0.1) |
| Local/regional | 3 (0.1) |
| Other/unknown | 5 (0.1) |
| Setting | |
| Inpatient | 101 (2.1) |
| Outpatient | 4629 (98) |
| Surgical specialty | |
| Plastics | 4667 (99) |
| Genera surgery | 53 (1.1) |
| Other | 10 (0.2) |
| Year of surgery | |
| 2008 | 85 (1.8) |
| 2009 | 100 (2.1) |
| 2010 | 98 (2.1) |
| 2011 | 52 (1.1) |
| 2012 | 149 (3.2) |
| 2013 | 396 (8.4) |
| 2014 | 559 (12) |
| 2015 | 654 (14) |
| 2016 | 517 (11) |
| 2017 | 466 (9.9) |
| 2018 | 630 (13) |
| 2019 | 619 (13) |
| 2020 | 405 (8.6) |
| Characteristics . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Type of surgery | |
| Breast augmentation, of which: | 2537 (54) |
| With prosthetic implant | 2507 (53) |
| Without prosthetic implant | 24 (0.5) |
| Isolated augmentation | 2386 (50) |
| + Capsule procedure | 45 (1.0) |
| + Implant removal | 59 (1.2) |
| + Capsule procedure + implant removal | 34 (0.7) |
| + Other/unknown procedure | 13 (0.3) |
| Breast augmentation + mastopexy, of which | 241 (5.1) |
| Isolated augmentation + mastopexy | 206 (4.4) |
| + Capsule procedure | 6 (0.1) |
| + Implant removal | 15 (0.3) |
| + Capsule procedure + implant removal | 11 (0.2) |
| + Other/unknown procedure | 3 (0.1) |
| Mastopexy, of which | 232 (4.9) |
| Isolated mastopexy | 175 (3.7) |
| + Capsule procedure | 26 (0.5) |
| + Implant removal | 16 (0.3) |
| + Capsule procedure + implant removal | 8 (0.2) |
| + Other/unknown procedure | 7 (0.1) |
| Breast reduction, of which | 354 (7.5) |
| Isolated reduction | 341 (7.2) |
| + Capsule procedure | 4 (0.1) |
| + Other/unknown procedure | 9 (0.2) |
| Breast reduction + mastopexy, of which | 26 (0.5) |
| Isolated reduction + mastopexy | 25 (0.5) |
| + Capsule procedure | 1 (0.0) |
| Breast augmentation + breast reduction | 6 (0.1) |
| Breast reconstruction, of which | 1140 (24) |
| Immediate implant-based, of which | 707 (20) |
| Isolated immediate implant-based breast reconstruction | 144 (4.0) |
| Immediate implant-based breast reconstruction + Mastopexy | 202 (5.6) |
| Implant replacement | 142 (4.0) |
| Implant replacement + mastopexy | 35 (1.0) |
| Implant replacement + capsulotomy/capsulectomy | 29 (0.8) |
| Capsulotomy/capsulectomy | 111 (3.1) |
| Capsulotomy/capsulectomy + mastopexy | 27 (0.8) |
| Other revision procedures | 17 (0.5) |
| Delayed implant-based, of which | 154 (4.3) |
| Isolated delayed implant-based breast reconstruction | 142 (4.0) |
| Revision of delayed implant-based breast reconstruction | 12 (0.3) |
| Using tissue expander | 37 (1.0) |
| Immediate autologous | 3 (0.1) |
| Delayed autologous | 17 (0.5) |
| Not further specified revision procedures | 115 (3.2) |
| Other/unknown | 107 (3.0) |
| Isolated implant removal | 57 (1.2) |
| Capsulectomy/capsulotomy, of which | 50 (1.1) |
| Isolated capsulectomy/capsulotomy | 46 (1.0) |
| + Other/unknown procedure | 4 (0.1) |
| Other | 62 (1.3) |
| Type of anesthesia | |
| General | 4688 (99) |
| Monitored anesthesia care | 18 (0.4) |
| Epidural/spinal | 7 (0.1) |
| Local/regional | 3 (0.1) |
| Other/unknown | 5 (0.1) |
| Setting | |
| Inpatient | 101 (2.1) |
| Outpatient | 4629 (98) |
| Surgical specialty | |
| Plastics | 4667 (99) |
| Genera surgery | 53 (1.1) |
| Other | 10 (0.2) |
| Year of surgery | |
| 2008 | 85 (1.8) |
| 2009 | 100 (2.1) |
| 2010 | 98 (2.1) |
| 2011 | 52 (1.1) |
| 2012 | 149 (3.2) |
| 2013 | 396 (8.4) |
| 2014 | 559 (12) |
| 2015 | 654 (14) |
| 2016 | 517 (11) |
| 2017 | 466 (9.9) |
| 2018 | 630 (13) |
| 2019 | 619 (13) |
| 2020 | 405 (8.6) |
Reported as n (%).
| Characteristics . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Type of surgery | |
| Breast augmentation, of which: | 2537 (54) |
| With prosthetic implant | 2507 (53) |
| Without prosthetic implant | 24 (0.5) |
| Isolated augmentation | 2386 (50) |
| + Capsule procedure | 45 (1.0) |
| + Implant removal | 59 (1.2) |
| + Capsule procedure + implant removal | 34 (0.7) |
| + Other/unknown procedure | 13 (0.3) |
| Breast augmentation + mastopexy, of which | 241 (5.1) |
| Isolated augmentation + mastopexy | 206 (4.4) |
| + Capsule procedure | 6 (0.1) |
| + Implant removal | 15 (0.3) |
| + Capsule procedure + implant removal | 11 (0.2) |
| + Other/unknown procedure | 3 (0.1) |
| Mastopexy, of which | 232 (4.9) |
| Isolated mastopexy | 175 (3.7) |
| + Capsule procedure | 26 (0.5) |
| + Implant removal | 16 (0.3) |
| + Capsule procedure + implant removal | 8 (0.2) |
| + Other/unknown procedure | 7 (0.1) |
| Breast reduction, of which | 354 (7.5) |
| Isolated reduction | 341 (7.2) |
| + Capsule procedure | 4 (0.1) |
| + Other/unknown procedure | 9 (0.2) |
| Breast reduction + mastopexy, of which | 26 (0.5) |
| Isolated reduction + mastopexy | 25 (0.5) |
| + Capsule procedure | 1 (0.0) |
| Breast augmentation + breast reduction | 6 (0.1) |
| Breast reconstruction, of which | 1140 (24) |
| Immediate implant-based, of which | 707 (20) |
| Isolated immediate implant-based breast reconstruction | 144 (4.0) |
| Immediate implant-based breast reconstruction + Mastopexy | 202 (5.6) |
| Implant replacement | 142 (4.0) |
| Implant replacement + mastopexy | 35 (1.0) |
| Implant replacement + capsulotomy/capsulectomy | 29 (0.8) |
| Capsulotomy/capsulectomy | 111 (3.1) |
| Capsulotomy/capsulectomy + mastopexy | 27 (0.8) |
| Other revision procedures | 17 (0.5) |
| Delayed implant-based, of which | 154 (4.3) |
| Isolated delayed implant-based breast reconstruction | 142 (4.0) |
| Revision of delayed implant-based breast reconstruction | 12 (0.3) |
| Using tissue expander | 37 (1.0) |
| Immediate autologous | 3 (0.1) |
| Delayed autologous | 17 (0.5) |
| Not further specified revision procedures | 115 (3.2) |
| Other/unknown | 107 (3.0) |
| Isolated implant removal | 57 (1.2) |
| Capsulectomy/capsulotomy, of which | 50 (1.1) |
| Isolated capsulectomy/capsulotomy | 46 (1.0) |
| + Other/unknown procedure | 4 (0.1) |
| Other | 62 (1.3) |
| Type of anesthesia | |
| General | 4688 (99) |
| Monitored anesthesia care | 18 (0.4) |
| Epidural/spinal | 7 (0.1) |
| Local/regional | 3 (0.1) |
| Other/unknown | 5 (0.1) |
| Setting | |
| Inpatient | 101 (2.1) |
| Outpatient | 4629 (98) |
| Surgical specialty | |
| Plastics | 4667 (99) |
| Genera surgery | 53 (1.1) |
| Other | 10 (0.2) |
| Year of surgery | |
| 2008 | 85 (1.8) |
| 2009 | 100 (2.1) |
| 2010 | 98 (2.1) |
| 2011 | 52 (1.1) |
| 2012 | 149 (3.2) |
| 2013 | 396 (8.4) |
| 2014 | 559 (12) |
| 2015 | 654 (14) |
| 2016 | 517 (11) |
| 2017 | 466 (9.9) |
| 2018 | 630 (13) |
| 2019 | 619 (13) |
| 2020 | 405 (8.6) |
| Characteristics . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Type of surgery | |
| Breast augmentation, of which: | 2537 (54) |
| With prosthetic implant | 2507 (53) |
| Without prosthetic implant | 24 (0.5) |
| Isolated augmentation | 2386 (50) |
| + Capsule procedure | 45 (1.0) |
| + Implant removal | 59 (1.2) |
| + Capsule procedure + implant removal | 34 (0.7) |
| + Other/unknown procedure | 13 (0.3) |
| Breast augmentation + mastopexy, of which | 241 (5.1) |
| Isolated augmentation + mastopexy | 206 (4.4) |
| + Capsule procedure | 6 (0.1) |
| + Implant removal | 15 (0.3) |
| + Capsule procedure + implant removal | 11 (0.2) |
| + Other/unknown procedure | 3 (0.1) |
| Mastopexy, of which | 232 (4.9) |
| Isolated mastopexy | 175 (3.7) |
| + Capsule procedure | 26 (0.5) |
| + Implant removal | 16 (0.3) |
| + Capsule procedure + implant removal | 8 (0.2) |
| + Other/unknown procedure | 7 (0.1) |
| Breast reduction, of which | 354 (7.5) |
| Isolated reduction | 341 (7.2) |
| + Capsule procedure | 4 (0.1) |
| + Other/unknown procedure | 9 (0.2) |
| Breast reduction + mastopexy, of which | 26 (0.5) |
| Isolated reduction + mastopexy | 25 (0.5) |
| + Capsule procedure | 1 (0.0) |
| Breast augmentation + breast reduction | 6 (0.1) |
| Breast reconstruction, of which | 1140 (24) |
| Immediate implant-based, of which | 707 (20) |
| Isolated immediate implant-based breast reconstruction | 144 (4.0) |
| Immediate implant-based breast reconstruction + Mastopexy | 202 (5.6) |
| Implant replacement | 142 (4.0) |
| Implant replacement + mastopexy | 35 (1.0) |
| Implant replacement + capsulotomy/capsulectomy | 29 (0.8) |
| Capsulotomy/capsulectomy | 111 (3.1) |
| Capsulotomy/capsulectomy + mastopexy | 27 (0.8) |
| Other revision procedures | 17 (0.5) |
| Delayed implant-based, of which | 154 (4.3) |
| Isolated delayed implant-based breast reconstruction | 142 (4.0) |
| Revision of delayed implant-based breast reconstruction | 12 (0.3) |
| Using tissue expander | 37 (1.0) |
| Immediate autologous | 3 (0.1) |
| Delayed autologous | 17 (0.5) |
| Not further specified revision procedures | 115 (3.2) |
| Other/unknown | 107 (3.0) |
| Isolated implant removal | 57 (1.2) |
| Capsulectomy/capsulotomy, of which | 50 (1.1) |
| Isolated capsulectomy/capsulotomy | 46 (1.0) |
| + Other/unknown procedure | 4 (0.1) |
| Other | 62 (1.3) |
| Type of anesthesia | |
| General | 4688 (99) |
| Monitored anesthesia care | 18 (0.4) |
| Epidural/spinal | 7 (0.1) |
| Local/regional | 3 (0.1) |
| Other/unknown | 5 (0.1) |
| Setting | |
| Inpatient | 101 (2.1) |
| Outpatient | 4629 (98) |
| Surgical specialty | |
| Plastics | 4667 (99) |
| Genera surgery | 53 (1.1) |
| Other | 10 (0.2) |
| Year of surgery | |
| 2008 | 85 (1.8) |
| 2009 | 100 (2.1) |
| 2010 | 98 (2.1) |
| 2011 | 52 (1.1) |
| 2012 | 149 (3.2) |
| 2013 | 396 (8.4) |
| 2014 | 559 (12) |
| 2015 | 654 (14) |
| 2016 | 517 (11) |
| 2017 | 466 (9.9) |
| 2018 | 630 (13) |
| 2019 | 619 (13) |
| 2020 | 405 (8.6) |
Reported as n (%).
For in-depth assessment, we manually analyzed all cases of CBS and classified them into one of the following types of surgeries: breast augmentation, breast augmentation with concurrent mastopexy, mastopexy, breast reduction, breast reduction with concurrent mastopexy, breast augmentation with concurrent breast reduction (“reductive augmentation”), breast reconstruction, capsule procedures with concurrent implant removal, isolated implant removal, isolated capsulectomy/capsulotomy, and other.10 Next, we refined this classification system by specifying whether concurrently to the aforementioned main procedures a capsule procedure, an implant removal, capsule procedure plus implant removal, or another breast surgery-related procedure was performed. Notably, when classifying and naming the individual types of surgery, we closely adhered to the nomenclature recorded in the NSQIP database. A more precise specification, for example of reconstructive procedures, was not possible due to the limited case information entered. The surgical characteristics, including the classification pattern and frequency of each surgical type, are summarized in Table 2.
3. Thirty-day postoperative outcomes included the length of hospital stay (LOS) and the destination of discharge (home, rehab, separate acute care, skilled care, and other/unknown). LOS is calculated as the difference in days between the date of admission and the date of discharge. In other words, if the patient was discharged on the same day of surgery, LOS = 0 is entered. Accordingly, for overnight stays, LOS > 0 is registered. Any complications were defined as the occurrence of any of mortality, reoperation, readmission or unplanned readmission, and surgical or medical complications. For further analyses, we considered all surgical complications recorded in the NSQIP database (ie, superficial and deep incisional site infection, organ space infection, wound disruption/dehiscence, and bleeding transfusions) occurring at least once. Likewise, while analyzing all medical complications reported in the NSQIP database (ie, pneumonia, reintubation, pulmonary embolism, ventilator use for more than 48 hours, renal insufficiency, acute renal failure, infection of the urinary tract, cerebrovascular incident/stroke, cardiac arrest, myocardial infarction, deep vein thrombosis/thrombophlebitis, sepsis and septic shock), we concentrated on those of which at least 1 case has been reported. Detailed information on postoperative outcomes following CBS is displayed in Tables 3 to 5.
| Outcome . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Length of hospital stay (days) | 0.2 [2.4] |
| Operative time, mean (minutes) | 101 [68] |
| Any complication | 96 (2.0) |
| Mortality within 30 days | 0 (0.0) |
| Reoperation | 50 (1.1) |
| Readmission | 18 (0.4) |
| Unplanned readmission | 16 (0.3) |
| Surgical complication | 36 (0.8) |
| Superficial incisional infection | 22 (0.5) |
| Deep incisional infection | 4 (0.1) |
| Organ space infection | 2 (0.0) |
| Dehiscence | 11 (0.2) |
| Medical complication | 15 (0.3) |
| Pneumonia | 2 (0.0) |
| Pulmonary embolism | 2 (0.0) |
| Urinary tract infection | 10 (0.2) |
| Deep vein thrombosis/thrombophlebitis | 1 (0.0) |
| Discharge destination | |
| Home | 4438 (94) |
| Rehab | 1 (0.0) |
| Separate acute care | 3 (0.1) |
| Skilled care | 4 (0.1) |
| Other/unknown | 284 (6.0) |
| Outcome . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Length of hospital stay (days) | 0.2 [2.4] |
| Operative time, mean (minutes) | 101 [68] |
| Any complication | 96 (2.0) |
| Mortality within 30 days | 0 (0.0) |
| Reoperation | 50 (1.1) |
| Readmission | 18 (0.4) |
| Unplanned readmission | 16 (0.3) |
| Surgical complication | 36 (0.8) |
| Superficial incisional infection | 22 (0.5) |
| Deep incisional infection | 4 (0.1) |
| Organ space infection | 2 (0.0) |
| Dehiscence | 11 (0.2) |
| Medical complication | 15 (0.3) |
| Pneumonia | 2 (0.0) |
| Pulmonary embolism | 2 (0.0) |
| Urinary tract infection | 10 (0.2) |
| Deep vein thrombosis/thrombophlebitis | 1 (0.0) |
| Discharge destination | |
| Home | 4438 (94) |
| Rehab | 1 (0.0) |
| Separate acute care | 3 (0.1) |
| Skilled care | 4 (0.1) |
| Other/unknown | 284 (6.0) |
Reported as mean [standard deviation] or n (%).
| Outcome . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Length of hospital stay (days) | 0.2 [2.4] |
| Operative time, mean (minutes) | 101 [68] |
| Any complication | 96 (2.0) |
| Mortality within 30 days | 0 (0.0) |
| Reoperation | 50 (1.1) |
| Readmission | 18 (0.4) |
| Unplanned readmission | 16 (0.3) |
| Surgical complication | 36 (0.8) |
| Superficial incisional infection | 22 (0.5) |
| Deep incisional infection | 4 (0.1) |
| Organ space infection | 2 (0.0) |
| Dehiscence | 11 (0.2) |
| Medical complication | 15 (0.3) |
| Pneumonia | 2 (0.0) |
| Pulmonary embolism | 2 (0.0) |
| Urinary tract infection | 10 (0.2) |
| Deep vein thrombosis/thrombophlebitis | 1 (0.0) |
| Discharge destination | |
| Home | 4438 (94) |
| Rehab | 1 (0.0) |
| Separate acute care | 3 (0.1) |
| Skilled care | 4 (0.1) |
| Other/unknown | 284 (6.0) |
| Outcome . | Cosmetic breast surgery (n = 4730) . |
|---|---|
| Length of hospital stay (days) | 0.2 [2.4] |
| Operative time, mean (minutes) | 101 [68] |
| Any complication | 96 (2.0) |
| Mortality within 30 days | 0 (0.0) |
| Reoperation | 50 (1.1) |
| Readmission | 18 (0.4) |
| Unplanned readmission | 16 (0.3) |
| Surgical complication | 36 (0.8) |
| Superficial incisional infection | 22 (0.5) |
| Deep incisional infection | 4 (0.1) |
| Organ space infection | 2 (0.0) |
| Dehiscence | 11 (0.2) |
| Medical complication | 15 (0.3) |
| Pneumonia | 2 (0.0) |
| Pulmonary embolism | 2 (0.0) |
| Urinary tract infection | 10 (0.2) |
| Deep vein thrombosis/thrombophlebitis | 1 (0.0) |
| Discharge destination | |
| Home | 4438 (94) |
| Rehab | 1 (0.0) |
| Separate acute care | 3 (0.1) |
| Skilled care | 4 (0.1) |
| Other/unknown | 284 (6.0) |
Reported as mean [standard deviation] or n (%).
Distribution of Procedures With the Type-Specific Occurrence of Any Complication
| Type of surgery . | Total . | Any complication . | Any complication/total(%) . |
|---|---|---|---|
| Breast augmentation, of which: | 2537 | 41 | 1.6 |
| Isolated augmentation | 2386 | 37 | 1.6 |
| + Capsule procedure | 45 | 2 | 4.4 |
| + Implant removal | 59 | 1 | 1.7 |
| + Capsule procedure + implant removal | 34 | 1 | 2.9 |
| + Other/unknown procedure | 13 | 0 | 0.0 |
| Breast augmentation + mastopexy, of which: | 241 | 4 | 1.7 |
| Isolated augmentation + mastopexy | 206 | 2 | 1.0 |
| + Capsule procedure | 6 | 0 | 0.0 |
| + Implant removal | 15 | 0 | 0.0 |
| + Capsule procedure + implant removal | 11 | 2 | 18.2 |
| + Other/unknown procedure | 3 | 0 | 0.0 |
| Mastopexy, of which: | 232 | 6 | 2.6 |
| Isolated mastopexy | 175 | 2 | 1.1 |
| + Capsule procedure | 26 | 2 | 7.7 |
| + Implant removal | 16 | 2 | 12.5 |
| + Capsule procedure + implant removal | 8 | 0 | 0.0 |
| + Other/unknown procedure | 7 | 0 | 0.0 |
| Breast reduction, of which: | 354 | 19 | 5.4 |
| Isolated reduction | 341 | 18 | 5.3 |
| + Capsule procedure | 4 | 0 | 0.0 |
| + Other/unknown procedure | 9 | 1 | 11.1 |
| Breast reduction + mastopexy, of which: | 26 | 2 | 7.7 |
| Isolated reduction + mastopexy | 25 | 2 | 8.0 |
| + Capsule procedure | 1 | 0 | 0.0 |
| Breast augmentation + breast reduction | 6 | 1 | 16.7 |
| Breast reconstruction, of which: | 1140 | 19 | 1.7 |
| Immediate implant-based, of which | 707 | 12 | 1.7 |
| Isolated immediate implant-based | 144 | 3 | 2.1 |
| + Mastopexy | 202 | 5 | 2.5 |
| + Implant replacement | 142 | 2 | 1.4 |
| + Implant replacement + mastopexy | 35 | 0 | 0.0 |
| + Implant replacement + capsule procedure | 29 | 1 | 3.4 |
| + Capsule procedure | 111 | 0 | 0.0 |
| + Capsule procedure + mastopexy | 27 | 1 | 3.7 |
| + Other revision procedures | 17 | 0 | 0.0 |
| Delayed implant-based, of which | 154 | 1 | 0.6 |
| Isolated delayed implant-based | 142 | 1 | 0.7 |
| Revision of delayed implant-based | 12 | 0 | 0.0 |
| Using tissue expander | 37 | 2 | 5.4 |
| Immediate autologous | 3 | 2 | 67.0 |
| Delayed autologous | 17 | 1 | 5.9 |
| Not further specified revision procedures | 115 | 1 | 0.9 |
| Other/unknown | 107 | 0 | 0.0 |
| Capsule + removal procedure | 25 | 1 | 4.0 |
| Isolated implant removal | 57 | 0 | 0.0 |
| Capsulectomy/capsulotomy, of which: | 50 | 1 | 2.0 |
| Isolated capsulectomy/capsulotomy | 46 | 1 | 2.2 |
| + Other/unknown procedure | 4 | 0 | 0.0 |
| Other | 62 | 2 | 3.2 |
| Type of surgery . | Total . | Any complication . | Any complication/total(%) . |
|---|---|---|---|
| Breast augmentation, of which: | 2537 | 41 | 1.6 |
| Isolated augmentation | 2386 | 37 | 1.6 |
| + Capsule procedure | 45 | 2 | 4.4 |
| + Implant removal | 59 | 1 | 1.7 |
| + Capsule procedure + implant removal | 34 | 1 | 2.9 |
| + Other/unknown procedure | 13 | 0 | 0.0 |
| Breast augmentation + mastopexy, of which: | 241 | 4 | 1.7 |
| Isolated augmentation + mastopexy | 206 | 2 | 1.0 |
| + Capsule procedure | 6 | 0 | 0.0 |
| + Implant removal | 15 | 0 | 0.0 |
| + Capsule procedure + implant removal | 11 | 2 | 18.2 |
| + Other/unknown procedure | 3 | 0 | 0.0 |
| Mastopexy, of which: | 232 | 6 | 2.6 |
| Isolated mastopexy | 175 | 2 | 1.1 |
| + Capsule procedure | 26 | 2 | 7.7 |
| + Implant removal | 16 | 2 | 12.5 |
| + Capsule procedure + implant removal | 8 | 0 | 0.0 |
| + Other/unknown procedure | 7 | 0 | 0.0 |
| Breast reduction, of which: | 354 | 19 | 5.4 |
| Isolated reduction | 341 | 18 | 5.3 |
| + Capsule procedure | 4 | 0 | 0.0 |
| + Other/unknown procedure | 9 | 1 | 11.1 |
| Breast reduction + mastopexy, of which: | 26 | 2 | 7.7 |
| Isolated reduction + mastopexy | 25 | 2 | 8.0 |
| + Capsule procedure | 1 | 0 | 0.0 |
| Breast augmentation + breast reduction | 6 | 1 | 16.7 |
| Breast reconstruction, of which: | 1140 | 19 | 1.7 |
| Immediate implant-based, of which | 707 | 12 | 1.7 |
| Isolated immediate implant-based | 144 | 3 | 2.1 |
| + Mastopexy | 202 | 5 | 2.5 |
| + Implant replacement | 142 | 2 | 1.4 |
| + Implant replacement + mastopexy | 35 | 0 | 0.0 |
| + Implant replacement + capsule procedure | 29 | 1 | 3.4 |
| + Capsule procedure | 111 | 0 | 0.0 |
| + Capsule procedure + mastopexy | 27 | 1 | 3.7 |
| + Other revision procedures | 17 | 0 | 0.0 |
| Delayed implant-based, of which | 154 | 1 | 0.6 |
| Isolated delayed implant-based | 142 | 1 | 0.7 |
| Revision of delayed implant-based | 12 | 0 | 0.0 |
| Using tissue expander | 37 | 2 | 5.4 |
| Immediate autologous | 3 | 2 | 67.0 |
| Delayed autologous | 17 | 1 | 5.9 |
| Not further specified revision procedures | 115 | 1 | 0.9 |
| Other/unknown | 107 | 0 | 0.0 |
| Capsule + removal procedure | 25 | 1 | 4.0 |
| Isolated implant removal | 57 | 0 | 0.0 |
| Capsulectomy/capsulotomy, of which: | 50 | 1 | 2.0 |
| Isolated capsulectomy/capsulotomy | 46 | 1 | 2.2 |
| + Other/unknown procedure | 4 | 0 | 0.0 |
| Other | 62 | 2 | 3.2 |
Distribution of Procedures With the Type-Specific Occurrence of Any Complication
| Type of surgery . | Total . | Any complication . | Any complication/total(%) . |
|---|---|---|---|
| Breast augmentation, of which: | 2537 | 41 | 1.6 |
| Isolated augmentation | 2386 | 37 | 1.6 |
| + Capsule procedure | 45 | 2 | 4.4 |
| + Implant removal | 59 | 1 | 1.7 |
| + Capsule procedure + implant removal | 34 | 1 | 2.9 |
| + Other/unknown procedure | 13 | 0 | 0.0 |
| Breast augmentation + mastopexy, of which: | 241 | 4 | 1.7 |
| Isolated augmentation + mastopexy | 206 | 2 | 1.0 |
| + Capsule procedure | 6 | 0 | 0.0 |
| + Implant removal | 15 | 0 | 0.0 |
| + Capsule procedure + implant removal | 11 | 2 | 18.2 |
| + Other/unknown procedure | 3 | 0 | 0.0 |
| Mastopexy, of which: | 232 | 6 | 2.6 |
| Isolated mastopexy | 175 | 2 | 1.1 |
| + Capsule procedure | 26 | 2 | 7.7 |
| + Implant removal | 16 | 2 | 12.5 |
| + Capsule procedure + implant removal | 8 | 0 | 0.0 |
| + Other/unknown procedure | 7 | 0 | 0.0 |
| Breast reduction, of which: | 354 | 19 | 5.4 |
| Isolated reduction | 341 | 18 | 5.3 |
| + Capsule procedure | 4 | 0 | 0.0 |
| + Other/unknown procedure | 9 | 1 | 11.1 |
| Breast reduction + mastopexy, of which: | 26 | 2 | 7.7 |
| Isolated reduction + mastopexy | 25 | 2 | 8.0 |
| + Capsule procedure | 1 | 0 | 0.0 |
| Breast augmentation + breast reduction | 6 | 1 | 16.7 |
| Breast reconstruction, of which: | 1140 | 19 | 1.7 |
| Immediate implant-based, of which | 707 | 12 | 1.7 |
| Isolated immediate implant-based | 144 | 3 | 2.1 |
| + Mastopexy | 202 | 5 | 2.5 |
| + Implant replacement | 142 | 2 | 1.4 |
| + Implant replacement + mastopexy | 35 | 0 | 0.0 |
| + Implant replacement + capsule procedure | 29 | 1 | 3.4 |
| + Capsule procedure | 111 | 0 | 0.0 |
| + Capsule procedure + mastopexy | 27 | 1 | 3.7 |
| + Other revision procedures | 17 | 0 | 0.0 |
| Delayed implant-based, of which | 154 | 1 | 0.6 |
| Isolated delayed implant-based | 142 | 1 | 0.7 |
| Revision of delayed implant-based | 12 | 0 | 0.0 |
| Using tissue expander | 37 | 2 | 5.4 |
| Immediate autologous | 3 | 2 | 67.0 |
| Delayed autologous | 17 | 1 | 5.9 |
| Not further specified revision procedures | 115 | 1 | 0.9 |
| Other/unknown | 107 | 0 | 0.0 |
| Capsule + removal procedure | 25 | 1 | 4.0 |
| Isolated implant removal | 57 | 0 | 0.0 |
| Capsulectomy/capsulotomy, of which: | 50 | 1 | 2.0 |
| Isolated capsulectomy/capsulotomy | 46 | 1 | 2.2 |
| + Other/unknown procedure | 4 | 0 | 0.0 |
| Other | 62 | 2 | 3.2 |
| Type of surgery . | Total . | Any complication . | Any complication/total(%) . |
|---|---|---|---|
| Breast augmentation, of which: | 2537 | 41 | 1.6 |
| Isolated augmentation | 2386 | 37 | 1.6 |
| + Capsule procedure | 45 | 2 | 4.4 |
| + Implant removal | 59 | 1 | 1.7 |
| + Capsule procedure + implant removal | 34 | 1 | 2.9 |
| + Other/unknown procedure | 13 | 0 | 0.0 |
| Breast augmentation + mastopexy, of which: | 241 | 4 | 1.7 |
| Isolated augmentation + mastopexy | 206 | 2 | 1.0 |
| + Capsule procedure | 6 | 0 | 0.0 |
| + Implant removal | 15 | 0 | 0.0 |
| + Capsule procedure + implant removal | 11 | 2 | 18.2 |
| + Other/unknown procedure | 3 | 0 | 0.0 |
| Mastopexy, of which: | 232 | 6 | 2.6 |
| Isolated mastopexy | 175 | 2 | 1.1 |
| + Capsule procedure | 26 | 2 | 7.7 |
| + Implant removal | 16 | 2 | 12.5 |
| + Capsule procedure + implant removal | 8 | 0 | 0.0 |
| + Other/unknown procedure | 7 | 0 | 0.0 |
| Breast reduction, of which: | 354 | 19 | 5.4 |
| Isolated reduction | 341 | 18 | 5.3 |
| + Capsule procedure | 4 | 0 | 0.0 |
| + Other/unknown procedure | 9 | 1 | 11.1 |
| Breast reduction + mastopexy, of which: | 26 | 2 | 7.7 |
| Isolated reduction + mastopexy | 25 | 2 | 8.0 |
| + Capsule procedure | 1 | 0 | 0.0 |
| Breast augmentation + breast reduction | 6 | 1 | 16.7 |
| Breast reconstruction, of which: | 1140 | 19 | 1.7 |
| Immediate implant-based, of which | 707 | 12 | 1.7 |
| Isolated immediate implant-based | 144 | 3 | 2.1 |
| + Mastopexy | 202 | 5 | 2.5 |
| + Implant replacement | 142 | 2 | 1.4 |
| + Implant replacement + mastopexy | 35 | 0 | 0.0 |
| + Implant replacement + capsule procedure | 29 | 1 | 3.4 |
| + Capsule procedure | 111 | 0 | 0.0 |
| + Capsule procedure + mastopexy | 27 | 1 | 3.7 |
| + Other revision procedures | 17 | 0 | 0.0 |
| Delayed implant-based, of which | 154 | 1 | 0.6 |
| Isolated delayed implant-based | 142 | 1 | 0.7 |
| Revision of delayed implant-based | 12 | 0 | 0.0 |
| Using tissue expander | 37 | 2 | 5.4 |
| Immediate autologous | 3 | 2 | 67.0 |
| Delayed autologous | 17 | 1 | 5.9 |
| Not further specified revision procedures | 115 | 1 | 0.9 |
| Other/unknown | 107 | 0 | 0.0 |
| Capsule + removal procedure | 25 | 1 | 4.0 |
| Isolated implant removal | 57 | 0 | 0.0 |
| Capsulectomy/capsulotomy, of which: | 50 | 1 | 2.0 |
| Isolated capsulectomy/capsulotomy | 46 | 1 | 2.2 |
| + Other/unknown procedure | 4 | 0 | 0.0 |
| Other | 62 | 2 | 3.2 |
| Case . | Reoperation . | Readmission . | Surgical complications . | Medical complications . | No. of patients, when >1 . |
|---|---|---|---|---|---|
| Breast augmentation | |||||
| + Removal intact mammary implant | UTI | ||||
| + Open periprosthetic capsulotomy | Dehiscence | ||||
| + Open periprosthetic capsulotomy | X | 2 | |||
| No concurrent procedure | X | 18 | |||
| No concurrent procedure | SII | 3 | |||
| No concurrent procedure | UTI | 6 | |||
| No concurrent procedure | PE | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X | X UP | SII | ||
| No concurrent procedure | X | ||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | DII | ||||
| No concurrent procedure | X | X UP | OSI | ||
| No concurrent procedure | SII, Dehiscence | ||||
| Breast augmentation + mastopexy | |||||
| + Open periprosthetic capsulotomy, implant removal | X | OSI | |||
| + Open periprosthetic capsulectomy, implant removal | X | X UP | SII | ||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X | ||||
| Mastopexy | |||||
| + Periprosthetic capsulectomy | X | X UP | Dehiscence | ||
| + Periprosthetic capsulectomy | SII | ||||
| + Implant removal | UTI | ||||
| + Implant removal | SII | ||||
| No concurrent procedure | X | ||||
| No concurrent procedure | SII | ||||
| Breast reduction | |||||
| + Nipple/areola reconstruction | SII | ||||
| + Tissue grafts other | SII | ||||
| No concurrent procedure | X | 4 | |||
| No concurrent procedure | SII | 7 | |||
| No concurrent procedure | SII, Dehiscence | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | X | ||||
| No concurrent procedure | X | X UP | SII, Dehiscence | ||
| No concurrent procedure | X | X UP | Dehiscence | ||
| Breast reduction + mastopexy | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | SII | ||||
| Breast augmentation + breast reduction | |||||
| No concurrent procedure | Dehiscence | ||||
| Breast reconstruction | |||||
| Isolated immediate implant-based | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | UTI | ||||
| No concurrent procedure | × UP | PE | |||
| IIBR + mastopexy | |||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | SII | 2 | |||
| No concurrent procedure | × | 2 | |||
| IIBR implant replacement | |||||
| Immediate prosthesis, mastectomy, implant removal | × | 2 | |||
| IIBR implant replacement + capsulotomy/capsulectomy | |||||
| No concurrent procedure | × | × UP | |||
| IIBR capsulotomy/capsulectomy + mastopexy | |||||
| No concurrent procedure | × | ||||
| Isolated delayed implant-based breast reconstruction | |||||
| + Drainage of hematoma/seroma/fluid | × | ||||
| Breast reconstruction using tissue expander | |||||
| No concurrent procedure | × | DII | |||
| No concurrent procedure | × | ||||
| Immediate autologous breast reconstruction | |||||
| Latissimus dorsi muscle flap | UTI | ||||
| Transverse rectus abdominis muscle flap | × | ||||
| Delayed autologous breast reconstruction | |||||
| Muscle/myocutaneous flap | DVT | ||||
| Not further specified revision procedures | |||||
| + Areola Reconstruction, mastopexy | × | ||||
| Others | |||||
| Including each procedure entered | |||||
| Periprosthetic capsulectomy | × | × UP | |||
| Open periprosthetic capsulotomy, implant removal | × | ||||
| Complete mastectomy | × UP | DII | |||
| Subcutaneous mastectomy | × | × UP | |||
| Case . | Reoperation . | Readmission . | Surgical complications . | Medical complications . | No. of patients, when >1 . |
|---|---|---|---|---|---|
| Breast augmentation | |||||
| + Removal intact mammary implant | UTI | ||||
| + Open periprosthetic capsulotomy | Dehiscence | ||||
| + Open periprosthetic capsulotomy | X | 2 | |||
| No concurrent procedure | X | 18 | |||
| No concurrent procedure | SII | 3 | |||
| No concurrent procedure | UTI | 6 | |||
| No concurrent procedure | PE | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X | X UP | SII | ||
| No concurrent procedure | X | ||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | DII | ||||
| No concurrent procedure | X | X UP | OSI | ||
| No concurrent procedure | SII, Dehiscence | ||||
| Breast augmentation + mastopexy | |||||
| + Open periprosthetic capsulotomy, implant removal | X | OSI | |||
| + Open periprosthetic capsulectomy, implant removal | X | X UP | SII | ||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X | ||||
| Mastopexy | |||||
| + Periprosthetic capsulectomy | X | X UP | Dehiscence | ||
| + Periprosthetic capsulectomy | SII | ||||
| + Implant removal | UTI | ||||
| + Implant removal | SII | ||||
| No concurrent procedure | X | ||||
| No concurrent procedure | SII | ||||
| Breast reduction | |||||
| + Nipple/areola reconstruction | SII | ||||
| + Tissue grafts other | SII | ||||
| No concurrent procedure | X | 4 | |||
| No concurrent procedure | SII | 7 | |||
| No concurrent procedure | SII, Dehiscence | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | X | ||||
| No concurrent procedure | X | X UP | SII, Dehiscence | ||
| No concurrent procedure | X | X UP | Dehiscence | ||
| Breast reduction + mastopexy | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | SII | ||||
| Breast augmentation + breast reduction | |||||
| No concurrent procedure | Dehiscence | ||||
| Breast reconstruction | |||||
| Isolated immediate implant-based | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | UTI | ||||
| No concurrent procedure | × UP | PE | |||
| IIBR + mastopexy | |||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | SII | 2 | |||
| No concurrent procedure | × | 2 | |||
| IIBR implant replacement | |||||
| Immediate prosthesis, mastectomy, implant removal | × | 2 | |||
| IIBR implant replacement + capsulotomy/capsulectomy | |||||
| No concurrent procedure | × | × UP | |||
| IIBR capsulotomy/capsulectomy + mastopexy | |||||
| No concurrent procedure | × | ||||
| Isolated delayed implant-based breast reconstruction | |||||
| + Drainage of hematoma/seroma/fluid | × | ||||
| Breast reconstruction using tissue expander | |||||
| No concurrent procedure | × | DII | |||
| No concurrent procedure | × | ||||
| Immediate autologous breast reconstruction | |||||
| Latissimus dorsi muscle flap | UTI | ||||
| Transverse rectus abdominis muscle flap | × | ||||
| Delayed autologous breast reconstruction | |||||
| Muscle/myocutaneous flap | DVT | ||||
| Not further specified revision procedures | |||||
| + Areola Reconstruction, mastopexy | × | ||||
| Others | |||||
| Including each procedure entered | |||||
| Periprosthetic capsulectomy | × | × UP | |||
| Open periprosthetic capsulotomy, implant removal | × | ||||
| Complete mastectomy | × UP | DII | |||
| Subcutaneous mastectomy | × | × UP | |||
Adverse events occurred in 96 cases (2.0%). DII, deep incisional infection; DVT, deep venous thrombosis; IIBR, isolated immediate implant-based breast reconstruction; OSI, organ space infection; PE, pulmonary embolism; SII, superficial incisional infection; UP, unplanned; UTI, urinary tract infection.
| Case . | Reoperation . | Readmission . | Surgical complications . | Medical complications . | No. of patients, when >1 . |
|---|---|---|---|---|---|
| Breast augmentation | |||||
| + Removal intact mammary implant | UTI | ||||
| + Open periprosthetic capsulotomy | Dehiscence | ||||
| + Open periprosthetic capsulotomy | X | 2 | |||
| No concurrent procedure | X | 18 | |||
| No concurrent procedure | SII | 3 | |||
| No concurrent procedure | UTI | 6 | |||
| No concurrent procedure | PE | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X | X UP | SII | ||
| No concurrent procedure | X | ||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | DII | ||||
| No concurrent procedure | X | X UP | OSI | ||
| No concurrent procedure | SII, Dehiscence | ||||
| Breast augmentation + mastopexy | |||||
| + Open periprosthetic capsulotomy, implant removal | X | OSI | |||
| + Open periprosthetic capsulectomy, implant removal | X | X UP | SII | ||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X | ||||
| Mastopexy | |||||
| + Periprosthetic capsulectomy | X | X UP | Dehiscence | ||
| + Periprosthetic capsulectomy | SII | ||||
| + Implant removal | UTI | ||||
| + Implant removal | SII | ||||
| No concurrent procedure | X | ||||
| No concurrent procedure | SII | ||||
| Breast reduction | |||||
| + Nipple/areola reconstruction | SII | ||||
| + Tissue grafts other | SII | ||||
| No concurrent procedure | X | 4 | |||
| No concurrent procedure | SII | 7 | |||
| No concurrent procedure | SII, Dehiscence | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | X | ||||
| No concurrent procedure | X | X UP | SII, Dehiscence | ||
| No concurrent procedure | X | X UP | Dehiscence | ||
| Breast reduction + mastopexy | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | SII | ||||
| Breast augmentation + breast reduction | |||||
| No concurrent procedure | Dehiscence | ||||
| Breast reconstruction | |||||
| Isolated immediate implant-based | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | UTI | ||||
| No concurrent procedure | × UP | PE | |||
| IIBR + mastopexy | |||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | SII | 2 | |||
| No concurrent procedure | × | 2 | |||
| IIBR implant replacement | |||||
| Immediate prosthesis, mastectomy, implant removal | × | 2 | |||
| IIBR implant replacement + capsulotomy/capsulectomy | |||||
| No concurrent procedure | × | × UP | |||
| IIBR capsulotomy/capsulectomy + mastopexy | |||||
| No concurrent procedure | × | ||||
| Isolated delayed implant-based breast reconstruction | |||||
| + Drainage of hematoma/seroma/fluid | × | ||||
| Breast reconstruction using tissue expander | |||||
| No concurrent procedure | × | DII | |||
| No concurrent procedure | × | ||||
| Immediate autologous breast reconstruction | |||||
| Latissimus dorsi muscle flap | UTI | ||||
| Transverse rectus abdominis muscle flap | × | ||||
| Delayed autologous breast reconstruction | |||||
| Muscle/myocutaneous flap | DVT | ||||
| Not further specified revision procedures | |||||
| + Areola Reconstruction, mastopexy | × | ||||
| Others | |||||
| Including each procedure entered | |||||
| Periprosthetic capsulectomy | × | × UP | |||
| Open periprosthetic capsulotomy, implant removal | × | ||||
| Complete mastectomy | × UP | DII | |||
| Subcutaneous mastectomy | × | × UP | |||
| Case . | Reoperation . | Readmission . | Surgical complications . | Medical complications . | No. of patients, when >1 . |
|---|---|---|---|---|---|
| Breast augmentation | |||||
| + Removal intact mammary implant | UTI | ||||
| + Open periprosthetic capsulotomy | Dehiscence | ||||
| + Open periprosthetic capsulotomy | X | 2 | |||
| No concurrent procedure | X | 18 | |||
| No concurrent procedure | SII | 3 | |||
| No concurrent procedure | UTI | 6 | |||
| No concurrent procedure | PE | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X | X UP | SII | ||
| No concurrent procedure | X | ||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | DII | ||||
| No concurrent procedure | X | X UP | OSI | ||
| No concurrent procedure | SII, Dehiscence | ||||
| Breast augmentation + mastopexy | |||||
| + Open periprosthetic capsulotomy, implant removal | X | OSI | |||
| + Open periprosthetic capsulectomy, implant removal | X | X UP | SII | ||
| No concurrent procedure | X UP | ||||
| No concurrent procedure | X | ||||
| Mastopexy | |||||
| + Periprosthetic capsulectomy | X | X UP | Dehiscence | ||
| + Periprosthetic capsulectomy | SII | ||||
| + Implant removal | UTI | ||||
| + Implant removal | SII | ||||
| No concurrent procedure | X | ||||
| No concurrent procedure | SII | ||||
| Breast reduction | |||||
| + Nipple/areola reconstruction | SII | ||||
| + Tissue grafts other | SII | ||||
| No concurrent procedure | X | 4 | |||
| No concurrent procedure | SII | 7 | |||
| No concurrent procedure | SII, Dehiscence | ||||
| No concurrent procedure | X | Dehiscence | |||
| No concurrent procedure | X UP | Pneumonia | |||
| No concurrent procedure | X | ||||
| No concurrent procedure | X | X UP | SII, Dehiscence | ||
| No concurrent procedure | X | X UP | Dehiscence | ||
| Breast reduction + mastopexy | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | SII | ||||
| Breast augmentation + breast reduction | |||||
| No concurrent procedure | Dehiscence | ||||
| Breast reconstruction | |||||
| Isolated immediate implant-based | |||||
| No concurrent procedure | × | ||||
| No concurrent procedure | UTI | ||||
| No concurrent procedure | × UP | PE | |||
| IIBR + mastopexy | |||||
| No concurrent procedure | Dehiscence | ||||
| No concurrent procedure | SII | 2 | |||
| No concurrent procedure | × | 2 | |||
| IIBR implant replacement | |||||
| Immediate prosthesis, mastectomy, implant removal | × | 2 | |||
| IIBR implant replacement + capsulotomy/capsulectomy | |||||
| No concurrent procedure | × | × UP | |||
| IIBR capsulotomy/capsulectomy + mastopexy | |||||
| No concurrent procedure | × | ||||
| Isolated delayed implant-based breast reconstruction | |||||
| + Drainage of hematoma/seroma/fluid | × | ||||
| Breast reconstruction using tissue expander | |||||
| No concurrent procedure | × | DII | |||
| No concurrent procedure | × | ||||
| Immediate autologous breast reconstruction | |||||
| Latissimus dorsi muscle flap | UTI | ||||
| Transverse rectus abdominis muscle flap | × | ||||
| Delayed autologous breast reconstruction | |||||
| Muscle/myocutaneous flap | DVT | ||||
| Not further specified revision procedures | |||||
| + Areola Reconstruction, mastopexy | × | ||||
| Others | |||||
| Including each procedure entered | |||||
| Periprosthetic capsulectomy | × | × UP | |||
| Open periprosthetic capsulotomy, implant removal | × | ||||
| Complete mastectomy | × UP | DII | |||
| Subcutaneous mastectomy | × | × UP | |||
Adverse events occurred in 96 cases (2.0%). DII, deep incisional infection; DVT, deep venous thrombosis; IIBR, isolated immediate implant-based breast reconstruction; OSI, organ space infection; PE, pulmonary embolism; SII, superficial incisional infection; UP, unplanned; UTI, urinary tract infection.
Statistical Analysis
Data were gathered and saved in an electronic laboratory notebook (LabArchives, LLC; San Marcos, CA) and evaluated using GraphPad Prism version 9.00 for macOS (GraphPad Software; La Jolla, CA). Continuous variables are stated as mean [standard deviation, SD] values and were analyzed with independent t-tests. To measure differences in categoric variables, Pearson's chi square was applied. In cases with fewer than 10 events, Fisher's exact test was used. Statistical significance was defined at P < .05. To identify risk factors for complications, univariable subgroup analysis was performed, partitioning the cohort into 3 groups according to the occurrence of any complications, surgical complications, and medical complications. Multivariable regression was conducted by including all variables that were found to be significant predictors of the occurrence of a complication, thus eliminating confounding factors. Rate of occurrence of any complications was also compared within and between procedures using Pearson's chi square. Within procedures a comparison was made between isolated surgery and surgery performed concurrently with other procedures such as capsule procedures or implant removal. Between procedures a comparison was made between the major groups of procedures: augmentation, augmentation with mastopexy, mastopexy, reduction, reduction with mastopexy, augmentation with reduction, and reconstruction.
RESULTS
Patient Demographics
The study population included 4730 female patients who underwent CBS over a 13-year review period (2008-2020). The mean [SD] patient age and BMI were 40 [13] years and 24 [4.5] kg/m2, respectively. White patients (n = 3876; 82%) represented the majority of our patient cohort. Obesity (n = 480; 10%) accounted for the most common comorbidity, while 11% (n = 506) of patients were current smokers (Figure 1). Detailed demographic data are presented in Table 1.
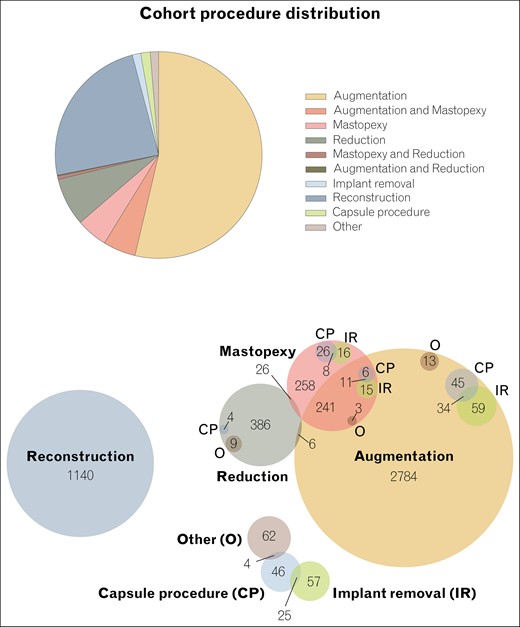
Distribution of procedures. In total, the cohort includes 4730 cases.
Surgical Characteristics
When excluding cases concomitant with mastopexy or reduction, augmentation mammoplasty accounted for 54% (n = 2539). Breast reconstruction listed as a cosmetic procedure accounted for 24% (n = 1137) of cases. Furthermore, there were 232 (4.9%) mastopexy cases (excluding concomitant augmentation or reduction) and 354 (7.5%) breast reduction procedures (excluding concomitant augmentation or mastopexy). Table 2 and Figure 1 display surgical characteristics in detail.
Perioperative Outcomes
Mean operation time was 101 [68] minutes and postoperative LOS was 0.2 [2.4] days on average with 94% (n = 4438) of patients discharged home afterwards (Table 3).
Postoperative Surgical and Medical Outcomes
No case of death was reported within the 30-day postoperative period, while 50 (1.1%) patients returned to the operating room. There were complications (at least 1 of: reoperation, readmission, and surgical or medical complication) in 2.0% (n = 96) of cases. Further details are provided in Tables 3 to 5. The surgical complication rate was 0.8% (n = 36) with superficial incisional infection (n = 22; 0.5%) accounting for the majority of surgical adverse events. Medical complications were rare and occurred in 0.3% (n = 15) of cases.
Age >65 years (P = .002), obesity (P < .0001), and diabetes (P = .04) were identified as risk factors for occurrence of any complication. A significant correlation between occurrence of any complications and inpatient procedures was noted (P < .0001). Age >65 years (P = .02), obesity (P = .03), diabetes (P = .01), history of COPD (P = .002) and CHF (P < .0001), nicotine abuse in the past year (P = .003), the setting (inpatient status; P = .007) and increased ASA score (P < .0001) were predictors of surgical complications (Table 6). Multivariable analysis confirmed that COPD and obesity Class 1 and 3 were independent risk factors for occurrence of any complication (P = .0005, .0003 and <.0001, respectively). Inpatient status was also confirmed to be associated with increased complications (P < .0001). In addition to COPD and obesity Class 1 and 3 (all P < .0001), multiple procedures (P = .02) and smoking (P = .005) were confirmed as independent risk factors for occurrence of any surgical complication. Dyspnea (P = .03), obesity Class 2 (P < .0001), partial dependency (P = .005), and inpatient procedures (P < .0001) were less likely to be associated with a home discharge, while hypertension (P = .02), underweight (P = .0004), and normal BMI (P < .0001) were more likely to be associated with a home discharge (Table 7).
| Characteristic . | Any complication . | P-value . | Surgical complication . | P-value . | Medical complication . | P-value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 96) . | No (n = 4634) . | Yes (n = 36) . | No (n = 4694) . | Yes (n = 15) . | No (n = 4715) . | ||||
| Demographics | |||||||||
| Age (years) | 44 [14] | 40 [13] | .002 | 45 [15] | 40 [13] | .02 | 43 [12] | 40 [13] | .42 |
| BMI (kg/m2) | 27 [7] | 24 [4] | <.0001 | 32 [7] | 24 [4] | <.0001 | 24 [5] | 24 [5] | .83 |
| Race | .21 | .008 | .77 | ||||||
| American Indian/Alaskan native | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | |||
| Asian | 2 (2.1) | 126 (2.7) | 1 (2.8) | 127 (2.7) | 0 (0.0) | 128 (2.7) | |||
| Native Hawaiian/Pacific Islander | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | |||
| Black/African American | 5 (5.2) | 195 (4.2) | 0 (0.0) | 200 (4.3) | 1 (6.7) | 199 (4.2) | |||
| White | 71 (74.0) | 3805 (82.1) | 24 (66.7) | 3852 (82.1) | 14 (93.3) | 3862 (81.9) | |||
| Other or unknown | 18 (18.8) | 493 (10.6) | 11 (30.6) | 500 (10.7) | 0 (0.0) | 511 (10.8) | |||
| Setting | 4543 (98.0) | <.0001 | .007 | .27 | |||||
| Outpatient | 86 (89.6) | 32 (88.9) | 4597 (97.9) | 14 (93.3) | 4615 (97.9) | ||||
| Inpatient | 10 (10.4) | 91 (2.0) | 4 (11.1) | 97 (2.1) | 1 (6.7) | 100 (2.1) | |||
| Preop health/comorbidities | |||||||||
| Diabetes | 4 (4.2) | 63 (1.4) | .04 | 3 (8.3) | 64 (1.4) | .01 | 0 (0.0) | 67 (1.4) | >.99 |
| Insulin-treated diabetes | 3 (3.1) | 20 (0.4) | .01 | 2 (5.5) | 21 (0.4) | .01 | 0 (0.0) | 23 (0.5) | >.99 |
| COPD | 2 (2.1) | 7 (0.2) | .01 | 2 (5.5) | 7 (0.1) | .002 | 0 (0.0) | 9 (0.2) | >.99 |
| Obesity | 30 (31.3) | 450 (9.7) | <.0001 | 21 (58.3) | 459 (9.8) | .03 | 1 (6.7) | 326 (6.9) | >.99 |
| Hypertension | 11 (11.5) | 316 (6.8) | .10 | 6 (16.7) | 321 (6.8) | .16 | 0 (0.0) | 22 (0.5) | >.99 |
| Dyspnea | 1 (1.0) | 21 (0.5) | .36 | 1 (2.8) | 21 (0.4) | >.99 | 0 (0.0) | 2 (0.0) | >.99 |
| CHF | 0 (0.0) | 2 (0.0) | .99 | 0 (0.0) | 2 (0.0) | <.0001 | 3 (20.0) | 477 (10.1) | .19 |
| Current smoker | 15 (15.6) | 490 (10.6) | .13 | 10 (27.8) | 495 (10.5) | .003 | 0 (0.0) | 505 (10.7) | .40 |
| Corticosteroid use | 0 (0.0) | 35 (0.8) | .99 | 0 (0.0) | 35 (0.7) | >.99 | 0 (0.0) | 35 (0.7) | >.99 |
| Wound infection | 0 (0.0) | 1 (0.0) | .99 | 0 (0.0) | 1 (0.0) | >.99 | 0 (0.0) | 1 (0.0) | .12 |
| ASA class | <.0001 | .84 | |||||||
| 1. No disturbance | 31 (32.3) | 2290 (49.4) | .001 | 8 (22.2) | 2313 (49.3) | 6 (40.0) | 2315 (49.1) | ||
| 2. Mild disturbance | 57 (59.4) | 2185 (47.2) | 22 (61.1) | 2220 (47.3) | 8 (53.3) | 2234 (47.4) | |||
| 3. Severe disturbance | 8 (8.3) | 152 (3.3) | 6 (16.7) | 154 (3.3) | 1 (6.7) | 159 (3.4) | |||
| 4. Life threatening | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | |||
| Wound class | .11 | .57 | .99 | ||||||
| 1. Clean | 93 (96.9) | 4591 (99.1) | 35 (97.2) | 4649 (99.0) | 15 (100.0) | 4669 (99.0) | |||
| 2. Clean/contaminated | 2 (2.1) | 34 (0.7) | 1 (2.8) | 35 (0.7) | 0 (0.0) | 36 (0.8) | |||
| 3. Contaminated | 1 (1.0) | 8 (0.2) | 0 (0.0) | 9 (0.2) | 0 (0.0) | 9 (0.2) | |||
| 4. Dirty/infected | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | |||
| Functional status | 4619 | .88 | .09 | .06 | |||||
| Independent | 95 (99.0) | 1 (0.0) | 36 (100) | 4678 (99.7) | 15 (100.0) | 4699 (99.7) | |||
| Partially/totally dependent | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | ||||
| Characteristic . | Any complication . | P-value . | Surgical complication . | P-value . | Medical complication . | P-value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 96) . | No (n = 4634) . | Yes (n = 36) . | No (n = 4694) . | Yes (n = 15) . | No (n = 4715) . | ||||
| Demographics | |||||||||
| Age (years) | 44 [14] | 40 [13] | .002 | 45 [15] | 40 [13] | .02 | 43 [12] | 40 [13] | .42 |
| BMI (kg/m2) | 27 [7] | 24 [4] | <.0001 | 32 [7] | 24 [4] | <.0001 | 24 [5] | 24 [5] | .83 |
| Race | .21 | .008 | .77 | ||||||
| American Indian/Alaskan native | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | |||
| Asian | 2 (2.1) | 126 (2.7) | 1 (2.8) | 127 (2.7) | 0 (0.0) | 128 (2.7) | |||
| Native Hawaiian/Pacific Islander | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | |||
| Black/African American | 5 (5.2) | 195 (4.2) | 0 (0.0) | 200 (4.3) | 1 (6.7) | 199 (4.2) | |||
| White | 71 (74.0) | 3805 (82.1) | 24 (66.7) | 3852 (82.1) | 14 (93.3) | 3862 (81.9) | |||
| Other or unknown | 18 (18.8) | 493 (10.6) | 11 (30.6) | 500 (10.7) | 0 (0.0) | 511 (10.8) | |||
| Setting | 4543 (98.0) | <.0001 | .007 | .27 | |||||
| Outpatient | 86 (89.6) | 32 (88.9) | 4597 (97.9) | 14 (93.3) | 4615 (97.9) | ||||
| Inpatient | 10 (10.4) | 91 (2.0) | 4 (11.1) | 97 (2.1) | 1 (6.7) | 100 (2.1) | |||
| Preop health/comorbidities | |||||||||
| Diabetes | 4 (4.2) | 63 (1.4) | .04 | 3 (8.3) | 64 (1.4) | .01 | 0 (0.0) | 67 (1.4) | >.99 |
| Insulin-treated diabetes | 3 (3.1) | 20 (0.4) | .01 | 2 (5.5) | 21 (0.4) | .01 | 0 (0.0) | 23 (0.5) | >.99 |
| COPD | 2 (2.1) | 7 (0.2) | .01 | 2 (5.5) | 7 (0.1) | .002 | 0 (0.0) | 9 (0.2) | >.99 |
| Obesity | 30 (31.3) | 450 (9.7) | <.0001 | 21 (58.3) | 459 (9.8) | .03 | 1 (6.7) | 326 (6.9) | >.99 |
| Hypertension | 11 (11.5) | 316 (6.8) | .10 | 6 (16.7) | 321 (6.8) | .16 | 0 (0.0) | 22 (0.5) | >.99 |
| Dyspnea | 1 (1.0) | 21 (0.5) | .36 | 1 (2.8) | 21 (0.4) | >.99 | 0 (0.0) | 2 (0.0) | >.99 |
| CHF | 0 (0.0) | 2 (0.0) | .99 | 0 (0.0) | 2 (0.0) | <.0001 | 3 (20.0) | 477 (10.1) | .19 |
| Current smoker | 15 (15.6) | 490 (10.6) | .13 | 10 (27.8) | 495 (10.5) | .003 | 0 (0.0) | 505 (10.7) | .40 |
| Corticosteroid use | 0 (0.0) | 35 (0.8) | .99 | 0 (0.0) | 35 (0.7) | >.99 | 0 (0.0) | 35 (0.7) | >.99 |
| Wound infection | 0 (0.0) | 1 (0.0) | .99 | 0 (0.0) | 1 (0.0) | >.99 | 0 (0.0) | 1 (0.0) | .12 |
| ASA class | <.0001 | .84 | |||||||
| 1. No disturbance | 31 (32.3) | 2290 (49.4) | .001 | 8 (22.2) | 2313 (49.3) | 6 (40.0) | 2315 (49.1) | ||
| 2. Mild disturbance | 57 (59.4) | 2185 (47.2) | 22 (61.1) | 2220 (47.3) | 8 (53.3) | 2234 (47.4) | |||
| 3. Severe disturbance | 8 (8.3) | 152 (3.3) | 6 (16.7) | 154 (3.3) | 1 (6.7) | 159 (3.4) | |||
| 4. Life threatening | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | |||
| Wound class | .11 | .57 | .99 | ||||||
| 1. Clean | 93 (96.9) | 4591 (99.1) | 35 (97.2) | 4649 (99.0) | 15 (100.0) | 4669 (99.0) | |||
| 2. Clean/contaminated | 2 (2.1) | 34 (0.7) | 1 (2.8) | 35 (0.7) | 0 (0.0) | 36 (0.8) | |||
| 3. Contaminated | 1 (1.0) | 8 (0.2) | 0 (0.0) | 9 (0.2) | 0 (0.0) | 9 (0.2) | |||
| 4. Dirty/infected | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | |||
| Functional status | 4619 | .88 | .09 | .06 | |||||
| Independent | 95 (99.0) | 1 (0.0) | 36 (100) | 4678 (99.7) | 15 (100.0) | 4699 (99.7) | |||
| Partially/totally dependent | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | ||||
Reported as mean [standard deviation] or n (%). ASA, American Society of Anesthesiology; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
| Characteristic . | Any complication . | P-value . | Surgical complication . | P-value . | Medical complication . | P-value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 96) . | No (n = 4634) . | Yes (n = 36) . | No (n = 4694) . | Yes (n = 15) . | No (n = 4715) . | ||||
| Demographics | |||||||||
| Age (years) | 44 [14] | 40 [13] | .002 | 45 [15] | 40 [13] | .02 | 43 [12] | 40 [13] | .42 |
| BMI (kg/m2) | 27 [7] | 24 [4] | <.0001 | 32 [7] | 24 [4] | <.0001 | 24 [5] | 24 [5] | .83 |
| Race | .21 | .008 | .77 | ||||||
| American Indian/Alaskan native | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | |||
| Asian | 2 (2.1) | 126 (2.7) | 1 (2.8) | 127 (2.7) | 0 (0.0) | 128 (2.7) | |||
| Native Hawaiian/Pacific Islander | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | |||
| Black/African American | 5 (5.2) | 195 (4.2) | 0 (0.0) | 200 (4.3) | 1 (6.7) | 199 (4.2) | |||
| White | 71 (74.0) | 3805 (82.1) | 24 (66.7) | 3852 (82.1) | 14 (93.3) | 3862 (81.9) | |||
| Other or unknown | 18 (18.8) | 493 (10.6) | 11 (30.6) | 500 (10.7) | 0 (0.0) | 511 (10.8) | |||
| Setting | 4543 (98.0) | <.0001 | .007 | .27 | |||||
| Outpatient | 86 (89.6) | 32 (88.9) | 4597 (97.9) | 14 (93.3) | 4615 (97.9) | ||||
| Inpatient | 10 (10.4) | 91 (2.0) | 4 (11.1) | 97 (2.1) | 1 (6.7) | 100 (2.1) | |||
| Preop health/comorbidities | |||||||||
| Diabetes | 4 (4.2) | 63 (1.4) | .04 | 3 (8.3) | 64 (1.4) | .01 | 0 (0.0) | 67 (1.4) | >.99 |
| Insulin-treated diabetes | 3 (3.1) | 20 (0.4) | .01 | 2 (5.5) | 21 (0.4) | .01 | 0 (0.0) | 23 (0.5) | >.99 |
| COPD | 2 (2.1) | 7 (0.2) | .01 | 2 (5.5) | 7 (0.1) | .002 | 0 (0.0) | 9 (0.2) | >.99 |
| Obesity | 30 (31.3) | 450 (9.7) | <.0001 | 21 (58.3) | 459 (9.8) | .03 | 1 (6.7) | 326 (6.9) | >.99 |
| Hypertension | 11 (11.5) | 316 (6.8) | .10 | 6 (16.7) | 321 (6.8) | .16 | 0 (0.0) | 22 (0.5) | >.99 |
| Dyspnea | 1 (1.0) | 21 (0.5) | .36 | 1 (2.8) | 21 (0.4) | >.99 | 0 (0.0) | 2 (0.0) | >.99 |
| CHF | 0 (0.0) | 2 (0.0) | .99 | 0 (0.0) | 2 (0.0) | <.0001 | 3 (20.0) | 477 (10.1) | .19 |
| Current smoker | 15 (15.6) | 490 (10.6) | .13 | 10 (27.8) | 495 (10.5) | .003 | 0 (0.0) | 505 (10.7) | .40 |
| Corticosteroid use | 0 (0.0) | 35 (0.8) | .99 | 0 (0.0) | 35 (0.7) | >.99 | 0 (0.0) | 35 (0.7) | >.99 |
| Wound infection | 0 (0.0) | 1 (0.0) | .99 | 0 (0.0) | 1 (0.0) | >.99 | 0 (0.0) | 1 (0.0) | .12 |
| ASA class | <.0001 | .84 | |||||||
| 1. No disturbance | 31 (32.3) | 2290 (49.4) | .001 | 8 (22.2) | 2313 (49.3) | 6 (40.0) | 2315 (49.1) | ||
| 2. Mild disturbance | 57 (59.4) | 2185 (47.2) | 22 (61.1) | 2220 (47.3) | 8 (53.3) | 2234 (47.4) | |||
| 3. Severe disturbance | 8 (8.3) | 152 (3.3) | 6 (16.7) | 154 (3.3) | 1 (6.7) | 159 (3.4) | |||
| 4. Life threatening | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | |||
| Wound class | .11 | .57 | .99 | ||||||
| 1. Clean | 93 (96.9) | 4591 (99.1) | 35 (97.2) | 4649 (99.0) | 15 (100.0) | 4669 (99.0) | |||
| 2. Clean/contaminated | 2 (2.1) | 34 (0.7) | 1 (2.8) | 35 (0.7) | 0 (0.0) | 36 (0.8) | |||
| 3. Contaminated | 1 (1.0) | 8 (0.2) | 0 (0.0) | 9 (0.2) | 0 (0.0) | 9 (0.2) | |||
| 4. Dirty/infected | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | |||
| Functional status | 4619 | .88 | .09 | .06 | |||||
| Independent | 95 (99.0) | 1 (0.0) | 36 (100) | 4678 (99.7) | 15 (100.0) | 4699 (99.7) | |||
| Partially/totally dependent | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | ||||
| Characteristic . | Any complication . | P-value . | Surgical complication . | P-value . | Medical complication . | P-value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 96) . | No (n = 4634) . | Yes (n = 36) . | No (n = 4694) . | Yes (n = 15) . | No (n = 4715) . | ||||
| Demographics | |||||||||
| Age (years) | 44 [14] | 40 [13] | .002 | 45 [15] | 40 [13] | .02 | 43 [12] | 40 [13] | .42 |
| BMI (kg/m2) | 27 [7] | 24 [4] | <.0001 | 32 [7] | 24 [4] | <.0001 | 24 [5] | 24 [5] | .83 |
| Race | .21 | .008 | .77 | ||||||
| American Indian/Alaskan native | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | 0 (0.0) | 12 (0.3) | |||
| Asian | 2 (2.1) | 126 (2.7) | 1 (2.8) | 127 (2.7) | 0 (0.0) | 128 (2.7) | |||
| Native Hawaiian/Pacific Islander | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.1) | |||
| Black/African American | 5 (5.2) | 195 (4.2) | 0 (0.0) | 200 (4.3) | 1 (6.7) | 199 (4.2) | |||
| White | 71 (74.0) | 3805 (82.1) | 24 (66.7) | 3852 (82.1) | 14 (93.3) | 3862 (81.9) | |||
| Other or unknown | 18 (18.8) | 493 (10.6) | 11 (30.6) | 500 (10.7) | 0 (0.0) | 511 (10.8) | |||
| Setting | 4543 (98.0) | <.0001 | .007 | .27 | |||||
| Outpatient | 86 (89.6) | 32 (88.9) | 4597 (97.9) | 14 (93.3) | 4615 (97.9) | ||||
| Inpatient | 10 (10.4) | 91 (2.0) | 4 (11.1) | 97 (2.1) | 1 (6.7) | 100 (2.1) | |||
| Preop health/comorbidities | |||||||||
| Diabetes | 4 (4.2) | 63 (1.4) | .04 | 3 (8.3) | 64 (1.4) | .01 | 0 (0.0) | 67 (1.4) | >.99 |
| Insulin-treated diabetes | 3 (3.1) | 20 (0.4) | .01 | 2 (5.5) | 21 (0.4) | .01 | 0 (0.0) | 23 (0.5) | >.99 |
| COPD | 2 (2.1) | 7 (0.2) | .01 | 2 (5.5) | 7 (0.1) | .002 | 0 (0.0) | 9 (0.2) | >.99 |
| Obesity | 30 (31.3) | 450 (9.7) | <.0001 | 21 (58.3) | 459 (9.8) | .03 | 1 (6.7) | 326 (6.9) | >.99 |
| Hypertension | 11 (11.5) | 316 (6.8) | .10 | 6 (16.7) | 321 (6.8) | .16 | 0 (0.0) | 22 (0.5) | >.99 |
| Dyspnea | 1 (1.0) | 21 (0.5) | .36 | 1 (2.8) | 21 (0.4) | >.99 | 0 (0.0) | 2 (0.0) | >.99 |
| CHF | 0 (0.0) | 2 (0.0) | .99 | 0 (0.0) | 2 (0.0) | <.0001 | 3 (20.0) | 477 (10.1) | .19 |
| Current smoker | 15 (15.6) | 490 (10.6) | .13 | 10 (27.8) | 495 (10.5) | .003 | 0 (0.0) | 505 (10.7) | .40 |
| Corticosteroid use | 0 (0.0) | 35 (0.8) | .99 | 0 (0.0) | 35 (0.7) | >.99 | 0 (0.0) | 35 (0.7) | >.99 |
| Wound infection | 0 (0.0) | 1 (0.0) | .99 | 0 (0.0) | 1 (0.0) | >.99 | 0 (0.0) | 1 (0.0) | .12 |
| ASA class | <.0001 | .84 | |||||||
| 1. No disturbance | 31 (32.3) | 2290 (49.4) | .001 | 8 (22.2) | 2313 (49.3) | 6 (40.0) | 2315 (49.1) | ||
| 2. Mild disturbance | 57 (59.4) | 2185 (47.2) | 22 (61.1) | 2220 (47.3) | 8 (53.3) | 2234 (47.4) | |||
| 3. Severe disturbance | 8 (8.3) | 152 (3.3) | 6 (16.7) | 154 (3.3) | 1 (6.7) | 159 (3.4) | |||
| 4. Life threatening | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | |||
| Wound class | .11 | .57 | .99 | ||||||
| 1. Clean | 93 (96.9) | 4591 (99.1) | 35 (97.2) | 4649 (99.0) | 15 (100.0) | 4669 (99.0) | |||
| 2. Clean/contaminated | 2 (2.1) | 34 (0.7) | 1 (2.8) | 35 (0.7) | 0 (0.0) | 36 (0.8) | |||
| 3. Contaminated | 1 (1.0) | 8 (0.2) | 0 (0.0) | 9 (0.2) | 0 (0.0) | 9 (0.2) | |||
| 4. Dirty/infected | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | |||
| Functional status | 4619 | .88 | .09 | .06 | |||||
| Independent | 95 (99.0) | 1 (0.0) | 36 (100) | 4678 (99.7) | 15 (100.0) | 4699 (99.7) | |||
| Partially/totally dependent | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.0) | ||||
Reported as mean [standard deviation] or n (%). ASA, American Society of Anesthesiology; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
Multivariable Assessment of Any, Surgical, or Medical Complication Occurrence for All Cosmetic Breast Surgery Patients
| Risk factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Any complications | |||
| COPD | 0.17 | 0.07-0.26 | .0005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.01-0.05 | .0003 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.14 | 0.10-0.19 | <.0001 |
| Inpatient | 0.06 | 0.03-0.09 | <.0001 |
| Surgical complications | |||
| Multiple procedures | 0.01 | 0.00-0.02 | .02 |
| COPD | 0.18 | 0.12-0.24 | <.0001 |
| Current smoker | 0.01 | 0.00-0.02 | .005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.02-0.04 | <.0001 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.13 | 0.10-0.16 | <.0001 |
| Discharge destination | |||
| Hypertension | −0.24 | −0.44-0.06 | .02 |
| Dyspnea | 0.71 | 0.04-1.38 | 0.03 |
| Underweight: BMI < 18.5 kg/m2 | −0.39 | −0.60-0.18 | .0004 |
| Normal weight: BMI = 18.5-24.9 kg/m2 | −0.23 | −0.33-0.12 | <.0001 |
| Obesity Class 2: BMI = 35-39.9 kg/m2 | 0.78 | 0.47-1.09 | <.0001 |
| Partial dependency | 4.36 | 1.34-7.39 | 0.005 |
| Inpatient | 1.50 | 1.19-1.81 | <.0001 |
| Risk factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Any complications | |||
| COPD | 0.17 | 0.07-0.26 | .0005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.01-0.05 | .0003 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.14 | 0.10-0.19 | <.0001 |
| Inpatient | 0.06 | 0.03-0.09 | <.0001 |
| Surgical complications | |||
| Multiple procedures | 0.01 | 0.00-0.02 | .02 |
| COPD | 0.18 | 0.12-0.24 | <.0001 |
| Current smoker | 0.01 | 0.00-0.02 | .005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.02-0.04 | <.0001 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.13 | 0.10-0.16 | <.0001 |
| Discharge destination | |||
| Hypertension | −0.24 | −0.44-0.06 | .02 |
| Dyspnea | 0.71 | 0.04-1.38 | 0.03 |
| Underweight: BMI < 18.5 kg/m2 | −0.39 | −0.60-0.18 | .0004 |
| Normal weight: BMI = 18.5-24.9 kg/m2 | −0.23 | −0.33-0.12 | <.0001 |
| Obesity Class 2: BMI = 35-39.9 kg/m2 | 0.78 | 0.47-1.09 | <.0001 |
| Partial dependency | 4.36 | 1.34-7.39 | 0.005 |
| Inpatient | 1.50 | 1.19-1.81 | <.0001 |
COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Multivariable Assessment of Any, Surgical, or Medical Complication Occurrence for All Cosmetic Breast Surgery Patients
| Risk factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Any complications | |||
| COPD | 0.17 | 0.07-0.26 | .0005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.01-0.05 | .0003 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.14 | 0.10-0.19 | <.0001 |
| Inpatient | 0.06 | 0.03-0.09 | <.0001 |
| Surgical complications | |||
| Multiple procedures | 0.01 | 0.00-0.02 | .02 |
| COPD | 0.18 | 0.12-0.24 | <.0001 |
| Current smoker | 0.01 | 0.00-0.02 | .005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.02-0.04 | <.0001 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.13 | 0.10-0.16 | <.0001 |
| Discharge destination | |||
| Hypertension | −0.24 | −0.44-0.06 | .02 |
| Dyspnea | 0.71 | 0.04-1.38 | 0.03 |
| Underweight: BMI < 18.5 kg/m2 | −0.39 | −0.60-0.18 | .0004 |
| Normal weight: BMI = 18.5-24.9 kg/m2 | −0.23 | −0.33-0.12 | <.0001 |
| Obesity Class 2: BMI = 35-39.9 kg/m2 | 0.78 | 0.47-1.09 | <.0001 |
| Partial dependency | 4.36 | 1.34-7.39 | 0.005 |
| Inpatient | 1.50 | 1.19-1.81 | <.0001 |
| Risk factors . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Any complications | |||
| COPD | 0.17 | 0.07-0.26 | .0005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.01-0.05 | .0003 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.14 | 0.10-0.19 | <.0001 |
| Inpatient | 0.06 | 0.03-0.09 | <.0001 |
| Surgical complications | |||
| Multiple procedures | 0.01 | 0.00-0.02 | .02 |
| COPD | 0.18 | 0.12-0.24 | <.0001 |
| Current smoker | 0.01 | 0.00-0.02 | .005 |
| Obesity Class 1: BMI = 30-34.9 kg/m2 | 0.03 | 0.02-0.04 | <.0001 |
| Extreme Obesity Class 3: BMI > 40 kg/m2 | 0.13 | 0.10-0.16 | <.0001 |
| Discharge destination | |||
| Hypertension | −0.24 | −0.44-0.06 | .02 |
| Dyspnea | 0.71 | 0.04-1.38 | 0.03 |
| Underweight: BMI < 18.5 kg/m2 | −0.39 | −0.60-0.18 | .0004 |
| Normal weight: BMI = 18.5-24.9 kg/m2 | −0.23 | −0.33-0.12 | <.0001 |
| Obesity Class 2: BMI = 35-39.9 kg/m2 | 0.78 | 0.47-1.09 | <.0001 |
| Partial dependency | 4.36 | 1.34-7.39 | 0.005 |
| Inpatient | 1.50 | 1.19-1.81 | <.0001 |
COPD, chronic obstructive pulmonary disease; OR, odds ratio.
To establish a correlation pattern between type of procedure performed and occurrence of any complication we first looked at the total number of complications occurred and found that the majority of complications were seen in patients undergoing augmentation, followed by patients undergoing reduction or cosmetic reconstruction (Figure 2A; Table 4). This was not surprising given that our cohort largely comprised of breast augmentation patients. We therefore proceeded to establish a complication occurrence rate (calculated as number of complications within a procedure category over total number of patients receiving that specific procedure). Comparison of complication occurrence rates between procedures identified that reduction procedures, when performed as isolated cases, that is without any other concurrent minor procedures such as implant removal, had significantly higher complication rates than isolated augmentation (P < .0001), isolated mastopexy (P = .03), isolated reconstruction (P = .001), and combined augmentation and mastopexy (P = .01) (Figure 2A; Table 4). Comparison of complication occurrence rates within procedures identified that isolated combined augmentation and mastopexy had a significantly lower rate than combined augmentation and mastopexy that was performed concurrently with a capsular procedure and an implant removal (P = .01). Likewise, isolated mastopexy was associated with a significantly lower rate of complications than mastopexy that was performed concurrently with an implant removal (P = .04) (Figure 2B; Table 4). Analysis of the complication rates within the reconstruction group identified that immediate autologous reconstruction was associated with the highest rates of complications, significantly higher than all other reconstruction groups (Figure 2B; Table 4). Of the 1140 reconstruction cases only 20 were autologous reconstruction, with only 3 immediate procedures. This may be due to the fact that surgeons are less likely to classify a reconstruction as cosmetic when it is an autologous reconstruction. Of the 3 patients undergoing immediate autologous reconstruction, 2 experienced complications, with 1 patient requiring reoperation and the other experiencing a urinary tract infection (Table 5).
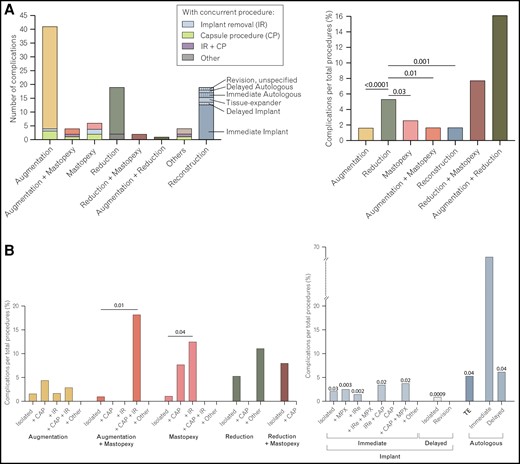
Complication occurrence in different cosmetic breast surgery procedures. (A) Comparison of complication occurrence between procedures. Comparison is presented as a total number of patients who experienced complications (left panel) and as a percentage of patients who experienced complications of the total number of procedures performed (right panel). The total number of isolated procedures per category was used as the total number of procedures performed. (B) Comparison of complication occurrence within procedures. The left panel displays the complication rates within the different procedures, when the procedures were isolated or performed in combination with other procedures. The right panel displays the complication rates within the reconstruction group. P-values displayed above each bar as compared to the immediate autologous group. The right panel displays the complication rates within the reconstruction group. P-values displayed above each bar as compared to the immediate autologous group. CAP, capsule procedure; IR, implant removal; IRe, implant replacement; MPX, mastopexy; TE, tissue expander.
DISCUSSION
Varying cross-cultural, personal, and medical variables mandate an individualized approach towards CBS in female patients.11 Big data databases such as the ACS-NSQIP database implement these variances and are considered helpful tools in identifying patterns to further improve clinical outcomes and refine patient selection.12 Thus, we accessed the ACS-NSQIP database and investigated predictive risk factors and (non)surgical complications, as well as 30-day postoperative outcomes of 4730 CBS cases performed in a hospital setting.
Outpatient CBS is of increasing popularity among plastic surgeons, with about 85% of breast reduction mammoplasties performed as an outpatient procedure.13 McManus et al outlined the potential cost savings of up to 78% in outpatient breast surgery.14 Margolese et al reported that outpatient breast surgery was associated with decreased postoperative psychological distress and reduced recovery times. Different reports concluded a comparable risk profile in inpatient vs outpatient procedure.15–17 Yet, these studies were mainly conducted at surgical centers and/or private practices. In agreement with previous research, we analyzed CBS cases performed in large academic centers and found a significantly lower risk for complications in outpatient procedures. In our study, inpatient procedures accounted for 10% of adverse events, while only 2.1% of total CBS procedures were performed in an inpatient setting. This is remarkable given that patients who undergo CBS in academic centers are commonly considered more complex cases and plastic surgeons at such centers are therefore more sensitized for perioperative complications.9 However, our analysis suggests that further efforts are needed to reduce the complication rate in inpatient CBS procedures. If performing inpatient CBS procedures, plastic surgeons may introduce a closer, more knitted perioperative patient monitoring to detect and treat adverse clinical conditions early on or even exclude high-risk patients in the preoperative planning phase. Yet, it is important to note that inpatient procedures are commonly considered a valuable treatment option when patients are particularly vulnerable and therefore more prone to complications. Thus, this finding remains to be corroborated in larger-scale studies.
Concomitant procedures in CBS commonly include combined augmentation-mastopexy and augmentation-reduction.18–20 Advantages to combined procedures include lower costs, patient convenience, a single recovery period, and increased patient satisfaction.21 For combined augmentation-reduction surgery, Manero et al reported comparable complication rates to single procedures. Yet, their study was conducted at a single private clinic and based on a single-surgeon experience.18 Stevens et al calculated similar adverse events rates when breast surgery was combined with abdominoplasty and/or facial surgery vs abdominoplasty alone.21 Again, their study was based on a single-surgeon experience. Work by Stevens et al concluded the combined augmentation-mastopexy approach to be a safe alternative to single procedures.22 In agreement with previous research we found no significant difference between isolated procedures and combined procedures. Patients undergoing isolated augmentation or isolated mastopexy were seen to have similar complication rates as patients undergoing combined augmentation and mastopexy, augmentation and reduction, or reduction and mastopexy.
In contrast, multivariable analysis of the 4730 CBS cases included in our cohort revealed a significantly increased surgical complication risk for multiple concurrent minor procedures. Specifically, combined augmentation and mastopexy that was performed concurrently with a capsular procedure and an implant removal was associated with a higher rate of complications than isolated combined augmentation and mastopexy. Furthermore, mastopexy performed concurrently with an implant removal was associated with a significantly higher rate of complications than isolated mastopexy. Interestingly, in a 2017 study, Gupta et al analyzed the occurrence of complications following cosmetic surgery performed in office-based surgical suites, ambulatory surgery centers, and hospitals and found that patients operated in office-based surgical suites were significantly less likely to undergo combined procedures compared to patients in ambulatory surgery centers and hospitals. Furthermore, they found that procedures in office-based surgical suites were associated with significantly lower complication rates than those in ambulatory centers or hospitals.23
Given the more challenging patient cases of CBS in a hospital setting, it is imperative to thoroughly weigh the potential benefits of concomitant surgeries in view of the increased complication risk. Due to the trend towards combining more and more procedures to realize patient demands for overall enhanced aesthetics after a single surgical intervention, it is even more important for plastic surgeons to refine their patient selection for concomitant procedures and identify risk candidates in the preoperative planning phase.
The various surgical approaches that fall under the CBS umbrella have been associated with different complications rates. For example, Sforza et al calculated a complication rate of 0.8% when analyzing 5813 breast augmentations, while a review of 8929 breast augmentations found complication rates of 1.7%.24–27 Focusing on augmentation cases, we calculated a comparable complication rate of 0.9% which is considered an extremely low incidence of adverse events.27 However, regarding urinary tract infections in our patient sample, 70% (n = 7) occurred in mammoplasty augmentation patients. In 2015, a consensus statement from the American Association of Plastic Surgeons recommended the administration of perioperative prophylaxis for patients undergoing CBS. Yet, the recommendations only target the risk reduction of surgical-site infections.28 Therefore, the risk of perioperative urinary tract infections in breast augmentation surgery is not equally addressed with the current antibiotic regimens. Plastic surgeons could consider providing reminder systems for controlling the urine status, as well as early postoperative removal of catheters to reduce the risk for urinary tract infection.29 Although our finding remains to be investigated in larger-scale studies, plastic surgeons should be aware of the possible link mammoplasty augmentations and urinary tract infections, since such infections have been shown to impair surgery outcomes.30
For breast reduction surgery, a wide range of complication rates is reported in the scientific literature. An analysis of 2492 breast reduction surgeries by Gust et al reported complication rates of 4.5%.31 The Breast Reduction Assessment: Value and Outcomes (BRAVO) study of 179 reduction cases calculated an incidence rate of adverse events of 43%.32 Setälä et al calculated incidence rates of 52% when reviewing 273 cases, while Shah et al reported complications in 23% of 306 patients.33,34 Winter et al attributed the wide range of complication rates to the lack of a standardized classification of complications.35 In our study sample, we found adverse events in 19 of 354 cases (5.4%) (Table 4; Figure 2A). The complication occurrence rate in patients undergoing a reduction procedure was found to be significantly higher than that in patients undergoing an augmentation, mastopexy, reconstruction, or combined augmentation and mastopexy. One possible reason (in combination with the increased breast size itself) for the elevated complication rates in breast reduction surgery might be the inverted T/keyhole incision pattern commonly used for reduction surgery of larger breasts.36 This incision technique can lead to the “bottoming out” sign (ie, a lengthening of the distance between the nipple and the inframammary fold) which hinders blood supply for the lower breast region and therefore increases the risk of postoperative complications. Hudson and Moodley have demonstrated the benefits of parenchymal sutures to reinforce the inframammary fold and reduce complications.37
Interestingly, although patients undergoing reconstruction tend to have a more complicated medical history, for example, previous treatment with radiotherapy or chemotherapy, the complication rates in this cohort were not significantly higher than the rates in the other procedure groups. Therefore, although these patients are classically considered more high risk, the complication rates of reconstruction were similar to those for nonreconstructive augmentation. Furthermore, reconstruction was shown to have lower complication rates than concomitant reduction and mastopexy or concomitant augmentation and reduction, as well as significantly lower rates of complications than reduction. It is noteworthy that 2 of the 3 patients undergoing immediate autologous reconstruction experienced complications. This is in agreement with previous research. In a 2017 prospective, multicenter study, Yoon et al compared complications between immediate and delayed breast reconstruction and found that the immediate cohort had significantly higher failure rates, while delayed reconstruction was associated with lower rates of any and major complications. Furthermore, the group identified that the delayed autologous patients experienced lower rates of complications compared with immediate autologous patients, a phenomenon they did not note for implant patients.38
Surgical site infections represent a tremendous burden to the healthcare system and amount to additional hospital costs exceeding US$900 million.39 The increased overall surgical-site morbidity in breast reduction has led to the development of the Baltodano Breast Reduction Score, a validated risk stratification tool.40 However, our study is the first to pinpoint the surgical-site morbidity since we found 50% of superficial incisional infections (11/22) to occur in breast reductions, which as a cohort only accounted for 7.5% of all CBS cases included. Superficial incisional infections are considered entry ports for bacteria such as Staphylococcus aureus, which results in severe wound infections and impaired surgical outcomes.41 This finding carries clinical translational potential, as plastic surgeons might pay increased attention to keystones of operating room hygiene and consider adapted surgical techniques for wound closure in breast reduction surgery. Both approaches have been shown to reduce the incidence of surgical site infections.41
In most cases, CBS is performed in nonacademic settings. Outcome research on risk factors and complication rates in CBS is commonly derived from retrospective analyses of single-institution, single-surgeon, or technique-specific medical records. Such curtailment may reduce research transferability and significance. Additionally, cosmetic-aesthetic surgery in particular must meet a broad spectrum of customized patient needs and preferences, with significant interindividual variability. By pooling patient data with geographic and institutional diversity, the analysis of the multicenter national ACS-NSQIP database can provide more robust risk factors and complication rates in CBS patients. Thus, the findings presented herein may help aesthetic surgeons to refine their patient counseling, optimize their perioperative workflow, and upgrade their risk-assessment repertoire.
LIMITATIONS
In this study we used a multi-institutional database to analyze a large, diverse cohort of CBS patients, but its limitations should be considered. The limitations can be broadly divided into 2 types: general and procedure-specific. General limitations associated with the NSQIP database have been previously extensively reported and include the database’s retrospective nature which is associated with inherent biases and confounders. In addition, the subjective nature of the data input, ie, the quality and accuracy of the data, depends on the individual filling in the data, and, therefore, depends on a subject's experience, knowledge, and skill.42 The national extent of the database has also been reported as a possible source of bias, as variations in quality may exist between, as well as within, institutions. Previous research has, however, identified low variance in the database's heterogeneity.43
Procedure-specific limitations are mainly due to the database's standardized nature of data collection which result in a lack of procedure-specific data. As such, the database lacks information on short-term (<30 days) procedure-specific outcomes such as hematoma, seroma, and excessive edema, and long-term (>30 days) procedure-specific outcomes such as contraction and flap or prosthesis failure. Satisfaction, a highly relevant variable in CBS, both in terms of patient and surgeon, is also not reported. The NSQIP’s lack of data on patient medical history, particularly in terms of previous chemotherapy or radiotherapy in the reconstructive cases, is a significant limitation of the database, and, hence, of our study. The long-term success of CBS depends on both aesthetic factors, such as presence and appearance of scarring, including scarring pigmentation, and breast symmetry, and functional factors, such as pain and sensation. Such outcomes are not reported on the database.44 Furthermore, some CBS experts may consider reconstructive breast surgery as not cosmetic. To provide a panoramic view on CBS, we included any reconstructive breast surgery that was coded as cosmetic on the database. It is, however, unclear why certain reconstructive cases are coded as cosmetic in NSQIP institutions, whereas others are not.
CONCLUSIONS
Our analysis of 4730 female CBS cases confirmed the positive safety profile of CBS performed in a hospital setting. We found inpatient CBSs and concomitant procedures to be predictive of perioperative complications. By implementing these points in their preoperative patient evaluation, plastic surgeons could refine their patient selection and identify risk cases.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Plastic Surgery Statistics Report 2020. Accessed November 5, 2022. https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
Author notes
Mr S Knoedler is a medical student, Division of Plastic Surgery, Department of Surgery, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Dr Kauke-Navarro is a plastic surgery resident, Department of Surgery, Division of Plastic Surgery, Yale School of Medicine, New Haven, CT, USA.
Dr Haug is a plastic surgery resident, Department of Hand, Plastic and Reconstructive Surgery, Microsurgery, Burn Trauma Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwigshafen, Germany.
Dr Broer is a professor, Department of Plastic, Reconstructive, Hand and Burn Surgery, Bogenhausen Academic Teaching Hospital Munich, Munich, Germany.
Dr Pomahac is a professor, Department of Surgery, Division of Plastic Surgery, Yale School of Medicine, New Haven, CT, USA.
Mr L Knoedler is a medical student, Division of Plastic and Reconstructive Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Dr Panayi is an instructor, Division of Plastic Surgery, Department of Surgery, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
- obesity
- smoking
- body mass index procedure
- diabetes mellitus
- chronic obstructive airway disease
- congestive heart failure
- dehiscence
- surgical complications
- anesthesiology
- inpatients
- patient readmission
- perioperative care
- reconstructive surgical procedures
- repeat surgery
- safety
- surgical procedures, operative
- infections
- mortality
- protective factors
- mastopexy
- breast surgery
- american college of surgeons
- surgical outcome
- national surgical quality improvement program



