-
PDF
- Split View
-
Views
-
Cite
Cite
Greg Bran, Maarten Van Genechten, Surgical Anatomy of the Ligamentous Attachments in the Superoposterior Scalp Region, Aesthetic Surgery Journal, Volume 43, Issue 11, November 2023, Pages NP825–NP831, https://doi.org/10.1093/asj/sjad249
Close - Share Icon Share
Abstract
Hairline-lowering surgery has become increasingly popular in recent years, but little investigation into the surgical anatomy of the scalp has been performed.
The aim of this study was to describe findings based on clinical observation and anatomic study of ligamentous attachments in the superoposterior region of the scalp.
Six fresh cadaveric heads were dissected to identify connective tissue structures in the superoposterior scalp region. The areas of interest were along the sagittal suture towards the lambda, the obelion, and around the lambdoid suture. The location and dimensions of identified connective tissue attachments were documented with reference to described skeletal landmarks.
Three distinct structures could be identified: (1) a cylindrical structure that sits at the posterior end of the sagittal suture with the parietal foramina in its base. This ligamentous structure extends from the pericranium into the galea, causes dimpling in the skin, and contains emissary veins. As this fulfills the criteria for an osseocutaneous retaining ligament, the term “cranial retaining ligament” is proposed. (2) Anterior to this ligament a connective tissue thickening was identified running along the sagittal suture and blending into the ligament, for which the term “sagittal adhesion” is proposed. (3) Another adhesion was identified just superior to the lambdoid suture, posterior to the retaining ligament, for which the term “supralambdoid adhesion” is proposed.
Identification and better understanding of ligamentous structures in the superoposterior scalp allows for a safer and more effective advancement of the scalp in hairline-lowering surgery, which is a benefit to both patients and surgeons.
See the Commentary on this article here.
More than 30 years have elapsed since the original descriptions of the retaining ligaments that fix facial soft tissue to key bony landmarks.1,2 The term “retaining ligament” was originally introduced by Furnas when describing such structures in the cheek.1 Knowledge of these facial structures is widely considered to be pivotal for a better understanding of the dynamic processes of facial aging and hence for facial rejuvenation surgery. The main significance of these consistent anatomic structures relates to the surgical release of these ligaments to eliminate restraining effects on the facial soft tissues. Complete release of these retaining ligaments achieves passive repositioning of the facial tissues, thereby creating the desired aesthetic outcome.1-4
The purpose of this article was to describe a connective tissue thickening in the superoposterior region of the scalp that has been identified consistently during hairline-lowering (HALO) surgery and was further investigated in anatomic dissections.
Retaining Ligaments of the Face
The retaining ligaments of the face form a network of robust fibrous attachments originating from the periosteum or deep facial fascia, and penetrate perpendicularly through all facial layers, inserting directly into the dermis.1-3 The facial layers are: (1) skin, (2) subcutaneous layer, (3) musculoaponeurotic, (4) subaponeurotic, containing ligaments and soft tissue spaces, and (5) deep fascia (periosteum on the facial skeleton and deep muscle fascia on the deep temporal and the parotid masseteric). Microscopically, each ligament has a tree-like distribution with a root-like thickening in Layer 5, that divides as it approaches the superficial musculoaponeurotic system (SMAS) (Layer 3) into a branching network which inserts into the dermis (described as retinacular cutis).5
Stuzin et al classified these facial ligaments as: (1) osseocutaneous ligaments (eg, zygomatic ligament) originating from Layer 5; or (2) fasciocutaneous ligaments (eg, masseteric ligament), which coalesce between the deep and the superficial fascias of the face.2,6 The discussion about what defines a true retaining ligament vs connective tissue thickenings identified as septa or adhesions was introduced by Knize and Moss et al.7,8 For matters of consistency, we refer to the following definitions of these different types of connective tissue thickenings as presented by Moss et al in their study of the anatomy of the temporal and periorbital regions: “true ligaments,” arrangements of fibrous tissue arising from the deep fascia or periosteum, crossing the sub-SMAS plane to the undersurface of the SMAS, where they divide into numerous branches that form attachments to the dermis; “septum,” a fibrous linear, membranous structure passing between the deep fascia/pericranium and the undersurface of the SMAS; “adhesion,” a low-density area of fibrous adhesion with a broader base between the deep fascia/pericranium and the superficial fascia.7,8
METHODS
Six cadaveric heads were dissected in an anatomic laboratory to identify connective tissue structures between the forehead and the neck along the area of the scalp. All dissections were performed on fresh cadavers of normal body mass. Dissections took place on February 26, 2023. Formalinized cadavers were excluded because the structures of interest would have been distorted. Dissection was performed under 2.5× loupe magnification according to a standardized technique. The incision commenced along the anterior hairline, with anteroposterior extension lateral to the temporal lines and interconnecting posteriorly along the posterior hairline in the region of the neck. Dissection started along the forehead and was performed bluntly in an anteroposterior vector in the subgaleal plane. Loose areolar tissue was gently dissected away, identifying the superior temporal septum bilaterally. Any dense connective tissue attachments between the deep and superficial tissues were preserved and further examined. The areas of interest were along the sagittal suture towards the lambda (the confluence of the sagittal suture with the lambdoid suture), the obelion (an area cranial to the confluence of both sutures), and around the area of the lambdoid suture. The precise location and dimension of identified connective tissue attachments were documented with reference to described skeletal landmarks.
RESULTS
Cranial Retaining Ligament
A continuous layer of superficial fascia comprises the galea occipitofrontalis on the scalp and the SMAS in the midface.8 The subgaleal plane contains mainly loose areolar tissue and can therefore be easily developed. The release of the superior temporal septum can equally be facilitated easily using blunt finger dissection. The swift development of the subgaleal plane is, however, restricted in all specimens in the area of the obelion. The obelion is a region of the skull referred to as a landmark along the posterior segment of the sagittal suture intersected by an imaginary line between the parietal foramina. Within this region a strong cylindrical structure can be identified, which is consistent in all cases, and can only be released by sharp dissection. The base of this ligament presents circular in shape with a diameter varying between 25 and 40 mm, being greater in men than in women (Figure 1). This ligamentous structure originates from the pericranium and inserts into the galea (Figure 2). It is robust enough to lift the entire head of a specimen with an otherwise fully released scalp flap in Layer 4 (video). Furthermore, we can observe restrictive dimpling in the skin when the scalp flap is lifted. Sharp dissection through the ligament allows identification of blood vessels/vascular structures in some specimens (Figure 3) with the parietal foramina presenting at its base (Figure 4). These vessels are emissary veins, which drain into the superior sagittal sinus. The combination of a consistent location, periosteal origin, cylindrical arrangement, vascular passage, and what seems to be dermal infiltration constitutes sufficient anatomic information to classify this structure as an osseocutaneous retaining ligament. Given its location, we propose naming this structure the “cranial retaining ligament” (CRL) (Figure 5).
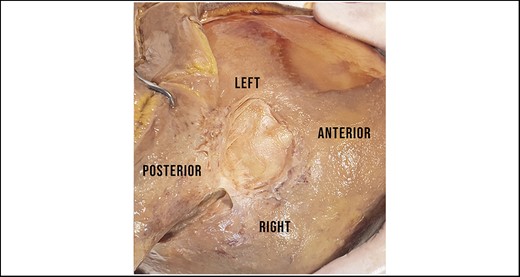
Identification of the strong ligamentous structure, which we suggest naming the “cranial retaining ligament.” Subgaleal dissection within this area always required sharp dissection. The base is circular in shape.
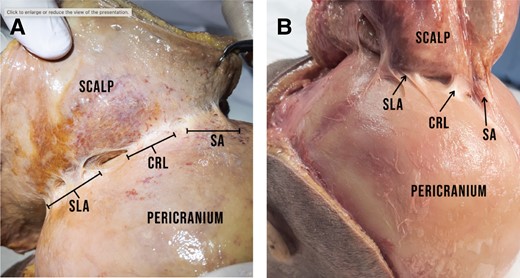
(A) The CRL originates from the pericranium and inserts into the galea. The photographs show 2 different specimens with the SA running anterior to the CRL. (B) Posterior to the CRL, the SLA can be seen. CRL, cranial retaining ligament; SA, sagittal adhesion; SLA, supralambdoid adhesion.
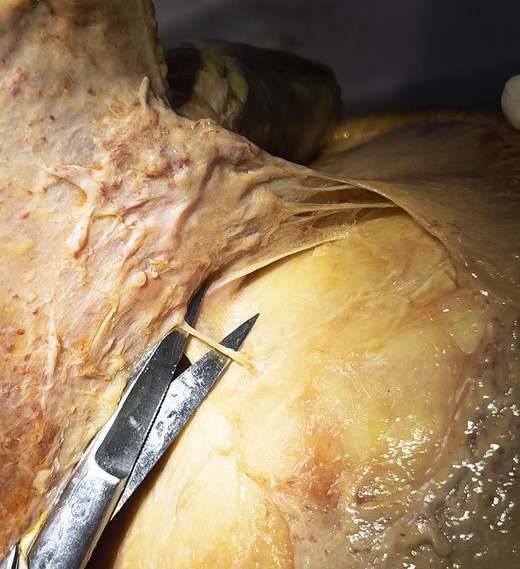
Identification of an emissary vein after full release of the pericranium at the base of the cranial retaining ligament (note the circular dissection through the periost).
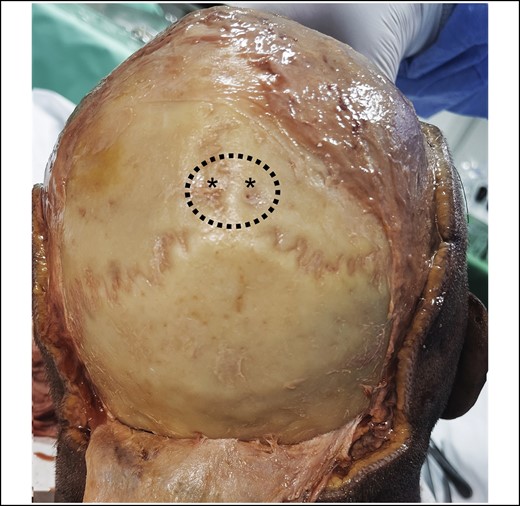
View on the posterior skull after full release of the entire pericranium. The dotted line represents the cylindrical base of the cranial retaining ligament. Note the parietal foramina (marked with asterisks) in the center of the base of the ligament. These are the exit points for the emissary veins, which are running through the cranial retaining ligament.
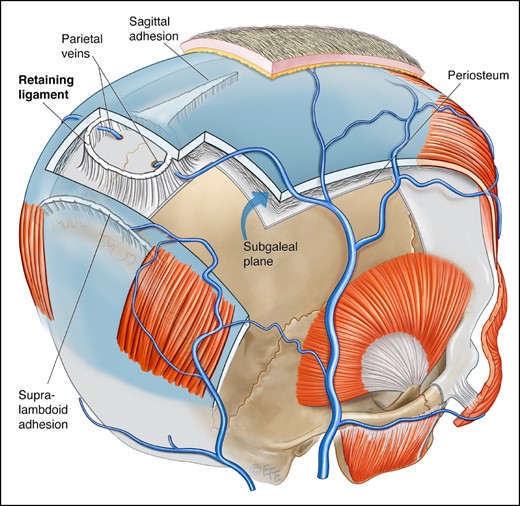
Schematic overview of the shape and localization of the described connective tissue thickenings. The emissary veins exit the parietal foramina in the base of the cranial retaining ligament. The saggital adhesion broadens in width as it is approaching the cranial retaining ligament. The supralambdoid adhesion is running posterocaudal to the retaining ligament. In some specimens a gap could be identified between the ligament and this adhesion; in other specimens there was a bridge of loose areolar tissue. Artwork created by and published with permission from Dr Levent Efe, CMI.
Sagittal Adhesion
Anterior to the CRL, most specimens show another connective tissue thickening. Although significantly less resistant to division than the CRL, this connective tissue thickening shows greater density than the surrounding loose areolar tissue. It can also be released bluntly. It runs along the superoposterior course of the sagittal suture towards the CRL. At its anterior beginning it is relatively narrow (approximately 5-12 mm in width) and broadens as it approximates the CRL posteriorly. Due to its course along the saggital suture we suggest naming this structure the “sagittal adhesion” (SA) (Figures 5, 6).
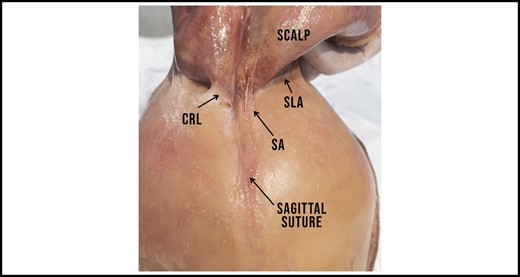
The SA starts in the vertex and runs across the saggital suture towards the CRL. Note how the adhesion gains in width along its superoposterior course. The SLA can be seen bilaterally, posterior to the CRL. CRL, cranial retaining ligament; SA, sagittal adhesion; SLA, supralambdoid adhesion.
The SA varies in size and density throughout the specimens but can be found consistently in every specimen. The SA transitions into the strong base of the CRL and hence does not allow for any further finger-assisted blunt dissection. The existence of the SA varies from individual to individual: in some cases it can be hardly unnoticeable, whereas in others it can present as a strong connection between pericranium and galea.
Supralambdoid Adhesion
Caudal to the CRL runs the lamboid suture in the posterior segment of the skull. We are able to identify an additional consistent connective tissue thickening that runs superior to the course of the lambdoid suture in an inverted-V shape. It forms a sheath of dense connective tissue between the pericranium and the galea. The width of the pericranial base of this connective tissue thickening varies between 4 and 12 mm. The density of this structure is comparable to the density of the SA. Given its position, we suggest naming it the “supralambdoid adhesion” (SLA) (Figure 7A, B). This adhesion gets wider as it approaches the midline, however—unlike the SA—it is not confluent with the CRL. In most cases a gap of approximately 15 to 25 mm can be identified between the posterior caudal segment of the CRL and the medial segment of the SLA. This gap is bridged with loose areolar tissue. In other cases the SLA runs close to the caudal segment of the CRL. However, we were unable to identify a confluence in any dissection.
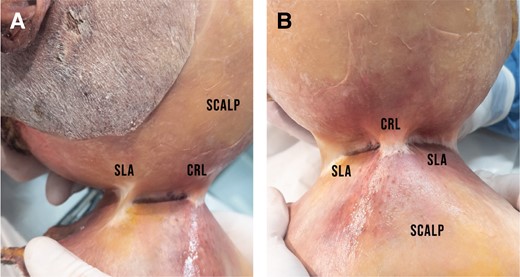
(A) View from the left side of the specimen showing the SLA posterior to the CRL. Just like the CRL, this connective tissue thickening originates from the pericranium and inserts into the galea. The SLA is less dense than the CRL and can be released by blunt dissection, whereas the CRL requires sharp release. (B) Same specimen, anterior view. The CRL is positioned superoanterior to the SLA which runs posterolaterally. CRL, cranial retaining ligament; SLA, supralambdoid adhesion.
Clinical Significance
The arrangement of the ligamentous fixation between the pericranium and the underside of the galea provides landmark information for the surgeon operating in this area. The CRL is a consistent zone of fixation at the posterior end of the sagittal suture.
Over the past 3 years (February 2021 to March 2023) the first author has performed 206 HALO surgeries for aesthetic reasons to shorten the vertical distance of overly high foreheads. The main factor for significant advancement during HALO surgery is scalp laxity. The latter depends on the connective tissue properties of the patient scalp; these tissues include the areolar tissue and the ligamentous attachments in Layer 4, between the pericranium and the galea. Individuals with a very high forehead require a full release of all the aforementioned structures. In many cases we encountered a condensation of connective tissue along the posterior course of the sagittal suture which could always be bluntly released. The further posterior we dissected, the broader this adhesion became. In every case which required more than average development of a scalp flap, we encountered a strong ligamentous attachment, the location of which correlated with the area of the obelion. This connective tissue thickening could only be released by sharp dissection (using sharp, curved Ramirez dissectors or a Metzenbaum). Based on clinical observations, venous bleeding could be observed in some cases after full release of the CRL. Bleeding stopped after compression with a gauze for few minutes. Venous bleeding did not occur in every case. Our clinical observations correlate with the anatomic findings in this study, as we were not able to identify emissary veins in each specimen.
Posterolateral to the CRL, further connective tissue attachments were identified, which could be released bluntly using a Jankauer suction tip. Clinical assessment of scalp laxity in bald men allowed us to observe dimpling in the area of the CRL when the scalp is being moved. This observation appears to support the existence of dermal attachments of the CRL through a subcutaneous fascial system equivalent to the retinacula cutis.3,5,8
To our knowledge these ligamentous attachments in the posterior scalp have not previously been described in detail. The only reference we were able to find was made by Epstein when presenting his surgical technique for HALO surgery: “Tumescence also facilitates dissection through and reduces bleeding from the fibrous bands connecting the galea to the periosteum that are located at the posterior.”9
DISCUSSION
An extensive amount of literature has been published defining the surgical anatomy of the zygomatic cutaneous ligaments when evaluating extended SMAS or sub-SMAS facelift techniques.2-4,10,11 The zygomatic cutaneous ligaments are described as fibrous septa along the inferior border of the posterior zygomatic arch, thickening into a strong, cylindrical structure at the body of the zygoma in close proximity to the zygomaticus major muscle.1,4,6,11 A transition zone between different connective tissue thickenings was also observed in our anatomic study. The weaker anteriorly positioned SA broadens before transitioning into the strong CRL.
The zygomatic retaining ligament is defined as an osseocutaneous ligament with attachments extending directly into the dermis. This manifests as skin dimpling when the skin flap is being elevated during facelift surgery.1,6,12 We were able to make the same observation during the dissection of the CRL, which suggests the presence of retinacular cutis within Layer 1 of this region. However, microscopic analysis will be required for histological proof of this clinical observation.
Retaining ligaments are known to provide guidance for the passage of neurovascular structures from deeper layers to more superficial layers. Facial nerves and perforator vessels can be identified in the zygomatic retaining ligament.1,8 In our dissection we were able to identify emissary veins running from the parietal foramina to more superficial, supragaleal layers. The presence of vascular structures within this connective tissue thickening further supports the classification of this deep attachment as a true retaining ligament. The presence of the parietal foramina within the base of the CRL also emphasizes the proximity of the ligament to the bone. During our dissection we were able to observe that the base of the ligament originates from the pericranium.
It is worth mentioning that we did not identify any nerves passing along the course of the CRL.
Just like in deep plane facelift surgery, complete release of all ligamentous attachments in the superoposterior scalp region allows a greater advancement of the anterior hairline. A current clinical study is underway to quantify the impact of the CRL on HALO surgery by measuring the maximum anterior advancement of the scalp flap before and after full release of mentioned structure.
We need to acknowledge that the number of specimens in the current study was relatively small. However, we were able to identify all anatomic structures consistently in each specimen. A greater spectrum of dissections would enhance our understanding of anatomic variations in terms of dimensions and strength of the connective tissues, as well as differences in size of the parietal foramina.
Future Studies
Grodinsky and Holyoke established the gold standard for investigating fascias. For a surgeon transitioning from the operating theatre to the anatomy laboratory, it appears to be natural to use established surgical techniques for dissection in multiple angles and planes to accumulate data.13 However, we consider this to be the first level of investigation, which needs to be followed by further anatomic research in concert with dye injection, arterial/venous/lymphatic injection, and histological analysis.14 The close proximity of facial nerve branches next to facial retaining cutaneous ligaments has been well documented, and even though no motoric equivalent was observed in our anatomic research, we could prove vascular existence within the described ligament. Understanding vascularization and the biomechanical features of the various described connective tissue thickenings will result in greater intraoperative safety as well as greater scalp mobilization. We hope that other surgeons and anatomists will be inspired to join forces in this endeavor. Similarly to the publications on well-established retaining ligaments, controversy will likely also arise about the CRL and the surrounding adhesions. However, we believe that it is only through such a focused exchange of thoughts that true knowledge can be formed, which will serve surgeons to create better and safer results for their patients, following the principle we all abide to: primum nil nocere.
CONCLUSIONS
We were able to identify a consistent, strong connective tissue thickening which provides structural guidance for emissary veins, originating from the pericranium and most likely inserting into the dermis. To our knowledge this cranial structure has not been previously examined or described as a true retaining ligament.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Acknowledgments
The authors would like to express their gratitude to Dr Olivier Beckers at the University of Hasselt, Belgium, for his valuable research assistance.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Dr Bran is a facial plastic surgeon in private practice in London, UK.
Dr Van Genechten is a facial plastic surgeon in private practice in Antwerp, Belgium.



