-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel Golpanian, George A Rahal, William J Rahal, Outpatient-Based High-Volume Liposuction: A Retrospective Review of 310 Consecutive Patients, Aesthetic Surgery Journal, Volume 43, Issue 11, November 2023, Pages 1310–1324, https://doi.org/10.1093/asj/sjad164
Close - Share Icon Share
Abstract
Currently, the definition of large-volume liposuction is the removal of 5 L or more of total aspirate. Higher volumes of lipoaspirate come into consideration with higher BMIs, because more than 5 L is often required to achieve a satisfactory aesthetic result. The boundaries of what lipoaspirate volume is considered safe are based on historical opinion and are constantly in question.
Because to date there have been no scientific data available to support a specific safe maximum volume of lipoaspirate, the authors discuss necessary conditions for safe high-volume lipoaspirate extraction.
This retrospective study included 310 patients who had liposuction of ≥5 L over a 30-month period. All patients had 360° liposuction alone or in combination with other procedures.
Patient ages ranged from 20 to 66 with a mean age of 38.5 (SD = 9.3). Average operative time was 202 minutes (SD = 83.1). Mean total aspirate was 7.5 L (SD = 1.9). An average of 1.84 L (SD = 0.69) of intravenous fluids and 8.99 L (SD = 1.47) of tumescent fluid were administered. Urine output was maintained above 0.5 mL/kg/hr. There were no major cardiopulmonary complications or cases requiring blood transfusion.
High-volume liposuction is safe if proper preoperative, intraoperative, and postoperative protocols and techniques are employed. The authors believe that this bias should be modified and that sharing their experience with high-volume liposuction may help guide other surgeons to incorporate this practice with confidence and safety for better patient outcomes.

The practice of liposuction among plastic surgeons has evolved immensely over the last 40 years. Liposuction, once aimed at correcting contour irregularities, has developed to encompass the treatment of larger and multiple areas of the body. As such, today it is often considered a major surgery. This is in large part due to a better understanding of the physiologic changes after liposuction as well as improved surgical technique. Not only has it progressed to being performed as an adjunct in combination procedures, but the extent of liposuction being completed in 1 setting has increased.
Currently, the definition of large-volume liposuction is the removal of 5 L or more of total aspirate in a single stage.1 Higher volumes of lipoaspirate come into consideration with higher-BMI patients, for whom more than 5 L is often required to achieve a satisfactory aesthetic result. The boundaries of the lipoaspirate volume considered to be safe are based on historical opinion and constantly in question.2 Some believe that serial staging of large-volume aspirations is safer.3 To date, there have been no scientific data available to support a specific maximum volume at which liposuction is no longer safe.1,2,4–6 Even without supporting data, high-volume liposuction has been spurned to lesser or greater degrees by the medical community. This spurning has been further compounded by the limits imposed by regulatory bodies, including The Aesthetic Society and the American Society of Plastic Surgeons (ASPS)—limits that the authors believe should be reconsidered.
Advancements in surgical care, technique, and equipment have allowed for these higher-aspirate volumes to be safely removed. It is paramount to have a comprehensive understanding of all perioperative factors that can contribute to increased complications and to control for these. In this article we discuss conditions for safe high-volume lipoaspirate extraction; including surgical technique and technology as well as perioperative care. Additionally, we discuss how high-volume liposuction came to be deemed generally unsafe or less safe, and why the authors believe that this bias should be modified. The authors will share their experience with high-volume liposuction to guide other surgeons in incorporating this practice with confidence and safety to achieve better patient outcomes.
METHODS
This single-surgeon retrospective study included patients who had liposuction from April 2019 to October 2021. Over this 30-month period, 586 cases were performed. Of these cases, 310 patients who had liposuction procedures with greater than 5 L of total aspirate were reviewed.
All patients in this study had liposuction under general anesthesia in 1 of the following situations: (1) 360° liposuction only; (2) 360° liposuction with gluteal fat transfer; (3) 360° liposuction with gluteal fat transfer and abdominoplasty; (5) 360° liposuction and liposuction of extremities (ie, arms/thighs), and/or neck; (6) 360° liposuction and breast procedure, such as a breast augmentation or mastopexy or augmentation/mastopexy. The term “360° liposuction” is defined as the liposuction of 8 areas bilaterally: the abdomen, mons pubis, bra line, upper back, middle back, waist/flank, presacral triangle, and hip roll (Figure 1). All patients obtained medical risk assessment before surgery. Written consent was provided, by which the patients agreed to the use and analysis of their data. Additionally, the World Medical Association's Declaration of Helsinki was followed.
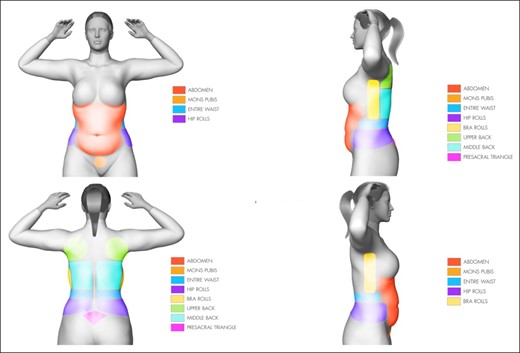
Treatment with 360° liposuction includes the following 8 areas: the abdomen, mons pubis, waist/flanks, hip rolls, bra rolls, upper back, middle back, and presacral triangle.
Preoperatively, patients were marked while standing. All patients underwent general anesthesia, had a Foley catheter placed, and had noninvasive blood pressure monitoring. Vital signs and urine output were continuously evaluated intraoperatively. Intraoperative fluid administration was given at the discretion of the surgeon and anesthesiologist based on vital hemodynamics and urine output. Specifically, it was limited to maintain a urine output of 0.5 per kg per hour. Conductive fabric warming was set at 43°C, employing the HotDog Patient Warming System (Augustine Temperature Management, LLC; Eden Prairie, MN). During surgery, the patient's body temperature was measured every 30 minutes. Patients were given a dose of weight-based preoperative antibiotics. Additionally, 1 g of intravenous tranexamic acid was administered.
Patients were initially supine, and then placed in each lateral position; the prone position was not employed. Tumescent solution consisted of 10 mL of 1% lidocaine and 2 mg of epinephrine (1:1000) added to 1 L of lactated Ringer solution, resulting in a 1:500,000 epinephrine solution.6,7 Solutions were warmed to 38°C with the ivNow fluid warmer (Enthermics Medical Systems; Menomonee Falls, WI). A superwet technique was utilized, and in some cases a tumescent technique was employed, with a 4-mm “exploded tip” cannula, utilizing the simultaneous separation and tumescence (SST) method.8 All patients underwent liposuction of at least 5000 mL total aspirate. Infiltration and liposuction were performed in the deep and superficial subcutaneous layers, and determination of the surgical endpoint was made with a “pinch-test” (1-1.5 cm) and visual comparison of symmetry. All liposuction was performed with power-assisted liposuction.
All cases were performed in an outpatient facility accredited by the American Association for Accreditation of Ambulatory Surgery Facilities (AAAASF). Postoperatively, patients were observed in the postanesthesia care unit (PACU) and then moved to an aftercare facility, which provided nurse monitoring for an additional 23-hour period once appropriate PACU criteria were met. Patients were followed up 5 to 7 days postoperatively and as long as 1 year postsurgery.
Statistical Methods
Demographic characteristics were expressed as mean and standard deviation (SD). A P value of less than 0.05 was considered statistically significant. All of the results for clinical and operative data comparisons were reported as mean and SD with statistical analysis utilizing chi-square and the t test. Statistical analyses were performed with the IBM Statistical Package for the Social Sciences (IBM SPSS 25.0; Armonk, NY).
RESULTS
A total of 310 patients underwent high-volume liposuction or extraction of ≥5 L of total aspirate within the study period. Patient ages ranged from 20 to 66 with a mean age of 38.5 (SD = 9.3). Females comprised 98.3% of the cohort, and 0.7% were males. Two hundred thirty-one patients were nonsmokers or former smokers for greater than 6 weeks. Only 4 patients had an American Society of Anesthesiologists (ASA) class of 3; the remainder of the cohort were either ASA class 1 or class 2.
Approximately two-thirds of the cohort did not have any comorbidities, and the remaining patients had either hypertension, type 2 diabetes, thyroid disease, or other abnormalities (Figure 2). Regardless of associated medical problems, all patients underwent appropriate medical risk assessment and optimization before surgery.
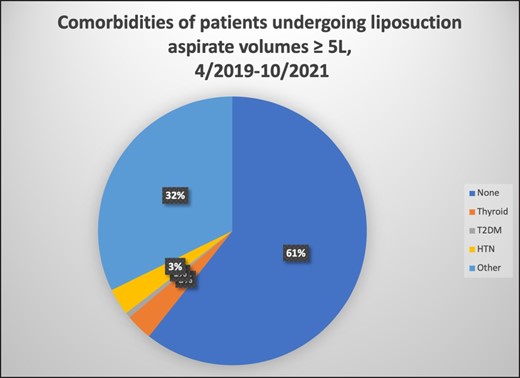
Patient comorbidities. HTN, hypertension; T2DM, type 2 diabetes mellitus.
All patients had fair to good skin tone and moderate to severe lipodystrophy at the surgical site. Mean BMI was 29.3 (SD = 4.7), and the majority of the cohort was in the 25 to 30 and 30 to 35 BMI groups (68.9%). The average operative time was 202 minutes (SD = 83.1). Mean total aspirate was 7.5 L (SD = 1.9) with a direct correlation to an increase in BMI (see Table 1). An average of 1.84 L (SD = 0.69) of intravenous fluids and 8.99 L (SD = 1.47) of tumescent fluid were administered. Urine output was carefully monitored and maintained at a mean value of 448 mL and above 0.5 mL/kg/hr.
| BMI . | Patients (n) . | Aspirate, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 6.357 | 1.400 |
| 25-30 | 136 | 6.794 | 1.222 |
| 30-35 | 67 | 8.607 | 1.769 |
| 35-40 | 36 | 9.269 | 1.978 |
| 40-45 | 6 | 10.783 | 1.278 |
| BMI . | Patients (n) . | Aspirate, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 6.357 | 1.400 |
| 25-30 | 136 | 6.794 | 1.222 |
| 30-35 | 67 | 8.607 | 1.769 |
| 35-40 | 36 | 9.269 | 1.978 |
| 40-45 | 6 | 10.783 | 1.278 |
No significant differences (P < .05).
| BMI . | Patients (n) . | Aspirate, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 6.357 | 1.400 |
| 25-30 | 136 | 6.794 | 1.222 |
| 30-35 | 67 | 8.607 | 1.769 |
| 35-40 | 36 | 9.269 | 1.978 |
| 40-45 | 6 | 10.783 | 1.278 |
| BMI . | Patients (n) . | Aspirate, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 6.357 | 1.400 |
| 25-30 | 136 | 6.794 | 1.222 |
| 30-35 | 67 | 8.607 | 1.769 |
| 35-40 | 36 | 9.269 | 1.978 |
| 40-45 | 6 | 10.783 | 1.278 |
No significant differences (P < .05).
Two patients (0.6%) were sent to the emergency room (for pain control and monitoring) and were later discharged with no need for admission. There were no major cardiopulmonary complications, including any myocardial infarction, cardiac failure, pulmonary edema, or venous thromboembolism. There were no cases requiring blood transfusion. The average follow-up time was at 13 weeks and follow-up ranged from 5 days to 12 months.
DISCUSSION
It is worth better understanding the reasons for marginalization of high-volume liposuction. Several highly publicized deaths in the office-based operating room setting, attributed to bleeding and prolonged operative times, resulted in ASPS patient safety committee guidelines in response to increased state regulatory awareness.9–11 The ASPS recommended that “regardless of the anesthetic route, large volume liposuction removal (greater than 5000 mL of total aspirate during a single procedure) should be performed in an acute care hospital or in a facility that is either accredited or licensed.”1 Following this trend, various states have espoused specific rules regarding maximum allowed volumes. By state, there is variability in both amount and type of aspirate. For instance, in the authors’ state of practice (California), it is mandated by law that any liposuction procedure involving a total aspirate of 5000 mL or more be performed in a general acute-care hospital or setting specified in Health and Safety Code Section 1248.1. This includes any ambulatory surgical center that is certified to participate in the Medicare program under Title XVIII (42 U.S.C. Sec. 1395 et seq.) of the federal Social Security Act, such as the AAAASF.12 In Oregon, liposuction of no more than 4000 mL of supernatant fat or 5% of total body weight (whichever is less) can be performed in an office-based setting.13 One of the strictest states is Florida, where no more than 4000 mL of supernatant fat may be removed by liposuction in the office setting, and no more than 1000 mL of supernatant fat when combined with another procedure or “if performed in a remote location from any other procedure.” More recently, Florida laws state that if more than 1000 mL of supernatant fat is removed in the office setting then a “Level II office surgery, or a Level III office surgery must register with the department unless the office is licensed as a facility under chapter 390 or chapter 395.”14–16
Each state has variability in its regulations, indicating the subjective nature of the guidelines. In addition, there is a lack of scientific evidence to support these seemingly arbitrary rules. The ASPS’s guidelines put forth by the committee were an “arbitrary decision that were based on the best available knowledge at the time.”17 Indeed, because the ASPS practice advisory on liposuction has recognized that there are no significant data supporting specific safe volumes, it is logical that the legal basis for such volumes is similarly unsubstantiated—by scientific evidence at least.1 With this lack of evidence, the maximum safe volume of lipoaspirate remains controversial in the current medical literature, and the legal extents of permissible liposuction on a state-by-state basis have remained inconsistent. These inconsistent and scientifically uncorroborated guidelines on the permissible volume of aspirate should be replaced by best practices agreed upon by the ASPS, medical community, and regulatory bodies. The best practices discussed below are intended to be a step toward consistent guidelines for the practice of safe high-volume liposuction.
Beyond these inconsistent guidelines and the lack of scientific evidence, the personal observation of the authors has been that surgeons who perform high-volume liposuction are subject to ostracization and the stigma of being “unsafe” surgeons. This labeling persists despite peer-reviewed publication of safe techniques for high-volume liposuction as well as presentations at national and international plastic surgery conferences. It begs the question of why, as a medical community, we still do not speak openly about high-volume liposuction and come together to set guidelines. A paradigm shift is overdue, one that can remove the stigma of “unsafe liposuction limits” and allow regulatory bodies to respectively update their standards.
High-volume liposuction has been well tolerated in healthy patients, as demonstrated by multiple studies in the plastic surgery literature.2,18,19 There is already a precedent for safety parameters to decrease complications.20 The complications of surgical blood loss, hypothermia, fluid overload, venous thromboembolism, pulmonary edema, and prolonged operative time can be properly mitigated by the advances in surgical technique, perioperative surgical care, and equipment.
This article does have potential limitations, however. First, the smaller sample size and the fact that it is retrospective and not a randomized, controlled study likely have effects on the power and robustness of this paper. Second, it does not include more specific measures (ie, hemoglobin level, hemodynamic studies with Swan-Ganz catheterization, etc.) to quantify the findings and allow for a more objective study. Last, although all patients underwent at least a 360° liposuction, there were also various combinations of procedures performed in a majority of the cases, and the lack of procedure stratification likely confounds results.
Nonetheless, studies to date have not addressed this topic head-on, even though many plastic surgeons have questioned the upper limit of what is considered “safe.” Currently, better care protocols exist, which allow us to perform liposuction safely beyond the limits established over 10 years ago. We believe that by sharing our experience with high-volume liposuction we can provide clearer objectivity in this field, contribute further to its knowledge base, and help guide plastic surgeons to perform safe surgery with aesthetically pleasing results in the high-volume patient population.
Bleeding
In the authors’ experience, the greatest factor in minimizing bleeding is surgical technique. First, our tumescent solution is introduced with the simultaneous separation and tumescent (SST) technique.8 The separation of the fat during infiltration allows for both faster vasoconstriction due to more contact of fat cells with epinephrine, as well as reduction in operative time.21,22 Vasoconstriction is observed in about 3 minutes in our experience. Additionally, we employ the 4-mm exploded-tip basket cannula connected to the power-assisted liposuction machine for a more efficient infiltration and separation of fat. Importantly, in accordance with Poiseuille's law, flow through a tube exponentially rises with an increase in the radius. That is, flow rate is inversely proportional to the cannula's radius to the fourth power. Similarly, fat elimination during liposuction increases with cannula diameter.21 We utilize the same 4-mm basket cannula for lipoaspiration, also supported by the fact that larger-bore cannulas improve fat viability during harvest.23 Traditional use of a small-diameter Klein cannula does not permit as efficient infiltration.24 Our tumescent solution contains 2 mg per liter of epinephrine rather than the conventional 1 mg per liter. This higher concentration leads to a more effective vasoconstriction. Although it has not been recommended to exceed 0.07 mg/kg of epinephrine, doses of up to 10 mg/kg have been safely described, with plasma levels reaching maximum levels at between 2 and 4 hours.6,7,25
To our knowledge, there are no case series demonstrating greater than 10 L of tumescence. In our case series, a subgroup of patients with a BMI of greater than 34 required more than 10 L of tumescence with 2 mg of epinephrine, which was well tolerated. Further, the authors believe that proper tumescence is the most important step overlooked in the successful performance of liposuction. During tumescence, it is important for the surgeon to visualize, in their “mind's eye,” all the anatomic planes: the deep and superficial fat compartments, which are separated by Scarpa's fascia. The superficial fat compartment is further subdivided into a deep, middle, and superficial layer. When a surgeon is infiltrating tumescent, it is important to distribute fluid correctly in all the surgical planes. If a surgeon under-infiltrates or does not infiltrate a specific plane and then proceeds to aspirate that area, the result will be increased bleeding.
In our series, the mean ratio of total tumescence to total aspirate was 1.23 (SD = 0.21). Further, when stratifying based on BMI, the mean tumescence-to-aspiration ratio trended lower but still was above 1 (see Table 2). This was likely due to our attempt to prevent fluid overload and lidocaine toxicity. When infiltrating tumescence, we adhered to a tumescent or superwet technique. Other techniques that employ a lower amount of tumescence increase bleeding, and lower amounts of yellow fat will be aspirated.
| BMI . | Patients (n) . | Tumescence, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 1.3689 | 0.2382 |
| 25-30 | 136 | 1.2638 | 0.1857 |
| 30-35 | 67 | 1.1416 | 0.1517 |
| 35-40 | 36 | 1.1166 | 0.1729 |
| 40-45 | 6 | 1.0471 | 0.0483 |
| BMI . | Patients (n) . | Tumescence, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 1.3689 | 0.2382 |
| 25-30 | 136 | 1.2638 | 0.1857 |
| 30-35 | 67 | 1.1416 | 0.1517 |
| 35-40 | 36 | 1.1166 | 0.1729 |
| 40-45 | 6 | 1.0471 | 0.0483 |
No significant differences (P < .05).
| BMI . | Patients (n) . | Tumescence, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 1.3689 | 0.2382 |
| 25-30 | 136 | 1.2638 | 0.1857 |
| 30-35 | 67 | 1.1416 | 0.1517 |
| 35-40 | 36 | 1.1166 | 0.1729 |
| 40-45 | 6 | 1.0471 | 0.0483 |
| BMI . | Patients (n) . | Tumescence, mean . | SD . |
|---|---|---|---|
| 20-25 | 49 | 1.3689 | 0.2382 |
| 25-30 | 136 | 1.2638 | 0.1857 |
| 30-35 | 67 | 1.1416 | 0.1517 |
| 35-40 | 36 | 1.1166 | 0.1729 |
| 40-45 | 6 | 1.0471 | 0.0483 |
No significant differences (P < .05).
Subsequently, after employing proper tumescent technique, the surgeon should aspirate in the same sequence that they infiltrated tumescence, because doing so allows one to aspirate areas that have been tumescent the longest amount of time. For example, in the abdomen, if one infiltrates tumescence in the deep then superficial layers, first in the lower abdomen and second in the upper abdomen, one should aspirate in the same sequential order. Not following the sequence and returning to an area recently tumescent can result in increased bleeding. As previously mentioned, we have observed that 3 minutes is an appropriate amount of time for vasoconstriction to occur when employing the SST technique.
We recommend aspirating the deep compartment of fat first, and then removing the deep and intermediate layers of the superficial compartment. This can be performed with both tactile and visual feedback, as well as the “mind's eye.” Leaving the superficial layer undisturbed is vital in preserving blood supply, and also lowering the risk of wound complications such as skin necrosis as well as contour irregularities.26 We did not observe any cases with postoperative skin necrosis, employing a technique similar to Cárdenas-Camarena, who performed liposuction of the deep plane and later the superficial plane with the pinch maneuver, also with cannula tips no larger than 5 mm.27
One can monitor the successful application of these tumescent and aspiration techniques by visually inspecting the canister. In the canister, one should observe the familiar yellow fat with very small amounts of blood. If the canister is tainted with large amounts of blood-tinged or bloody lipoaspirate, the likely culprit is improper tumescing, namely, aspirating areas that were not tumescent or under-infiltrated, as described above.
In low-volume liposuction, the absence of these techniques may not lead to problematic levels of blood loss. But to perform high-volume liposuction, it is imperative to minimize bleeding. This favorable outcome can be accomplished with the SST technique, as well as proper aspiration technique. Utilizing these best practices increases the safety profile of high-volume liposuction. Note that in our series no patients required blood transfusion.
Surgical application of tranexamic acid to reduce intraoperative bleeding has been rapidly adopted for use in various plastic surgery procedures, and recently in conjunction with liposuction.28–33-33 We administer a preoperative dose of intravenous tranexamic acid, which has been supported for utilization in large-volume liposuction cases to minimize blood loss and transfusion requirements.33 We support it as a best practice; however, we believe that in a high-volume setting tranexamic acid alone is not sufficient to mitigate blood loss due to improper technique.34
Hypothermia
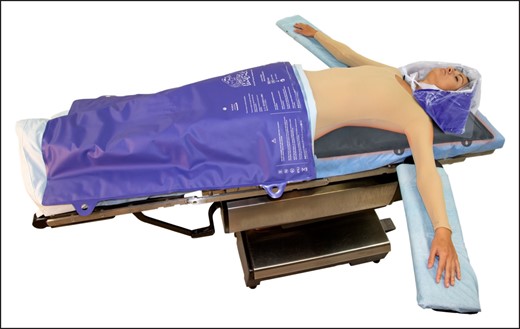
We employed the following active and passive warming strategies to maintain normothermia: preoperative warming with forced air warming (FAW), maintaining the operating room at a temperature of 72°F, active warming with the HotDog system, warming the intravenous (IV) solution with ivNow, and immediate warming postoperatively with the HotDog system (Figure 3).
HotDog system. Reprinted with permission from Augustine Surgical, Inc. (Eden Prairie, MN). Alphanumerics refer to product model number.
Evidence has clearly shown intraoperative hypothermia to be 1 of the most significant factors affecting coagulation and contributing to cardiopulmonary issues, postoperative wound infections, and delayed hospital discharges.35–38 Indeed, level 2 evidence supports maintaining patient normothermia to prevent coagulopathy and its related complications, such as further blood loss.39,40 The risk of hypothermia increases with operative time; core body temperature can decrease by almost 3°C in the first hour of surgery. Large-volume cases are at a higher risk because more surface area of the patient's body is exposed.26 Additionally, anesthetic effects on the autonomic nervous system contribute to lowering core body temperature during surgery.41 It is important to note that anesthetized patients do not maintain their normal thermoregulatory mechanisms, such as shivering or vasoconstriction to preserve core temperature, as nonanesthetized patients do.42
Hypothermia is attributed to multiple factors, including cutaneous heat loss, a reduction in metabolic heat production, and most significantly, core to periphery heat redistribution vis-à-vis anesthetic-induced vasodilation and inhibition of vasoconstriction.43,44-45 Cutaneous heat loss occurs through 4 main mechanisms: conduction, convection, radiation, and evaporation; convection and radiation contribute the most to these losses.46 Redistribution is responsible for over 80% of hypothermia during general anesthesia. Core hypothermia during general anesthesia occurs in 3 phases: initial rapid decrease, slow linear reduction, and a plateau phase.47,48 The initial rapid decrease phase is due to anesthesia-induced redistribution, which occurs within the first hour of induction. The second slow reduction phase is due to an imbalance between metabolic heat production and heat loss. The third plateau phase is stabilization of the core temperature due to a return of peripheral vasoconstriction.49
Although cutaneous heat losses may seem to only primarily contribute to the second and third phases, core to periphery redistribution in the first phase is dependent on a temperature gradient. Therefore, increasing the peripheral temperature can reduce this gradient and subsequently mitigate the massive core to periphery heat shift within the first hour.50 This is a crucial point, because during surgery the patient is poikilothermic and the body temperature is strongly dependent on environmental factors, such as warming devices and the operating room temperature.
Preoperatively we employ forced-air warming (FAW) with the 3M Bair Hugger System (Saint Paul, MN). Although we feel that the HotDog system is an overall better strategy for maintaining normothermia, practical considerations lend one to use FAWs preoperatively. Specifically, the HotDog is best utilized when a patient is lying down, and preoperatively the patient is usually not laying down at all times. Indeed, studies have shown that preoperative warming with FAWs for 30 minutes reduces the degree of core temperature decrease and decreases the incidence of intraoperative hypothermia.51,52
Intraoperatively, we employ the HotDog patient warming system, set at 43°C to maintain normothermia. The HotDog is a resistive heating device and its mechanism of action is through a combination of conductive and radiative heat transfer, which are the 2 largest contributors to cutaneous heat loss. Compared to FAWs, it has been shown to be 2.3 times more efficient in heat transfer.53 Not only is this related to its semiconductive polymer fabric properties but also the fact that more surface area participates in heat exchange (through simultaneous warming from above and below the patient), a limiting factor for FAWs. In a large study by Sun et al, 58,814 adults having surgery lasting greater than 1 hour who were warmed with FAW were evaluated.54 Almost half of all the patients failed to reach normothermia (36°C) within 2 hours of induction, and 25% failed to reach normothermia within 5 hours of induction. Of note, they reported that hypothermia was significantly associated with an increased transfusion requirement and prolonged hospitalization duration.
In the past, the W.J.R. has utilized FAWs, with patients’ intraoperative temperatures falling below 36°C. This is similar to Kenkel et al, who found a mean core temperature of 35.5°C in all patients undergoing moderate- to large-volume liposuction when using the Bair Hugger System FAWs intraoperatively.55 Changes in the W.J.R.'s practice to the combined conductive and radiative heat transfer of the HotDog system during surgery has allowed for maintenance of intraoperative normothermia. In our cohort, the mean core temperature was maintained at 36.3°C (SD = 1.16).
Multiple investigators have shown FAWs to have marginal intraoperative rewarming rates, with an average of only 0.1°C/hr.35,56–64 Although some studies have shown equally effective rewarming rates between FAWs and conductive heating devices, they only compared FAW blankets to conductive heating blankets or FAW blankets to conductive heating mattresses, not FAW blankets to conductive heating systems, like the HotDog system we utilize that has a heated blanket as well as a heated mattress.65–70 In a prospective, randomized trial by Sugai et al, the combinatory conductive and radiative heat transfer of the HotDog system demonstrated significantly better patient warming (0.35°C/hour vs 0.01°C/hour) compared to FAW blankets, when all other variables were held constant.71 Ohki et al reported that conductive fabric warming devices were particularly more superior than FAWs when preoperative warming was performed, a protocol we routinely employ.72
Another important distinction to make between the 2 devices is the air-free characteristic of the HotDog. Studies have also shown FAWs to disrupt the protective laminar flow ventilation systems in the operating room by creating hot air convection currents, or “waste heat,” which mobilizes floor contaminates into the sterile field.73–76 As one can imagine, this can pose serious safety and outcome implications for patients. This does not occur with conductive heat warming devices like the Hot Dog, because it does not rely on air convection. Recently, the US Centers for Disease Control and Prevention has warned that “Nothing that blows air should be in an operating theater, if possible.”77 Use of conductive and radiative heat transfer systems have been reported to lead to a 74% reduction in infection when FAWs were discontinued.73
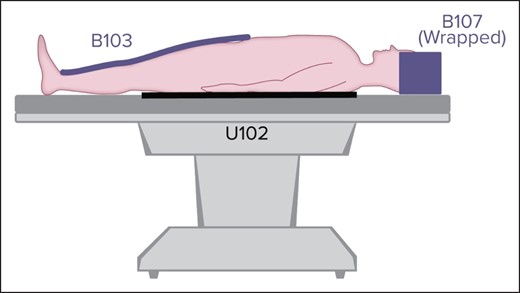
We employ 2 HotDog blankets and 1 mattress in all liposuction cases; 1 mattress is placed underneath the patient on the operating room table and covered with sterile drapes, 1 blanket covers the patient's lower body (from the lower third of the thigh to the ankle), and 1 covers the head (Figure 4). Additionally, we heat the infiltrating solution for tumescence and any intravenous fluids with the ivNow fluid warmer. This has been the practice of other plastic surgeons, and not doing so can also contribute to hypothermia.40,55,78
Three HotDog blankets. Note that we place the lower body blanket caudal to what is shown in this figure, extending from the lower third of the thigh to the ankle. Reprinted with permission from Augustine Surgical, Inc. (Eden Prairie, MN).
Low operating room temperatures lead to intraoperative hypothermia mostly through radiant heat loss.79 Some have recommended keeping the operating room ambient temperature set at 78° to 80° F for patient comfort and to help prevent intraoperative hypothermia, and others have stated that it is necessary to keep the temperature no less than 77° F in large-volume cases.80,81 Inconsistencies regarding the optimal operating room temperature are due to other variables involved in preserving normothermia, including cutaneous heat losses, as well as surgeon and staff discomfort and other heat exposures, such as operating room lights.46 Because radiation is 1 of the most significant mechanisms in cutaneous heat loss, it is prudent to raise the operating room temperature as a passive warming technique to lessen the temperature gradient and minimize heat loss. Because a mere temperature gradient of greater than 4°C between the skin and the environment can cause more heat to be lost than is produced, maintaining an ambient temperature of at least 75° F can effectively mitigate hypothermia during general anesthesia regardless of age.82,83-84 We keep the operating room temperature set at 72° F to maintain normothermia, which remains within the standard requirements of the Joint Commission.85
Postoperatively, we use only 1 HotDog blanket placed over the patient's torso. We find that this is the best approach to preserving patient warmth and comfort. When a second blanket is kept under the patient, they generally complain that they feel uncomfortable from being too warm.
In summary, we suggest all these steps to ensure patients maintain normothermia. Doing so can help mitigate the risks associated with hypothermia and reduce the risk of blood loss. As for Cavallini et al, “in no case was blood transfusion required, and no pathologic bleeding was observed in any case during surgery.”39 In our series, we believe that these practices contributed to the fact that no patients required postoperative blood transfusions.
Fluid Management
Because fluid overload and potential cardiopulmonary issues can arise after large-volume liposuction, the appropriate ratio of intravenous fluid and subcutaneous infiltrate to total lipoaspirate has been an area of interest.3,18,86,87 Although there is no consensus on the exact amount of necessary fluid resuscitation, different formulas have been devised to help guide surgeons and minimize complications.19,87–90
Originally, Trott et al suggested that liposuction patients with aspiration volumes of less than 4 L receive only maintenance fluids with subcutaneous wetting solution, whereas volumes greater than or equal to 4 L should additionally receive 0.25 mL of intravenous crystalloid per mL of aspirate removed after reaching 4000 mL.4 Although in this study volume overload was avoided, patients were slightly over-resuscitated, which could pose a significant threat to those with undiagnosed cardiopulmonary issues. This led to a modification of the fluid resuscitation guidelines of Rohrich et al, such that the cutoff threshold was increased to 5 L, delivering an additional 0.25 mL of intravenous crystalloid per mL of aspirate over 5000 mL.19 In these studies, the intraoperative fluid ratios, that is, the sum of maintenance intravenous fluids and wetting solution divided by total aspirate, were 1.4 and 1.2 in large-volume cases. With these studies as a reference point, others have developed their own fluid resuscitation protocols.91 In our cases series, the intraoperative fluid ratio was 1.49 (SD = 0.27). Similarly, Basile et al reported an intraoperative fluid ratio ranging from 0.98 to 2.1, with an average of 1.25.88
On average, most patients who underwent high-volume liposuction received 1 to 2 L of IV fluid during surgery. Assuming blood loss was kept at a minimum, patients should not require more intravenous fluids. All high-volume cases had a Foley catheter placed to monitor urinary output. Our goal was for patients to maintain a minimum of 0.5 cc/kg per hour while maintaining mean arterial pressures of at least 65 mmHg. This was in accordance with Trott et al, who recommended that maintenance fluid replacement be adjusted based on vital signs and urine output.4 All patients went to aftercare and received a maximum of an additional 1 L of fluid. Excess administration of intravenous fluids has been significantly associated with increased risk of complications after liposuction, including but not limited to congestive heart failure, pulmonary edema, and electrolyte imbalances.1,92–94 Our overall strategy was to avoid fluid overload. Because of the large amount of wetting solution utilized in these high-volume procedures, one has to be cautious of how much intravenous fluid is given to minimize third-spacing and eventual intravascular mobilization. Because it is estimated that 70% of subcutaneous wetting solution becomes intravascular, this can become extremely problematic, especially in a patient with cardiopulmonary morbidity.95
Venous Thromboembolism (VTE)
The risk of deep venous thrombosis (DVT) in general surgical patients has been reported to be 0.3, and of pulmonary embolism (PE) to range between 0.1 and 0.8—increasing to 2% to 3% in elective hip replacement cases.96,97 Although there have been studies on venous thromboembolism (VTE) in the plastic surgery literature, there is no true consensus due to the lack of enough information. Additionally, it has been suggested that the risk of VTE in plastic surgery is lower than that in the general surgery population, which has led plastic surgeons to search for the best practice for VTE prophylaxis.98–100 In 1998, the American Society of Plastic and Reconstructive Surgeons convened a task force on DVT because of the lack of data on DVT prophylaxis in plastic surgery patients. Although their risk stratification differed from the commonly accepted Caprini risk assessment model, they recommended that high-risk patients receive intermittent pneumatic compression devices. They also recommended consideration of low-molecular-weight heparin given preoperatively and daily postoperatively until the patient is ambulatory, weighing this against the risk of hematoma formation.101,102
VTE after liposuction has been a large area of interest. Rao et al reported 5 deaths after tumescent liposuction, however only 1 patient died of a fatal PE, and it occurred after liposuction of the lower extremities.103 The remaining fatalities were secondary to cardiopulmonary complications, and the authors surmised that lidocaine toxicity was at least partly related. High-volume liposuction is known to be a risk factor for VTE.2,104 Interestingly, although combining other general plastic surgery procedures with an abdominoplasty confers a higher risk of VTE, the risk is not increased with the addition of liposuction.105–108 Despite the fact that fatal PE has been cited as the most common cause of mortality after liposuction, large volumes can still be extracted safely in 1 setting by adopting appropriate risk stratification and perioperative practices.10,109,110
In our series, there was 1 case of nonfatal PE. All patients have intermittent pneumatic compression devices placed and functioning before anesthesia induction.87,111 We use the Caprini risk assessment model to determine which patients should receive VTE prophylaxis. Patients with a Caprini scores greater than 7 receive low-molecular-weight heparin intraoperatively as well as a direct factor Xa inhibitor postoperatively for 7 days, because this has been validated to confer a notable risk reduction in postoperative VTE.112,113 Further, all of our patients are instructed to ambulate immediately after surgery and wear sequential compression devices for the first 24 hours after surgery. We additionally administer prophylaxis to patients who have high-volume liposuction combined with abdominoplasty. Importantly, we did not observe any cases of postoperative hematoma, corroborated by the fact that thromboprophylaxis has not been shown to produce a clinically relevant or statistically significant increase in observed rates of reoperative hematoma in the plastic surgery literature.114
BMI Consideration
It is well known that obesity, defined as BMI ≥ 30, increases morbidity after plastic surgery, including surgical site infections and reoperation rates.115–118 Interestingly, a higher BMI confers a greater risk of major complications with cosmetic procedures than with reconstructive surgery.119
Although more studies on large-volume liposuction have included patients with BMIs above 30, few studies have examined liposuction in patients with a BMI above 40.120 Furthermore, studies have evaluated the physiological effects of high-volume liposuction on obese patients, including a reduction of inflammatory markers and improvement in insulin resistance, rather than safety itself.121,122 In our series, we removed an average of 9.5 L and 10.5 L of total aspirate on any patient with a BMI equal to or above 35 and 40, respectively. Even though we performed high-volume liposuction, we have not observed a higher rate of any complication in patients with a higher BMI. Still, this needs to be studied further. We believe that proper technique application can allow for safe removal of more than 5 L in high BMI patients. All of our patients obtain medical clearance as well.
It is also important to note that physiologic changes during liposuction can differ based on patient BMI, and therefore the overall size of the patient should be considered when determining the proper amount of lipoaspirate to be removed.123 The authors keeps the aspirate volume proportional to patient BMI. With each BMI increment of 5 (above 30), the mean lipoaspirate volume increases by 0.5 to 1 L. Patients with a BMI above 30 had a mean aspirate volume of 8.9 L, whereas those with a BMI above 40 had an average of 10.5 L removed (Table 3). Chow et al found that increasing both BMI and lipoaspirate volume conferred a “protective effect,” such that high-BMI patients can tolerate the removal of larger volumes than low-BMI patients, challenging the traditional concept that the an absolute volume exists.124 We believe that the relative volume is paramount and that a gradient of lipoaspirate volumes proportional to BMI should be employed.
| BMI . | Patients (n) . | Volume, mean . | SD . |
|---|---|---|---|
| Above 30 | 110 | 8.946 | 1.876 |
| Above 35 | 43 | 9.474 | 1.934 |
| Above 40 | 7 | 10.529 | 1.347 |
| BMI . | Patients (n) . | Volume, mean . | SD . |
|---|---|---|---|
| Above 30 | 110 | 8.946 | 1.876 |
| Above 35 | 43 | 9.474 | 1.934 |
| Above 40 | 7 | 10.529 | 1.347 |
No significant differences (P < .05).
| BMI . | Patients (n) . | Volume, mean . | SD . |
|---|---|---|---|
| Above 30 | 110 | 8.946 | 1.876 |
| Above 35 | 43 | 9.474 | 1.934 |
| Above 40 | 7 | 10.529 | 1.347 |
| BMI . | Patients (n) . | Volume, mean . | SD . |
|---|---|---|---|
| Above 30 | 110 | 8.946 | 1.876 |
| Above 35 | 43 | 9.474 | 1.934 |
| Above 40 | 7 | 10.529 | 1.347 |
No significant differences (P < .05).
Operative Time
Minimizing operative time is an important topic to address. Optimizing surgical technique in high-volume liposuction will result in greater efficiency in the operating room and fewer complications such as bleeding, hypothermia, and pulmonary embolisms.105 Surgeons need to be mindful of their time in the operating room. Although, as in any procedure, there is a learning curve, an operative time of 180 minutes for high-volume cases should be aimed for and can be achieved. This should apply to the liposuction portion of any combination procedure. In 2019, our mean operative time was 220 minutes (SD = 104.8), and this decreased by almost 40 minutes to 189 minutes (SD = 71.0) by 2021 (see Table 4). Of course, these times included all types of high-volume cases: 360° liposuction alone and in combination with other procedures. When strictly taking into account cases of only 360° liposuction and 360° liposuction with gluteal fat transfer, mean operative times in 2021 were lower, at 164 and 159 minutes, respectively, than 166 and 200 minutes, respectively, in 2019.
| Year . | Patients (n) . | Operative time (min), mean . | SD . |
|---|---|---|---|
| 2019 | 49 | 219.57 | 104.8 |
| 2020 | 92 | 210.78 | 83.6 |
| 2021 | 127 | 188.81 | 71.0 |
| Year . | Patients (n) . | Operative time (min), mean . | SD . |
|---|---|---|---|
| 2019 | 49 | 219.57 | 104.8 |
| 2020 | 92 | 210.78 | 83.6 |
| 2021 | 127 | 188.81 | 71.0 |
No significant differences (P < .05).
| Year . | Patients (n) . | Operative time (min), mean . | SD . |
|---|---|---|---|
| 2019 | 49 | 219.57 | 104.8 |
| 2020 | 92 | 210.78 | 83.6 |
| 2021 | 127 | 188.81 | 71.0 |
| Year . | Patients (n) . | Operative time (min), mean . | SD . |
|---|---|---|---|
| 2019 | 49 | 219.57 | 104.8 |
| 2020 | 92 | 210.78 | 83.6 |
| 2021 | 127 | 188.81 | 71.0 |
No significant differences (P < .05).
In agreement with our technique, level 1 and level 2 evidence has shown that power-assisted liposuction (PAL) in conjunction with the superwet technique, on an outpatient basis, is the preferred method.125–127-128 By reducing surgeon fatigue, PAL can improve efficiency and facilitate a decreased operating room time, especially in large-volume cases.1 In keeping with this, our mean operative time in high-volume cases was 202 minutes (SD = 83.1). By employing the previously described SST and aspiration techniques, we can monitor efficiency at an average rate of 100 mL per minute or more of lipoaspirate.
In cases when the patient's anatomy is highly fibrous, a rate no less than 75 mL per minute is maintained. Equally important, we believe the surgeon should confirm that the “to-and-fro” motion of the cannula is progressively moving through nontreated zones and advancing through each subcutaneous fat layer, so as not to inefficiently keep entering already suctioned areas.
In summary, the cumulative result of these techniques with concomitant reduction of operating time leads to the safer performance of high-volume liposuction. Our series’ low complication rates and absence of certain complications, such as the need for blood transfusions, support this conclusion.
Ambulatory Care
With regard to postoperative standard of care for high-volume liposuction, patients should be admitted to an aftercare facility for overnight hemodynamic and fluid monitoring. This was first validated by the ASPS Practice Advisory on Liposuction in 2003.129 Today, it is still recommended by the ASPS patient safety committee and is part of the general practice guidelines espoused by many plastic surgeons.2,20,123,130 Further, the ASPS safety committee clearly stated that “Regardless of the anesthetic route, large-volume liposuction (greater than 5000 cc of total aspirate) should be performed in an acute care hospital or in a facility that is either accredited or licensed.”1
Outpatient ambulatory centers not only maintain certain standards preoperatively and intraoperatively but also postoperatively for adequate patient monitoring.131 Additionally, each state provides state-specific regulations on liposuction extraction volumes and other related standards of care and practice. As stated earlier, the state of California requires high-volume cases to be performed in a setting specified in Health and Safety Code Section 1248.1, which includes AAAASF facilities.132 This procedure is followed by the authors. In our series, all patients who underwent high-volume liposuction were transferred to an overnight facility, where a Foley catheter was maintained and vital signs were monitored every hour, as recommended by the ASPS task force.1 This practice allows for the early identification of potential complications and prevents any delay in treatment.
Of our series’ 310 patients who went to aftercare, 2 were transferred to a hospital for further workup; 1 for tachycardia (heart rate over 120 beats per minute), and the other for shortness of breath. Both patients had unremarkable laboratory values and negative imaging and were discharged home from the emergency room.
CONCLUSION
High-volume liposuction can be safely performed when best practices are followed. Adhering to the aforementioned surgical principles and techniques minimizes blood loss, controls for hypothermia, and reduces operative time—all factors that may lead to morbidity and mortality after high-volume liposuction. In addition, our data show that when performed properly high-volume liposuction has exceedingly low complication rates.
This concept of safety poses a paradigm shift for high-volume liposuction. Commonly in the surgical community, if arbitrary volume limits have been exceeded, postoperative complications or deaths are automatically attributed to the “high-volume nature” of the case or surgeon error.10 We maintain that this is a confounding bias, and that postoperative morbidity is due not to the absolute volume of lipoaspirate but rather to improper surgical techniques or not having the appropriate amount of surgical training and certification.
It is important to implement specific, universally agreed upon guidelines for high-volume liposuction. Doing so will further advance the field of high-volume liposuction, give surgeons the ability to offer higher-BMI patients improved outcomes while maintaining their safety, and help lift the medical and legal stigma associated with the procedure.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
California Law. California Legislative Information. Accessed August 31, 2021. https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?lawCode=HSC§ionNum=1248.1
Author notes
Dr Golpanian is plastic surgeon in private practice in Beverly Hills, CA, USA.
Dr Rahal is plastic surgeon in private practice in Beverly Hills, CA, USA.
Mr Rahal is a practice consultant for a private practice in Los Angeles, CA, USA.



