-
PDF
- Split View
-
Views
-
Cite
Cite
Chak Yuen Fung, Jeong Heon Kim, Pei-Hsun Liao, Yong Ju Jang, Revision Rhinoplasty Using Glued Diced Costal Cartilage Shaped With Mold for Management of Complicated Silicone Rhinoplasty, Aesthetic Surgery Journal, Volume 43, Issue 11, November 2023, Pages 1237–1247, https://doi.org/10.1093/asj/sjad180
Close - Share Icon Share
Abstract
Complicated silicone nose is a common clinical problem. Selection of replacement material for revision dorsal augmentation is a challenging task.
The authors presented their experience in the use of molded glued diced cartilage graft (GDCG) for revision rhinoplasty in patients who had complicated silicone augmentation.
The authors performed a retrospective review of the medical records of 28 patients who underwent silicone implant removal and revision dorsal augmentation with costal cartilage at a tertiary center between February 1, 2018, and February 28, 2022. Patient demographics, surgical technique, anthropometric measurements, and complication data were retrieved and analyzed. Aesthetic outcome scoring and anthropometric measurements were performed.
Twenty-eight patients (9 males and 19 females) who underwent revision rhinoplasty with augmentation were reviewed. The principal indication for revision was cosmetic dissatisfaction. Mean postoperative follow-up duration was 18.3 months. All patients had revision dorsal augmentation with molded GDCG. Other key surgical techniques include the use of caudal septal extension and extended spreader and tip grafts. The majority of the patients were judged to have good or excellent outcomes (91.1%). There were significant percentage increases in dorsal height, radix height, nasal length, and nasal tip projection (2.78%, 2.26%, 7.53%, and 2.40%, respectively; P < .05) and reduction of nasal axis deviation of 1.15° (P < .05) postoperatively. Two patients had postoperative complications, including infection and cosmetic dissatisfaction.
Revision rhinoplasty following unsuccessful silicone augmentation is commonly encountered in the Asian population. Molded GDCG for revision dorsal augmentation is a reliable option that delivers good to excellent aesthetic outcomes with acceptable complication rates.

In contrast to the Western population, augmentation rhinoplasty is one of the most popular and frequently performed rhinoplasty procedures in the Asian population.1 Dorsal augmentation may be performed with autologous materials or alloplastic implants, with both types of materials having their pros and cons, which have been widely discussed and debated. Among the alloplastic materials, silicone seems to have stood the test of time despite its various potential problems and controversies. Many rhinoplasty surgeons will agree that silicone continues to be the alloplastic implant of choice for dorsal augmentation, especially in Asia.2
Silicone implants can cause complications seen with any alloplastic implants, such as deviation, displacement, infection, and extrusion.3 The overall rate of complications following silicone augmentation rhinoplasty varies from 4% to 36% in the literature.4 Because silicone is one of the most frequently utilized alloplastic implants in rhinoplasty, revision surgery following complicated silicone rhinoplasty is frequently encountered by rhinoplasty surgeons.
We recognize that revision surgery following a complicated silicone rhinoplasty presents its own set of unique challenges, and 1 example is the choice of implant material for revision augmentation. Many patients naturally expect a superior outcome from revision surgery, and the choice of implant material for revision augmentation must be carefully considered, such that, in addition to maintaining good performance, safety and the lowest risk of complications are ensured to prevent further frustrations for the patient. Although some surgeons and patients may decide to use alloplastic materials such as silicone again for the revision surgery, most surgeons will consider autologous materials at this point to avoid complications that have already occurred in the same patient. Commonly used autologous materials in revision cases include septal, auricular, and costal cartilage, and dermafat.5 However, in revision cases, septal or auricular cartilage may have already been utilized, and costal cartilage may be the only viable option, especially if dorsal augmentation is required, due to the strength and amount of material it provides.2,6,7
Various ways of preparing costal cartilage for dorsal augmentation have been described. These include the monobloc technique, laminated costal cartilage grafting, crushed cartilage, injection of free diced cartilage, diced cartilage wrapped with fascia, and glued diced cartilage.8-10 Among these techniques, the use of glued diced cartilage graft (GDCG) for dorsal augmentation has been gaining popularity.11,12 Y. J. J. has fabricated a novel mold (Jang Cartilage Mold, Nextcore; Ulsan, Republic of Korea) for shaping GDCG utilized in augmentation, which allows finer control of the shape and volume of the GDCG implant. Given the promising results with the novel mold in an earlier study, we were encouraged to expand the indication of this novel mold further.13 In this article, we aim to assess the performance of this novel mold when employed with GDCG in revision cases of complicated silicone rhinoplasty in Asian patients, focusing on its use for dorsal augmentation in revision scenarios and the overall aesthetic outcome. Although many studies have looked at the various problems associated with revision rhinoplasty following alloplastic implants and their management, to the best of our knowledge this is the first case series to investigate the surgical outcome of a specific surgical technique for revision augmentation following removal of silicone implants in the English literature.14-16
METHODS
Study Design
All procedures performed in studies involving human participants were approved by the Asan Medical Center internal review board and written consent was provided, by which the patients agreed to the use and analysis of their data. A retrospective review of the medical records was performed for patients who underwent silicone implant removal and revision dorsal augmentation with costal cartilage at the Asan Medical Center between February 1, 2018, and February 28, 2022. All patients who had a silicone augmentation implant removed and revision augmentation done in the same setting with the molded GDCG during this period were included in the study. Any patient with incomplete medical records or missing clinical photographs was excluded from the study. Patient demographics, surgical techniques, anthropometric measurements, indications for revision surgery, and complication data were retrieved and analyzed.
Novel Mold Used for Glued Diced Cartilage Graft (GDCG) Dorsal Augmentation
The mold utilized in the study was designed and developed by Y. J. J.13 The mold consisted of 3 parts: a base template, a guarding frame with side holes, and a compressor with a handle (Figure 1A). There were a range of size options available for this mold, ranging from 20 to 45 mm in length and 1.0 to 3.0 mm in thickness. The shape of this mold was designed by Y. J. J. and fabricated with 3-dimensional printing. After harvesting of costal cartilage, the cartilage was first manually diced into less than 0.5-mm fragments with a dermatome blade (Zimmer Surgical Inc., Dover, OH) (Figure 1B). The patient's dorsal length was measured, and, keeping in mind the patient's desired degree of augmentation, a preformed mold of the desired length and thickness was chosen. To begin the molding process, autologous fascia such as costal perichondrium or homologous fascia (Maxxeus allograft 40 × 60 mm; Community Tissue Services, Dayton, OH) were inserted into the mold as covering materials for the implant. The final size of the costal perichondrium or homologous fascia would be cut accordingly, because this covering was the same size as the area of the base template of the mold selected for use. One or 2 drops of the fibrin glue were painted onto the covering material and the guarding frame of the mold was assembled. Next, a thin layer of diced cartilage was placed on top of the fascia or perichondrium, a second layer of glue was painted, an additional layer of diced cartilage was laid on top, and glue was painted over the second layer of diced cartilage. The process was repeated until the guarding frame was filled with diced cartilage. The compressor of the mold was applied and pressed together. Through the small holes of the sidewall, excessive glue and fluid around the diced cartilage were drained and suctioned out. After the components of the mold were carefully disassembled, the graft for dorsal augmentation was obtained (Figure 1C, D, E).

(A) Jang Cartilage Mold for dorsal augmentation with glued diced cartilage grafting (GDCG). Three different components (left to right: base template, guarding frame with side holes, and compressor with handle) make up the mold. (B) GDCG being prepared from costal cartilage. (C-E) GDCG dorsal implant prepared with Jang Cartilage Mold: (C) dorsal surface with covering fascia, (D) ventral surface, and (E) lateral view with covering fascia on dorsal surface.
Surgical Technique
All the revision cases were performed by Y. J. J. All patients were under general anesthesia, and the external approach was with an inverted V-shaped transcolumellar incision extending to bilateral marginal incisions. After raising the skin and soft tissue envelope, the silicone implants were identified and removed. Extensive capsulectomy was performed with blunt and sharp dissection, assisted by microdebrider. Any deviation of the bony and cartilaginous septum was identified and addressed. Osteotomies were performed for patients with bony vault deviations. Costal cartilage was harvested in all patients in the series. Intraoperative assessment determined the type of grafts to employ to maintain structural integrity and ensure a good aesthetic outcome. Examples of these grafts included septal extension grafts, spreader grafts and extended spreader grafts, batten grafts, and tip grafting. Finally, all patients received dorsal augmentation with the GDCG shaped with the mold, with the detailed technique outlined above. The GDCG implants were not anchored or fixed to the dorsum; rather, postoperative taping and external nasal splinting with Aquaplast (WFR/Aquaplast Corp., Wyckoff, NJ) were placed for 1 week to prevent displacement. The various surgical techniques employed are summarized in Table 1.
| Techniquea . | No. of patients (n = 28) . |
|---|---|
| Dorsal augmentation with GDCG with mold | 28 |
| Septal extension graft | 27 |
| Spreader graft | 26 |
| Tip graft | 15 |
| Batten graft | 9 |
| Hump resection | 6 |
| Osteotomy | 4 |
| Columellar strut | 2 |
| Lateral crural onlay graft | 1 |
| Techniquea . | No. of patients (n = 28) . |
|---|---|
| Dorsal augmentation with GDCG with mold | 28 |
| Septal extension graft | 27 |
| Spreader graft | 26 |
| Tip graft | 15 |
| Batten graft | 9 |
| Hump resection | 6 |
| Osteotomy | 4 |
| Columellar strut | 2 |
| Lateral crural onlay graft | 1 |
These procedures are not mutually exclusive. GDCG, glued diced cartilage graft.
| Techniquea . | No. of patients (n = 28) . |
|---|---|
| Dorsal augmentation with GDCG with mold | 28 |
| Septal extension graft | 27 |
| Spreader graft | 26 |
| Tip graft | 15 |
| Batten graft | 9 |
| Hump resection | 6 |
| Osteotomy | 4 |
| Columellar strut | 2 |
| Lateral crural onlay graft | 1 |
| Techniquea . | No. of patients (n = 28) . |
|---|---|
| Dorsal augmentation with GDCG with mold | 28 |
| Septal extension graft | 27 |
| Spreader graft | 26 |
| Tip graft | 15 |
| Batten graft | 9 |
| Hump resection | 6 |
| Osteotomy | 4 |
| Columellar strut | 2 |
| Lateral crural onlay graft | 1 |
These procedures are not mutually exclusive. GDCG, glued diced cartilage graft.
Anthropometric Measurements
Anthropometric measurements were performed to assess surgical outcome objectively. Preoperative and postoperative frontal and lateral facial photographs in the Frankfort horizontal plane were analyzed.17 The measurements included dorsal height, radix height, nasal length, nasal tip projection (NTP), nasofrontal angle (NFA), and nasolabial angle (NLA), as well as the degree of external nasal axis deviation (Figure 2). To measure dorsal height, a line was drawn from medial canthus to the alar crease, and a perpendicular line was drawn from this line to reach the rhinion. This second line was the dorsal height. Using again the line drawn from the medial canthus to the alar crease, radix height was measured perpendicularly from this line to the nasion. Nasal length was measured from the nasion along the dorsum to the pronasale. Nasal tip projection was measured from a line drawn perpendicularly from the white line to the pronasale. A reference length was defined as the distance between the pupil and the lateral commissure. To account for the variability in the objective distance, size and magnification of each photograph, dorsal height, radix height, nasal length, and nasal tip projection were reported as a ratio to this reference line. The NFA was the angle between the line measuring nasal length along the dorsum and a line tangent to the glabella. The NLA was the angle between a line on the anterior surface of the columella and a line on the surface of the upper-lip skin. To measure external nasal axis deviation, the nasal axis was measured on frontal photographs (Figure 3). The angle formed between a vertical line starting at the midpoint of the interpupillary line and a line connecting that point with the nasal tip defining point was measured as the degree of external nasal axis deviation.18
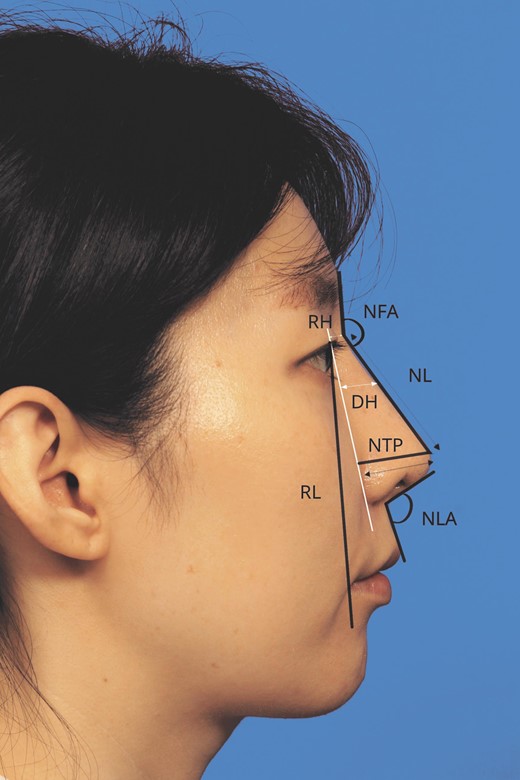
To measure dorsal height (DH), a line was drawn from the medial canthus to the alar crease, and a perpendicular line was drawn from this line to reach the rhinion. This second line was the dorsal height. Using again the line drawn from the medial canthus to the alar crease, radix height (RH) was measured perpendicularly from this line to the nasion. Nasal length (NL) was measured from the nasion along the dorsum to the pronasale. Nasal tip projection (NTP) was measured from a line drawn perpendicularly from the white line to the pronasale. A reference length (RL) defined as the distance between the pupil and the lateral commissure was measured. To account for the variability in the objective distance, size and magnification of each photograph, dorsal height, radix height, nasal length, and nasal tip projection were reported as a ratio to this reference line. The nasofrontal (NFA) was the angle between the line used to measure nasal length along the dorsum and a line tangent to the glabella. The nasolabial angle (NLA) was the angle between a line on the anterior surface of the columella and a line on the surface of the upper-lip skin.
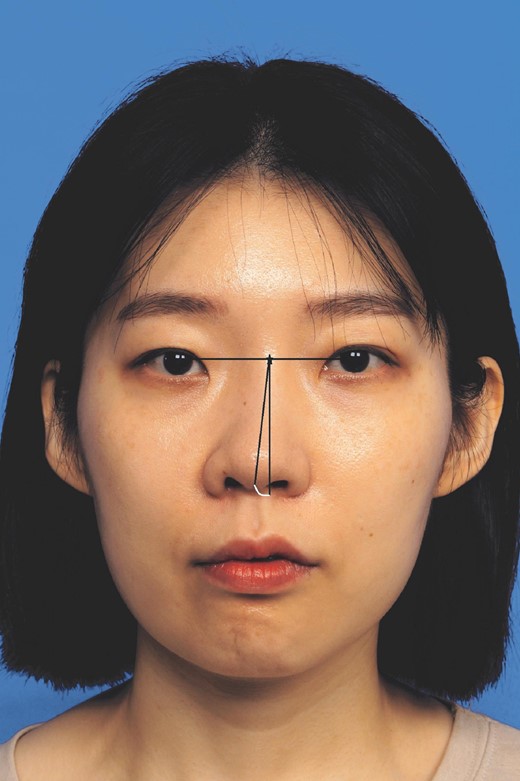
To measure external nasal axis deviation, the nasal axis was measured on frontal photographs. The angle formed between a vertical line starting at the midpoint of the interpupillary line and a line connecting that point with the nasal tip defining point was measured as the degree of external nasal axis deviation.
Aesthetic Outcome Evaluation
The aesthetic outcomes of the revision surgery were evaluated by 2 independent rhinoplasty surgeons who were not involved in care of the patients. The postoperative facial photographs taken at the last follow-up visit were compared with the preoperative facial photographs. The overall aesthetic outcome of each patient was graded as 1 (poor), 2 (fair), 3 (good), or 4 (excellent).
Statistical Analysis
Preoperative and postoperative anthropometric measurements were compared with the paired t test. Numerical data are presented as means and standard deviations (SD), and categorical variables are expressed as numbers (percentages, %). All statistical analyses were performed with SPSS for Windows, version 22.0 (IBM Corp., Armonk, NY). The criterion for statistical significance was set at P < .05.
RESULTS
Twenty-eight patients who underwent removal of the silicone implant and revision rhinoplasty with molded GDCG between February 1, 2018, and February 28, 2022 at a tertiary center were identified and included. The mean (SD) age of the patients was 40.1 (12.4) years (range, 20-58 years), with a 9:19 male to female ratio. The mean number of previous rhinoplasties performed was 1.75 (range 1-5). The mean duration (SD) of follow-up duration was 18.3 (11.7) months, with a range of 7 to 69 months. All the patients had aesthetic concerns when presenting to our clinic for revision rhinoplasty, as described in the preoperative diagnosis. Preoperative diagnoses included 21 deviated noses, 8 short and upturned noses, 6 hump noses, 1 extruded implant, and 1 case of chronic inflammation (Table 2). Patients could have more than 1 diagnosis.
| Characteristic . | Finding . |
|---|---|
| Patients, n | 28 |
| Gender | |
| Male:female, n (%) | 9:19 (32.1:67.9) |
| Age, mean, in years (SD) | 40.1 (±12.4) |
| No. of previous rhinoplasties, mean (SD) | 1.75 (±1.18) |
| Preoperative diagnosis (patients may have more than 1 diagnosis) | |
| Deviated nose | 21 |
| Short or upturned nose | 8 |
| Hump nose | 6 |
| Extruded implant | 1 |
| Chronic inflammation | 1 |
| Follow-up period, mean, in months (SD) | 18.3 (±11.7) |
| Characteristic . | Finding . |
|---|---|
| Patients, n | 28 |
| Gender | |
| Male:female, n (%) | 9:19 (32.1:67.9) |
| Age, mean, in years (SD) | 40.1 (±12.4) |
| No. of previous rhinoplasties, mean (SD) | 1.75 (±1.18) |
| Preoperative diagnosis (patients may have more than 1 diagnosis) | |
| Deviated nose | 21 |
| Short or upturned nose | 8 |
| Hump nose | 6 |
| Extruded implant | 1 |
| Chronic inflammation | 1 |
| Follow-up period, mean, in months (SD) | 18.3 (±11.7) |
SD, standard deviation.
| Characteristic . | Finding . |
|---|---|
| Patients, n | 28 |
| Gender | |
| Male:female, n (%) | 9:19 (32.1:67.9) |
| Age, mean, in years (SD) | 40.1 (±12.4) |
| No. of previous rhinoplasties, mean (SD) | 1.75 (±1.18) |
| Preoperative diagnosis (patients may have more than 1 diagnosis) | |
| Deviated nose | 21 |
| Short or upturned nose | 8 |
| Hump nose | 6 |
| Extruded implant | 1 |
| Chronic inflammation | 1 |
| Follow-up period, mean, in months (SD) | 18.3 (±11.7) |
| Characteristic . | Finding . |
|---|---|
| Patients, n | 28 |
| Gender | |
| Male:female, n (%) | 9:19 (32.1:67.9) |
| Age, mean, in years (SD) | 40.1 (±12.4) |
| No. of previous rhinoplasties, mean (SD) | 1.75 (±1.18) |
| Preoperative diagnosis (patients may have more than 1 diagnosis) | |
| Deviated nose | 21 |
| Short or upturned nose | 8 |
| Hump nose | 6 |
| Extruded implant | 1 |
| Chronic inflammation | 1 |
| Follow-up period, mean, in months (SD) | 18.3 (±11.7) |
SD, standard deviation.
Anthropometric Measurements
The preoperative to postoperative changes in anthropometric measurements are summarized in Table 3. The mean postoperative change in dorsal height was an increase of 2.78% (P < .05). For radix height, the change was an mean increase of 2.26% (P < .05). The increases were statistically significant. Postoperative change in nasal length and nasal tip projection also showed mean increases of 7.53% and 2.40%, respectively (P < .05). There was a mean reduction of the external nasal deviation angle of 1.15° (P < .05), which was statistically significant. Changes in the NLA and NFA did not achieve any statistical significance (P = .161, .150).
Preoperative and Postoperative Anthropometric Measurements in Patients Who Underwent Removal of Silicone Implant and Revision Rhinoplasty With GDCG With Mold
| Measurements . | Mean value ± SD . | P value . |
|---|---|---|
| Ratio of radix height | <.05 | |
| Preoperative | 10.4 ± 5.3 | |
| Postoperative (% increase) | 12.7 ± 4.9 (2.26) | |
| Ratio of dorsal height | <.05 | |
| Preoperative | 20.1 ± 4.3 | |
| Postoperative (% increase) | 22.8 ± 4.2 (2.78) | |
| Ratio of nasal length | <.05 | |
| Preoperative | 68.2 ± 6.6 | |
| Postoperative (% increase) | 75.7 ± 6.0 (7.53) | |
| Ratio of nasal tip projection | <.05 | |
| Preoperative | 26.9 ± 2.6 | |
| Postoperative (% increase) | 28.9 ± 3.0 (2.40) | |
| Nasofrontal angle (°) | .15 | |
| Preoperative | 144.2 ± 6.7 | |
| Postoperative | 145.7 ± 8.4 | |
| Nasolabial angle (°) | .16 | |
| Preoperative | 100.5 ± 12.2 | |
| Postoperative | 97.9 ± 10.6 | |
| Nasal axis (°) | <.05 | |
| Preoperative | 2.6 ± 1.5 | |
| Postoperative | 1.5 ± 1.0 |
| Measurements . | Mean value ± SD . | P value . |
|---|---|---|
| Ratio of radix height | <.05 | |
| Preoperative | 10.4 ± 5.3 | |
| Postoperative (% increase) | 12.7 ± 4.9 (2.26) | |
| Ratio of dorsal height | <.05 | |
| Preoperative | 20.1 ± 4.3 | |
| Postoperative (% increase) | 22.8 ± 4.2 (2.78) | |
| Ratio of nasal length | <.05 | |
| Preoperative | 68.2 ± 6.6 | |
| Postoperative (% increase) | 75.7 ± 6.0 (7.53) | |
| Ratio of nasal tip projection | <.05 | |
| Preoperative | 26.9 ± 2.6 | |
| Postoperative (% increase) | 28.9 ± 3.0 (2.40) | |
| Nasofrontal angle (°) | .15 | |
| Preoperative | 144.2 ± 6.7 | |
| Postoperative | 145.7 ± 8.4 | |
| Nasolabial angle (°) | .16 | |
| Preoperative | 100.5 ± 12.2 | |
| Postoperative | 97.9 ± 10.6 | |
| Nasal axis (°) | <.05 | |
| Preoperative | 2.6 ± 1.5 | |
| Postoperative | 1.5 ± 1.0 |
GDCG, glued diced cartilage graft; SD, standard deviation.
Preoperative and Postoperative Anthropometric Measurements in Patients Who Underwent Removal of Silicone Implant and Revision Rhinoplasty With GDCG With Mold
| Measurements . | Mean value ± SD . | P value . |
|---|---|---|
| Ratio of radix height | <.05 | |
| Preoperative | 10.4 ± 5.3 | |
| Postoperative (% increase) | 12.7 ± 4.9 (2.26) | |
| Ratio of dorsal height | <.05 | |
| Preoperative | 20.1 ± 4.3 | |
| Postoperative (% increase) | 22.8 ± 4.2 (2.78) | |
| Ratio of nasal length | <.05 | |
| Preoperative | 68.2 ± 6.6 | |
| Postoperative (% increase) | 75.7 ± 6.0 (7.53) | |
| Ratio of nasal tip projection | <.05 | |
| Preoperative | 26.9 ± 2.6 | |
| Postoperative (% increase) | 28.9 ± 3.0 (2.40) | |
| Nasofrontal angle (°) | .15 | |
| Preoperative | 144.2 ± 6.7 | |
| Postoperative | 145.7 ± 8.4 | |
| Nasolabial angle (°) | .16 | |
| Preoperative | 100.5 ± 12.2 | |
| Postoperative | 97.9 ± 10.6 | |
| Nasal axis (°) | <.05 | |
| Preoperative | 2.6 ± 1.5 | |
| Postoperative | 1.5 ± 1.0 |
| Measurements . | Mean value ± SD . | P value . |
|---|---|---|
| Ratio of radix height | <.05 | |
| Preoperative | 10.4 ± 5.3 | |
| Postoperative (% increase) | 12.7 ± 4.9 (2.26) | |
| Ratio of dorsal height | <.05 | |
| Preoperative | 20.1 ± 4.3 | |
| Postoperative (% increase) | 22.8 ± 4.2 (2.78) | |
| Ratio of nasal length | <.05 | |
| Preoperative | 68.2 ± 6.6 | |
| Postoperative (% increase) | 75.7 ± 6.0 (7.53) | |
| Ratio of nasal tip projection | <.05 | |
| Preoperative | 26.9 ± 2.6 | |
| Postoperative (% increase) | 28.9 ± 3.0 (2.40) | |
| Nasofrontal angle (°) | .15 | |
| Preoperative | 144.2 ± 6.7 | |
| Postoperative | 145.7 ± 8.4 | |
| Nasolabial angle (°) | .16 | |
| Preoperative | 100.5 ± 12.2 | |
| Postoperative | 97.9 ± 10.6 | |
| Nasal axis (°) | <.05 | |
| Preoperative | 2.6 ± 1.5 | |
| Postoperative | 1.5 ± 1.0 |
GDCG, glued diced cartilage graft; SD, standard deviation.
To further analyze the outcome in patients with specific aesthetic complications, 2 groups of patients were identified: patients with deviation or displacement (n = 21), and patients with a short, upturned nose (n = 8). When analyzing the subgroup of patients with preoperative deviation or displacement, the average reduction of the deviation angle was 1.06° (P < .05), again showing statistically significance. In the subgroup of patients with an upturned nose, there was an average reduction of the NLA of 8.30° (P = .333) and an average increase of nasal length of 5.83% (P = .153), but neither achieved statistical significance.
Aesthetic Outcome
Aesthetic outcome assessment by 2 independent rhinoplasty surgeons reported a mean (SD) score of 3.02 (0.59) on a scale of 1 to 4. The majority (91.1%) of the patients were judged to be in the good or excellent outcome categories.
Complications
Two out of 28 patients (7.14%) encountered complications and required revision surgery. The first patient had an infection, and surgery under local anesthesia was required for removal of inflamed and necrotic tissue and irrigation. The second patient complained of postoperative dorsal irregularities, and revision surgery was performed for graft trimming to improve the aesthetic outcome. There were no donor site complications encountered in this series of patients. Detailed below are case examples of 2 commonly encountered scenarios following silicone rhinoplasty—deviated nose and short or upturned nose.
Case 1: Deviated Nose
A 35-year-old lady presented to our clinic seeking revision rhinoplasty. She had had 1 previous rhinoplasty 12 years previously. She wanted to improve her external nasal deviation and right nasal obstruction. On examination, there was external deviation to the left in the upper two-thirds and right deviation in the lower one-third of the nose.
She underwent revision rhinoplasty with an open approach. After raising the skin and soft tissue envelope, the previously inserted L-shaped silicone implant was removed, along with any fibrous capsular tissue. Osteotomies were performed to correct any bony deviation. Costal cartilage was harvested, and this was fashioned into bilateral extended spreader grafts, a side-to-side caudal septal extension graft, and a batten graft and placed accordingly. A new dorsal implant was created with GDCG and the Jang Cartilage Mold. Tip augmentation was done with diced costal cartilage wrapped with perichondrium. A well-maintained, straight nasal dorsum and well-projected nasal tip were evident 12 months postoperatively. Figure 4 shows the patient's preoperative and postoperative clinical photographs.
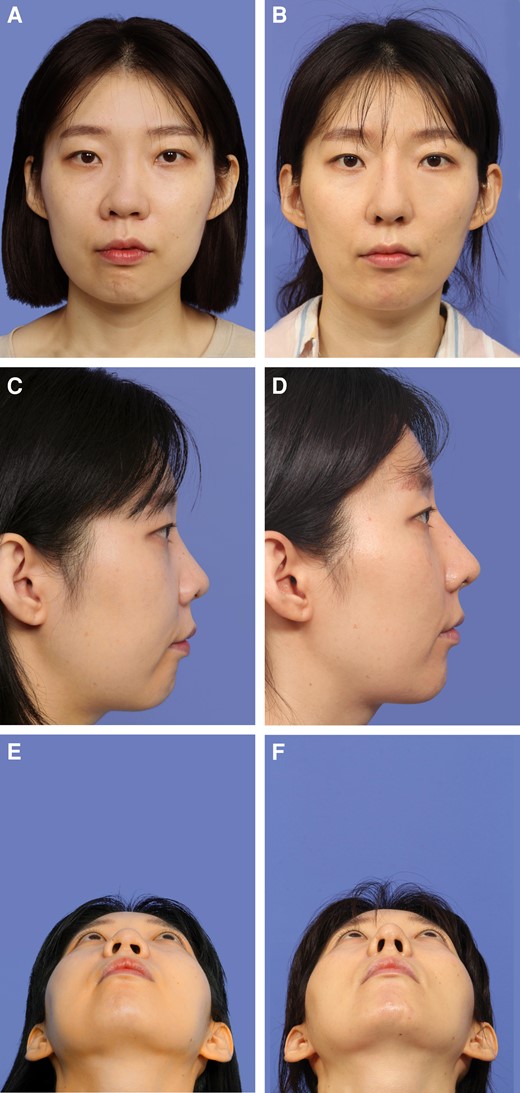
(A, C, E) A 35-year-old female presented with external nasal deviation following a silicone implant. She underwent removal of the silicone implant and revision rhinoplasty. (B, D, F) Postoperative photographs taken at 12 months demonstrated correction of the external nasal deviation in the (B) frontal view, and satisfactory dorsal augmentation and a smooth dorsum in the (D) lateral view. (F) Tip projection was also improved.
Case 2: Short, Upturned Nose
A 34-year-old lady presented to our clinic having had 2 previous rhinoplasties. The most recent surgery had been 5 years before presentation, in which silicone was used for dorsal augmentation. She wanted another revision surgery to improve her external nose appearance. On examination, her nose appeared relatively short with an upturned appearance. Her dorsum was low with a gentle concavity. The columella was tilted, with nostril asymmetry seen.
She underwent revision surgery with an open approach. The previously inserted silicone implant was removed with surrounding capsule. Other grafts from previous surgery were identified, including batten graft and tip grafts, and were removed. Costal cartilage was harvested. For this patient, bilateral extended spreader grafts and an end-to-end caudal septal extension graft were fashioned with costal cartilage and inserted. A new dorsal implant was created with GDCG covered with homologous fascia and a Jang Cartilage Mold. Diced cartilage was placed bilaterally lateral to the dorsal implant to fill up any potential space along the side wall. Last, crushed cartilage was inserted at the tip, covered with perichondrium. Postoperative facial photographs at 11 months demonstrated improvement of the upturned nose appearance, with a more acute nasolabial angle (a reduction of the NLA of 7.95°), reduced nostril show, satisfactory dorsal augmentation, and nostril symmetry. Figure 5 shows the patient's preoperative and postoperative clinical photographs.
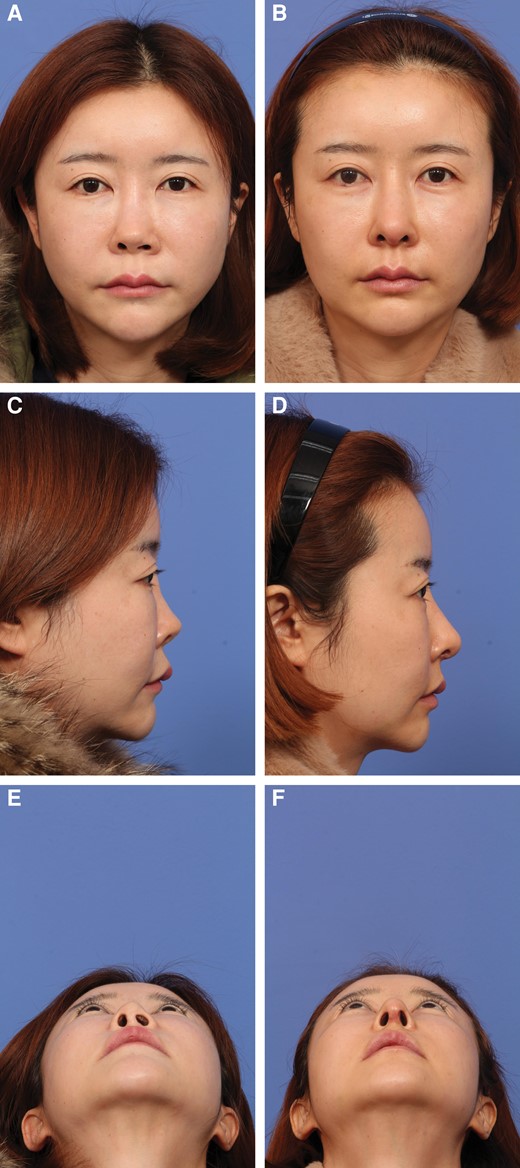
(A, C, E) A 34-year-old female presented with a short and upturned nose following silicone rhinoplasty. She underwent removal of the silicone implant and revision rhinoplasty. (B, D, F) The postoperative photographs were taken at 11 months. (B) In the frontal view, a straight and smooth dorsum is seen following revision surgery with molded glued diced cartilage grafting. Nostril asymmetry has been corrected. (D, F) In the lateral view, lengthening of the nose was achieved, with a more ideal tip position after derotation.
DISCUSSION
Our results have shown that molded GDCG as a grafting technique is reliable and can provide positive surgical outcomes, even in complicated silicone rhinoplasties that require revision dorsal augmentation. In revision rhinoplasty following the use of an alloplastic material such as silicone, most surgeons will prefer the use of an autologous material that is strong and has ample supply. Some surgeons may choose to perform revision augmentation with silicone or other alloplastic implants again, often due to ease of availability, familiarity, or technical limitations. As for autologous materials, dermafat has also been widely utilized for revision cases. However, a common concern with dermafat is resorption, which can be as high as 15% to 45% in some studies.5 Other issues with dermafat include donor site morbidities, and its inappropriateness as graft material for other structural support. In view of these issues with the above-mentioned materials, most surgeons will consider costal cartilage the ideal material in revision cases following the use of alloplastic materials. Some of the potential drawbacks of costal cartilage include donor site morbidities, additional surgical time, and implant-related complications such as warping, resorption, and infection.19 In our experience, harvesting of costal cartilage has minimal donor site morbidity, and in our series there were no donor site morbidities encountered. With increasing experience, harvesting did not add significant surgical time, typically requiring us only 10 to 15 minutes in uncomplicated cases. Therefore, in such complicated silicone rhinoplasty cases, our technique and material of choice is to completely replace the silicone implant with autologous costal cartilage.
Various methods have been employed for preparing costal cartilage for dorsal augmentation. Block costal cartilage, either in the form of a monobloc or in laminated form, has the benefit of having better structural integrity. However, warping is a concern when costal cartilage is used in this form, with studies showing an incidence from 3% to 26.1%.19 Other common complications include migration, infection, resorption, dorsal irregularities, and having an operated look due to the shrink-wrap phenomenon. Utilizing crushed cartilage for dorsal augmentation may avoid the problem of warping, but resorption was found to be significant, therefore employing it as the only augmentation material is not recommended.20 Diced costal cartilage overcomes the problem of warping and has been utilized in various ways, such as injecting free diced cartilage, diced cartilage wrapped with fascia, and GDCG, with the last method currently gaining widespread use.13 To shape the GDCG into a dorsal implant, many surgeons use a diagonally cut 3-mL syringe as a mold. However, when this method is employed, excess glue and fluid are often hard to remove from the GDCG, potentially overestimating the actual amount of cartilage placed in the dorsum. Furthermore, it can also lead to pebbling deformity of the dorsum, especially for thinned-skin individuals.13 With these options in mind, we concluded that the use of molded GDCG may be the most ideal option in this scenario. Molded GDCG has the benefits of autologous grafts, and at the same time avoids the problems of costal cartilage use such as warping. To solve some of the technical problems with GDCG use, Y. J. J. developed a novel mold, which allows finer control of the shape and volume when creating the GDCG implant. This novel mold has demonstrated satisfactory surgical and aesthetic outcome and provided many advantages over conventional techniques of cartilage grafting for dorsal augmentation in rhinoplasty.13 Our novel mold may be able to minimize the impact of resorption by removing excess fluid and glue during the molding process. Second, when the dorsal surface of the implant is covered by autologous perichondrium or homologous fascia during the molding process, the pebbling effect can be avoided.
To overcome some of the mentioned challenges in dorsal augmentation, other authors have derived new methods to improve on dorsal augmentation techniques in recent years. In a recent publication by Carles et al, a similar technique to ours has been described, with diced cartilage for dorsal augmentation, which also reported good aesthetic outcome.21 In that study, Y. J. J. developed a template for the creation of reproductive dorsal grafts with standard size and thickness utilizing diced cartilage and platelet-rich fibrin. One major difference between that study and our current study is that we focused solely on assessing the performance of our mold in complicated revision scenarios, after the favorable outcome seen in Y. J. J.'s previous study done on primary rhinoplasty cases.
In another recent study by Kovacevic et al, a “push up” technique has been described to manage saddle nose deformity and restore a normal dorsal line.22 The idea behind this technique originated in the recent resurgence of preservation rhinoplasty. The authors in the study explained that with this push-up technique the dorsum is reconstructed by reconstituting the subdorsal support and preserving the leading edge of the cartilaginous vault. The potential advantages of this over dorsal onlay implants include avoidance of graft show, minimizing the likelihood of potential warping of the rib cartilage, and a middle vault that complements the tip projection with no depression along the dorsum.
Daniel et al described their composite dorsal augmentation technique in a recent study.23 In their study, a combination of grafts from a single donor site was utilized, depending on the degree of augmentation required. The combination of grafts was broadly divided into a foundation layer, employing high spreader grafts and a dorsal gap graft, and an overlying contour layer of 3 possible types of fascia or diced fascia grafts. The authors proposed that with an incremental approach and different combinations, a highly individualized result might be achieved.
In our study, deviation was the most common aesthetic concern following augmentation rhinoplasty with a silicone implant, which was consistent with most studies. Lam et al reported displacement to be the most common complication encountered in 1079 rhinoplasties with silicone.24 Because our study aimed to specifically investigate the performance of molded GDCG as a dorsal implant, dorsal height and radix height were the important anthropometric measurements for outcome assessment. In our study, when comparing the preoperative and postoperative anthropometric measurements, there was an increase in both dorsal and radix height that was statistically significant, demonstrating that molded GDCG was able to provide satisfactory or better dorsal augmentation compared to silicone implants. Molded GDCG also managed to reduce nasal axis deviation in our study by an average of 1.15°, which was statistically significant. In addition, there was also a statistically significant increase in both nasal length and nasal tip projection. This suggests that molded GDCG may also have an important role in lengthening short noses and improving underprojected tips. Subgroup analysis of patients with preoperative deviation revealed similar results in terms of deviation correction. For the upturned nose subgroup, both a reduction of NLA and increase in nasal length were demonstrated, but this did not achieve statistical significance, likely due to the small number of patients. We believe that dorsal augmentation can assist in lengthening the nose by creating a new start point at the radix, and by improving the dorsal concavity it will also give the illusion of a longer-looking nose to the observer. Although the primary role of dorsal augmentation may not be to lengthen the nose or de-rotate the nasal tip, we find that there is often dorsal insufficiency that needs to be corrected after the lengthening and de-rotation process, especially in Asian noses. The dorsal implant is therefore essential to improving the overall harmony of the external nose in such cases.
Our results were able to demonstrate that molded GDCG was able to deliver significant dorsal augmentation and nasal axis deviation correction, as supported by the changes seen in the anthropometric measurements. Overall objective aesthetic outcome was also judged to be good to excellent in 91.1% of the patients.
The low rate of complication shows that molded GDCG is a safe technique when employed in revision settings. Both cases with complications were not directly attributed to GDCG use as a dorsal implant. Only 1 major complication was encountered, in which a patient had a postoperative infection, which originated from the septum and not the dorsum, unrelated to the molded GDCG implant. This patient required surgical debridement. There was 1 minor complication of aesthetic dissatisfaction, with the patient requiring revision surgery under local anesthesia to trim the caudal septal extension graft to improve dorsal irregularities. Again, this complication was not directly due to the molded GDCG implant. Overall, during the follow-up period, no revision surgery was requested by patients for poor aesthetic outcome related to dorsal augmentation with molded GDCG.
To the best of our knowledge, our study may be the first case series in English literature to evaluate the aesthetic outcome of a specific surgical technique for revision augmentation in complicated silicone rhinoplasty cases. However, despite the encouraging results, we recognize there were limitations to our study. The sample size of our study was relatively small, but given the fact that we were investigating a very selected group of complicated patients, we believe the study was still able to provide an insightful conclusion. The surgical techniques employed in the patients were not uniform, and some of the grafting techniques, such as spreader grafts and caudal septal extension grafts, may have played a role in enhancing the structural integrity of the nose and therefore helped support dorsal augmentation and deviation correction. As far as aesthetic outcome assessment, employing a patient-reported satisfaction outcome score may provide useful information. Although our mean follow-up period of 18.3 months should be considered reasonable given the various practical constraints, a longer follow-up period would be more ideal because some of the complications related to dorsal augmentation such as displacement, deviation, and graft resorption may occur months to years following revision surgery. Resorption in particular is a major concern for rhinoplasty surgeons utilizing diced cartilage for a dorsal implant.
The use of molded GDCG is a novel technique that continues to evolve. Our study supports its utility in complicated silicone rhinoplasties, which are a commonly encountered problem in the Asian population. Our study also showed that molded GDCG was able to overcome previously encountered challenges of costal cartilage such as warping and the problem of having excess glue in GDCG implants, and furthermore demonstrated that molded GDCG is robust enough to be utilized in both primary and revision rhinoplasty. We are hopeful that our study will support the use of molded GDCG for dorsal augmentation in different clinical situations, providing an additional option for dorsal augmentation in the armamentarium of rhinoplasty surgeons.
CONCLUSIONS
Revision rhinoplasty following unsuccessful silicone augmentation is commonly encountered in the Asian population. The majority of these patients present with deviation or displacement of silicone implants or with short or upturned noses. The use of molded GDCG for revision dorsal augmentation is a reliable option, especially in providing adequate dorsal augmentation and external nasal axis deviation correction. Overall, it delivers good to excellent aesthetic outcomes with low complication rates.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Dr Fung is a consultant, Department of Otorhinolaryngology, Head and Neck Surgery, Tan Tock Seng Hospital, Singapore.
Dr Kim is a resident, Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea.
Dr Jang is chairman and professor, Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea.
Dr Liao is a attending physician, Department of Otolaryngology, Head and Neck Surgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.



