-
PDF
- Split View
-
Views
-
Cite
Cite
Paul G Ruff, Gaurav Bharti, Joseph Hunstad, Bill Kortesis, Barry DiBernardo, Richard Gentile, Steven Cohen, Allison Martinez, Sachin M Shridharani, Safety and Efficacy of Renuvion Helium Plasma to Improve the Appearance of Loose Skin in the Neck and Submental Region, Aesthetic Surgery Journal, Volume 43, Issue 10, October 2023, Pages 1174–1188, https://doi.org/10.1093/asj/sjad055
Close - Share Icon Share
Abstract
Minimally invasive procedures that deliver thermal energy to subcutaneous tissue offer a solution when deciding between excisional and noninvasive options to address face and neck aging-related changes. A minimally invasive helium plasma device, Renuvion, was first utilized for subdermal tissue heating to reduce skin laxity under an FDA general clearance for cutting, coagulation, and ablation of soft tissue.
The purpose of this study was to demonstrate the safety and effectiveness of the helium plasma device for improving the appearance of loose skin in the neck and submental region.
Patients undergoing the procedure with the helium plasma device in the neck and submentum were studied. They were seen for 6 months following the procedure. The primary effectiveness endpoint for improvement in lax skin in the treatment area was determined by 2 of 3 blinded photographic reviewers. The primary safety endpoint was the level of pain after treatment.
The primary effectiveness endpoint was met; 82.5% demonstrated improvement at Day 180. The primary safety endpoint was met; 96.9% of patients experienced no pain to moderate pain to Day 7. There were no serious adverse events reported related to the study device or procedure.
The data demonstrate benefit to patients by improvement of the appearance of lax skin in the neck and submental region. Outcomes resulted in US Food and Drug Administration 510(k) clearance in July 2022, expanding indications for the device to include subcutaneous dermatological and aesthetic procedures to improve the appearance of loose skin in the neck and submental region.
초록
얼굴과 목 부위의 노화 관련 증상을 해결하기 위해 절제술과 비침습적 시술 중에 선택할 때 피하 조직에 열 에너지를 전달하는 최소한의 침습적 시술이 해결책을 제시합니다. Renuvion은 최소한의 침습적 헬륨 플라즈마 장치로, 연조직의 절단, 응고 및 절제에 대한 FDA의 일반 허가를 받아 피부 처짐을 완화하기 위한 피하 조직 가열에 처음 사용되었습니다.
이 연구의 목적은 목과 서브멘탈 부위의 처진 피부를 개선하는 데 있어 헬륨 플라즈마 장치의 안전성 및 효과를 입증하는 것입니다.
헬륨 플라즈마 장치로 목과 서브멘탈 부위에 시술을 받고 있는 환자를 대상으로 연구를 진행했습니다. 관찰은 시술 후 6개월 동안 진행되었습니다. 치료 부위의 처진 피부 개선에 대한 주 효과 평가지표는 맹검 사진 검토자 3명 중 2명이 결정했습니다. 주 안전성 평가지표는 치료 후 통증 정도였습니다.
주 유효성 평가지표가 충족되었으며 180일째에 82.5%가 개선된 것으로 나타났습니다. 주 안정성 평가지표가 충족되었으며 7일째에 96.9%의 환자가 통증이 없거나 경미한 통증을 경험했습니다. 연구 장치 또는 시술과 관련된 심각한 부작용은 보고되지 않았습니다.
목과 서브멘탈 부위의 처진 피부가 개선되어 환자에게 도움이 된다는 데이터가 나왔습니다. 이를 통해 2022년 7월 미국 식품의약국 510(k) 허가를 받아 목과 서브멘탈 부위의 처진 피부를 개선하는 피하 피부과학 및 미용 시술로 장치 사용 범위를 확장했습니다.

The effects of aging occur over the entire body but are most visibly evident in the appearance of the largest organ system, the skin. As one ages, collagen and elastin fibers in the hypodermis become sparse and increasingly disordered, resulting in aesthetically undesirable effects such as wrinkling and sagging associated with increased skin laxity.1,2 Age-related changes to the face and the neck are more apparent and more concerning for patients, because they are the anatomical areas first noted by themselves and others, as these areas above the clavicle are not typically covered by clothing. The increased use of real-time web camera communication during the COVID-19 pandemic has only served to increase awareness of physical features and the signs of facial and cervical aging.3 Although excisional facial and neck rejuvenation procedures can produce dramatic results, scar burden, recovery time, and other morbidities such as numbness have a significant impact on the popularity of these procedures.4 Noninvasive procedures involving transepidermal delivery of either ultrasound, light, or radiofrequency energy to reduce skin laxity are viable options but are accompanied by modest outcomes compared with excisional procedures.5-8
Minimally invasive devices that deliver thermal energy in the same subcutaneous tissue planes in which liposuction is performed have achieved the appropriate balance between excisional and noninvasive procedures. Laser-assisted liposuction (LAL) has been shown to be effective at producing more skin tightening than suction-assisted lipectomy alone.9,10 One study showed an average skin surface area reduction of 22% and 17% at 1- and 3-month follow-up visits, respectively, and an average of 26% skin tightening at 3 months when utilizing LAL to address abdominal skin laxity.11 In 2008, radiofrequency (RF) devices were introduced and have demonstrated efficacy in multiple body areas, including the abdomen, the arms, and the face and neck.4,12-16 One of the main challenges associated with the use of these minimally invasive devices to address skin laxity is the balance that must be achieved between heating the internal tissues enough to achieve the desired tissue/collagen contraction and maintaining safe external tissue temperatures. LAL and RF devices work on the principle of bulk tissue heating.4,11-17 With these devices, the energy is primarily directed into the dermis, and the device is activated to achieve subdermal tissue temperatures ranging from 45°C to 65°C across the entire volume of tissue or until the epidermal temperatures rise to levels that require pausing treatment to allow for cooling.4,11-18 The tissue being treated must be maintained within this range of subdermal temperatures for a minimum of 120 seconds for maximal collagen contraction to occur.19 Although these devices have proven effective in reducing skin laxity, the process of heating the tissue to the treatment temperature and maintaining that temperature for extended periods can be time consuming.20 In addition, during this process, the heat eventually conducts to the epidermis, requiring monitoring of epidermal temperatures to ensure that they do not exceed safe levels.4,11-18
In 2016, a minimally invasive helium-based plasma device (Renuvion; Apyx Medical, Clearwater, FL) was first utilized for subdermal tissue heating to reduce skin laxity. The system consists of an electrosurgical generator unit, a handpiece, and a supply of helium gas. RF energy is delivered to the handpiece by the generator to energize an electrode. When helium gas is passed over the energized electrode, a helium plasma is generated, which allows heat to be applied to tissue in 2 different and distinct ways. First, heat is generated by the actual production of the plasma beam itself through the ionization and rapid neutralization of the helium atoms. Second, because plasmas are very good electrical conductors, a portion of the RF energy passes from the electrode to the patient and heats tissue by passing current through the resistance of the tissue, a process known as joule heating. This method of heating of the tissue is different than the bulk heating of LAL and RF devices. As the tip of the device is drawn through the subdermal plane, new tissues are introduced to the energy, which allows the plasma beam to quickly alternate between treating the different tissues surrounding the tip of the device. At each new treatment location, the tissue is heated instantly to temperatures greater than 85°C for approximately 0.04 seconds.21 At 85°C, this is long enough to achieve maximal collagen contraction in the subdermal tissues.19 Unlike bulk tissue heating, the tissue surrounding the treatment location remains at cooler temperatures, resulting in rapid cooling after the application of the energy through conductive heat transfer. In the process, less heat is transferred to the epidermis, resulting in safe external temperatures without the need for epidermal temperature monitoring.20 This premise was validated in a recent study involving FLIR (forward-looking infrared) video footage showing lower epidermal temperatures for the instant-heating helium plasma device in comparison to a bulk-heating bipolar RF device.22
Until recently, all minimally invasive LAL, RF, and helium-based plasma devices have been cleared by the US Food and Drug Administration (FDA) for the intended uses of either electrocoagulation and hemostasis or the coagulation of soft tissues that ultimately results in the desired contraction of collagen.17,23,24 The purpose of this study was to demonstrate the safety and effectiveness of the helium plasma device for use in aesthetic procedures for the specific purpose of improving the appearance of loose skin in the neck and submental region.
METHODS
The study was a prospective, multicenter, evaluator-blinded, FDA investigational device exemption clinical trial to evaluate the safety and effectiveness of the Renuvion device alone for improving loose skin in the neck. No liposuction was performed in the study, and the study was completed in 2 phases. Phase 1 of the study began in December 2019, and the last patient in Phase 2 was seen for their final 180-day follow-up visit in February 2022. Phase 1 was conducted as an initial safety evaluation to identify any new risks associated with use of the device and to establish the expected treatment effects before starting Phase 2. Following IRB approval (Sterling IRB, Atlanta, GA), 17 patients were enrolled, treated, and followed through the 180-day postoperative time point across 3 investigational sites. Subcutaneous induration was identified as the only new risk related to the procedure in Phase 1, resulting from coagulated tissue and fat remaining in the treatment area, similar to the complication known to occur in other percutaneous procedures involving the delivery of energy to subcutaneous tissues.25,26 Before beginning Phase 2, the study protocol was amended to include aspiration of no more than 8 mL from the treated area to reduce the concentration of any residual fluids. Additional modifications were made to the protocol at this time to account for conducting the study during the COVID-19 pandemic and to improve the consistency of the before and after photographs. Because protocol changes were made after completion of Phase 1, a sample size of 65 patients was chosen for Phase 2, to ensure adequate numbers for statistical significance based on the results of the second phase alone. Following IRB approval of the protocol changes, the 65 patients were enrolled across 6 investigational sites in the US. All investigators had significant experience with the study device and other minimally invasive subdermal heating devices before participation in the study. This was a single-arm study in which all patients meeting the inclusion and exclusion criteria were treated with the study device. Written informed consent was obtained from all patients before study participation.
Eligible patients were males or females 35 to 65 years of age who were seeking a reduction in lax skin in their neck and submental region. Patients underwent pretreatment assessments for verification of eligibility for participation in the study. Patients with more than mild platysmal banding were excluded. Assessment of the platysmal bands was completed through comparison of baseline images of the patient performing a grimace with the Geister et al validated assessment scale for platysmal bands (Figure 1).27 Also, because the study procedure did not include liposuction to allow for assessment of the effectiveness of the study device by itself, patients with excessive subcutaneous fat in the treatment area were excluded. All patients meeting the eligibility requirements were enrolled in the study.
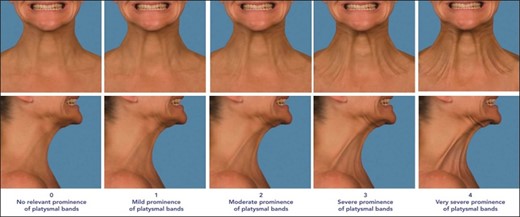
Geister validated assessment scale for platysmal bands (from left to right): Patient in the standard anatomical position anterior and right lateral view of “no relevant prominence of platysmal bands” or 0 on the scale; patient in the standard anatomical position anterior and right lateral view of “mild prominence of platysmal bands” or 1 on the scale; patient in the standard anatomical position anterior and right lateral view of “moderate prominence of platysmal bands” or 2 on the scale; patient in the standard anatomical position anterior and right lateral view of “severe prominence of platysmal bands” or 3 on the scale; and patient in the standard anatomical position anterior and right lateral view of “very severe prominence of platysmal bands” or 4 on the scale.28 Reproduced with permission from Geister TL et al 2013.
At the discretion of the investigators, optional preprocedure antibiotics (Keflex [Pragma Pharmaceuticals; Locust Valley, NY] 500 mg bid × 7 days or Z-Pak [Pfizer; New York, NY]), antianxiety medication (Ativan [Bausch Health Companies Inc.; Quebec, Canada] 2 mg or Valium [Genentec; San Francisco, CA] 10-20 mg), and/or pain medication administered within 90 minutes of the procedure (Norco [Allergan; Irvine, CA] 5-10 mg, hydrocodone 5-10 mg, or Ultram [Janssen Pharmaceuticals; Beerse, Belgium] 200 mg) were provided to the patients.
To support explanation of the helium plasma portion of the study treatment, a procedural video has been provided (Video, available online at www.aestheticsurgeryjournal.com). In addition, technique tips are provided below to ensure consistency in effectiveness and safety for all users of the technology. The steps of the helium plasma treatment portion of the procedure were as follows. The treatment area included the submental area and the bilateral tissue of the neck from the mandibular border to the posterior border of the sternocleidomastoid muscle, as shown in Figure 2.
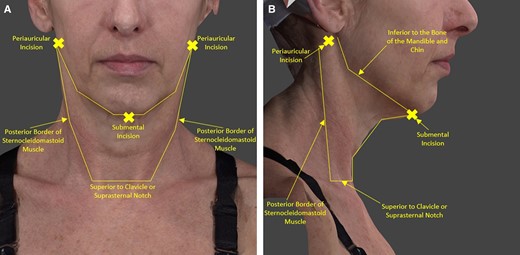
Treatment area and incisions: R/L periauricular incision and submental incision. (A) Patient in the standard anatomical position anterior view with graphics showing the treatment area and incisions. (B) Patient in the standard anatomical position right lateral view with graphics showing the treatment area and incisions.
One submental crease and 2 perilobular incisions were utilized to access the treatment area. The incisions should be made 3 to 4 cm in length to ensure they are large enough to allow for sufficient helium egress around the shaft of the device and through the other incisions during treatment. Ensuring sufficient helium egress reduces the amount of postprocedure crepitus associated with residual subcutaneous helium and avoids any risks associated with excessive helium accumulation in the treatment area.
By way of these 3 incisions, the entire treatment area was infused with 150 to 250 mL (or until adequate tissue turgor was achieved) of wetting solution comprised of normal saline or lactated Ringer’s solution with lidocaine and epinephrine. No sedation was employed in combination with the local anesthesia.
The amount of wetting solution infused should be no more and no less than what is typically placed in the neck and jawline area for suction-assisted lipectomy procedures. Once infused, after adequate time was allotted for the wetting solution to take effect, the treatment area was undermined with a 3- to 4-mm diameter cannula.
Sufficient undermining eases the passage of the helium plasma device and provides a clear pathway for helium to travel underneath the tissue and escape through the other incision sites. This facilitates helium egress from the treatment area. After undermining, the helium plasma treatment consisted of 4 to 6 treatment passes with settings of 70% power, 1.5 liters per minute of helium flow, and an activation speed of 1 to 3 cm.
Figure 3A, B provides an illustration of treatment strokes and passes. The helium plasma energy is applied to the subcutaneous tissue by activating the device while performing a series of single linear applications called strokes. A treatment pass is the accumulation of the sequential strokes needed to fully treat the tissue in 1 portion of the treatment area. Figure 3A depicts the placement of the treatment strokes (8 in this example) required to treat the lateral side of the neck from the right perilobular incision. These 8 strokes comprise 1 complete treatment pass for this portion of the treatment area. Figure 3B depicts the placement of the treatment strokes (7 in this example) required to treat the submental area from the incision in the submental crease. These 7 strokes comprise 1 complete treatment pass for this area.
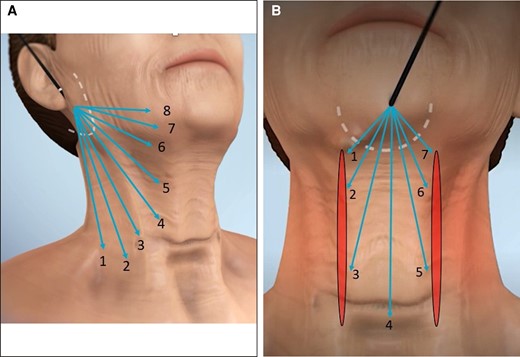
Illustration of strokes and passes for helium plasma treatment. (A) Three-dimensional generated anatomical model right oblique view illustrating the strokes and passes for helium plasma treatment. Produced by Canfield Scientific (Parsippany, NJ). (B) Three-dimensional generated anatomical model anterior view illustrating strokes and passes for helium plasma treatment. Produced by Canfield Scientific.
Care should be taken not to overlap treatment where the 2 lateral portions of the treatment area abut the submental portion (see areas indicated as borders to the treatment pass lines in Figure 3B). In addition, it is recommended to treat 1 lateral area, followed by the contralateral area, and then wait a few minutes for thermal relaxation of the tissue before proceeding with treatment in the submental area. This is a conservative approach that minimizes the risks of stacking energy delivery at the intersection of adjacent areas.
Strokes should be performed 2 to 3 cm apart to avoid performing multiple, subsequent strokes under the same tissue and to minimize the risk of thermal injury. Because treatment strokes converge at the incision site, application of the energy should be stopped as the tip of the device approaches the incision to avoid overtreatment in this area. White lines are provided on the shaft of the device to alert the user of the proximity of the tip to the incision. Activation should be stopped when the proximal white line on the shaft of the device becomes visible at the incision.
When performing a stroke, the treatment speed should be maintained at 1 to 3 cm. Treatment speeds slower than 1 cm increase the risks of overheating the tissue. Treatment speeds faster than 3 cm can decrease effectiveness results by not heating the tissue long enough to maximize contraction.
The study treatment consisted of 4 to 6 treatment passes in each of the bilateral areas of the neck and the submental area. The total number of passes performed should be based on the tissue quality of the patient. For patients with substantial dermal thinning and poor skin quality, one should consider reducing the number of passes to 4 to minimize the risk of cutaneous burn to the tissue. Following treatment with the study device, manual expression and surveillance aspiration with a 10-mL locking syringe were performed to evacuate any excess helium gas or residual fluid from the treatment area. Because this aspiration was not intended for fat removal, the amount was limited to an 8-mL maximum in the study.
The majority of residual fluid or coagulated tissue should be removed from the treatment area to minimize the risk of a postprocedure inflammatory response and subsequent subcutaneous induration. The maximum limit of 8 mL of aspirate was a condition imposed by the study protocol to minimize the potential impact of tissue removal on the effectiveness results. There is no need to limit the aspirate amount to 8 mL in standard practice.
Following the procedure, patients were directed to wear a neck compression garment with a Velcro chin strap for 22 hours per day (removing it only for showering or bathing) for the first 3 days. For days 4 to 21 postprocedure, patients were directed to continue to wear the compression garment at night. No postoperative physiotherapy was performed. Patients were seen for follow-up visits at 1, 7, 14, 30, 90, and 180 days postprocedure. Postprocedure assessments and 2-dimensional (2D) and 3-dimensional (3D) photographs were performed at each of the follow-up visits.
All baseline and follow-up photographs were taken with the Canfield Scientific, Inc. Vectra M4 Plus 3D imaging system (Parsipanny, NJ) and the same standardized photography views and lighting were employed throughout the study. To match the patient to their original baseline view, the study site staff utilized a ghost imaging feature in the camera system that overlayed the patient's live image with a translucent version of their baseline image. All images were reviewed by Canfield for quality assurance and retaken within the patient's visit window as needed to ensure consistency.
Effectiveness Endpoints
The primary effectiveness endpoint was improvement in the appearance of lax skin in the neck and submental region at 180 days postprocedure as determined by blinded independent photographic reviewers (IPRs). Three experienced IPRs who were not involved in the study treatments performed a review of the pretreatment and posttreatment image sets for each patient. The IPR review was conducted by Canfield independent of the study sponsor. The order of the pretreatment and posttreatment image sets were randomized, and the IPRs were blinded to the study visit associated with each image set. IPRs were asked to choose the image they thought to be the posttreatment image. Success was defined as selection of the correct posttreatment image by 2 out of the 3 reviewers. The percentage of patients with a correct posttreatment image selection was then calculated.
Additional effectiveness endpoints in the study included the following: quantitative measurement in the overall lift of the submental area at 180 days postprocedure. For this assessment, the right profile view of extracted 2D images was analyzed by Canfield image analysis software with marks at the lateral canthus of the eye, anterior nostril margin, and the chin attachment point of the neck to allow for the analysis of submental area, as shown in the box under the chin in Figure 4. An area reduction of more than 20 mm² from the baseline to the follow-up image was considered to be an improvement.
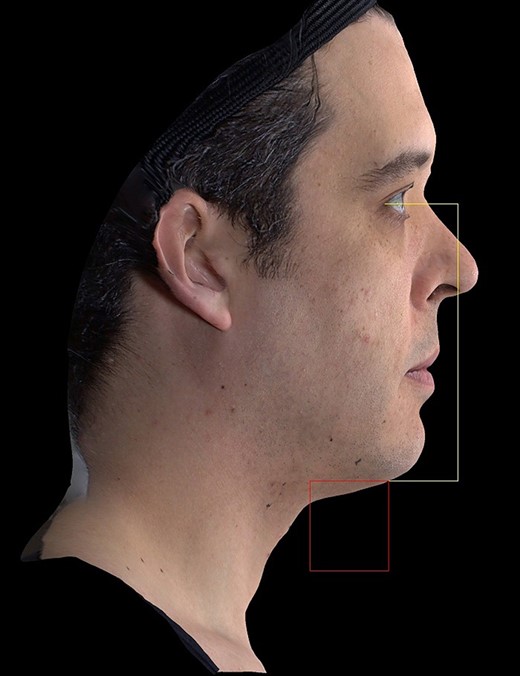
Quantitative assessment of submental area. Produced by Canfield Scientific (Parsippany, NJ).
For assessment of quantitative improvement in submental volume at 180 days postprocedure, a series of anatomically defined landmarks on the baseline 3D image was utilized to define the submental areas of interest for the volume difference measurements. The landmarks were transposed with marker-less tracking of points on the surface of the skin to the corresponding follow-up image (Figure 5). The Canfield image analysis software then determined the total change in submental volume by comparing the volume measured in the baseline and follow-up images.

Quantitative assessment of submental volume. (Left side) A 3-dimensional (3D) generated anatomical model right oblique view of the quantitative assessment of submental area. (Right side) A 3D generated anatomical model left oblique view of the quantitative assessment of submental area. Produced by Canfield Scientific (Parsippany, NJ).
Patient and investigator assessment of improvement in the appearance of the patient was accomplished with the Global Aesthetic Improvement Scale (GAIS). Patient satisfaction with the procedure was assessed with a study-specific questionnaire. The questionnaire has been provided in the Appendix, available online at www.aestheticsurgeryjournal.com.
Safety Endpoints
The primary safety endpoint was the level of pain and discomfort reported by the patient on an 11-point numeric rating scale (NRS), for which 0 was no pain and 10 was the most pain, through the 7-day follow-up visit. In this study, pain was defined as the average pain reported in the treatment area utilizing the following categories: none (score of 0), mild (score of 1-5), moderate (score of 6-7), and severe (score of 8-10).
All adverse events (AEs) and expected treatment effects (ETEs) observed by study patients, investigators, or other study staff from first exposure to the study product through the last study follow-up visit were recorded. An ETE was defined as any typical treatment side effect of the study device or procedure of mild to moderate severity and lasting a typical maximum duration. ETEs for this device and procedure were established in Phase 1 as mild to moderate discomfort/pain, edema, erythema, ecchymosis, temporary sensory nerve changes (touch sensitivity, itching, temporary numbness/tingling), transient migratory firmness, and temporary or transient crepitus. An AE was defined as any new medical problem, or exacerbation of an existing problem, experienced by a patient while enrolled in the study, whether or not it was considered device-related by the investigator.
RESULTS
A total of 65 patients were enrolled and underwent the study procedure in Phase 2. Of them, 9.2% (n = 6) were male and 90.8% (n = 59) were female, with an average age of 55.9 ± 6.3 (range 38-65 years of age) at the time of treatment. Most of the treated patients (93.8%, n = 61) were Caucasian/White, followed by 4.6% (n = 3) Hispanic or Latino, and 1.5% (n = 1) Asian. The average BMI was 25.4 ± 4.9 (range 17.1-47.8).
Following treatment, 63 patients completed their scheduled follow-up visits through to Day 90; 1 patient exited the study before the Day 30 follow-up visit, and 1 patient was lost to follow-up. Sixty-two patients returned for the Day 180 follow-up visit, and sixty-one of them had Day 180 images taken; 1 patient was unable to return for their Day 180 follow-up visit due to COVID-19, and 1 patient was unable to return in-person to the clinic, so they were seen virtually and were unable to have images taken.
Effectiveness Endpoints
The primary effectiveness endpoint of the study was met (Table 1). The observed proportion of patients achieving the primary effectiveness endpoint and demonstrating an improvement in the appearance of lax skin in the neck and submental region at Day 180 was 82.5% (n = 52; 97.5% 1-sided lower confidence level (CL) = 70.9%, P < .0001). For this Day 180 primary endpoint analysis, the Day 90 images were imputed forward for 2 patients who completed the study but did not have Day 180 images to bring the total number of patients included in the analysis to 63. The performance goal for the primary effectiveness endpoint was also met at the Day 90 follow-up time point with 76.2% (n = 48; 97.5% 1-sided lower CL = 63.8%, P = .0004) of patients achieving success based on the IPR review. These data provide evidence of continued improvement in skin laxity up to 6 months postprocedure. Based on results observed by the investigators for both study and nonstudy patients, additional improvement in skin laxity is expected to continue up to 1 year postprocedure. Representative results are shown in Figures 6-9.
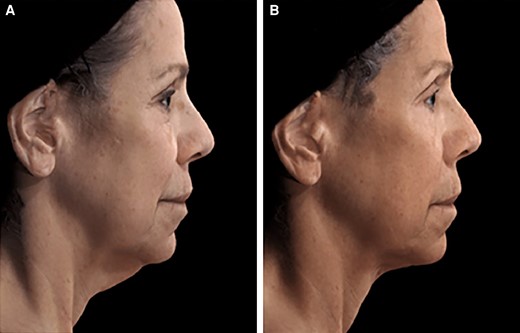
A 65-year-old female patient seeking improvement for lax tissue in the neck and submental region who underwent helium plasma treatment only. (A) Patient in standard anatomical position right lateral view preoperative image. (B) Patient in standard anatomical position right lateral view postoperative image taken at Day 180 follow-up visit.
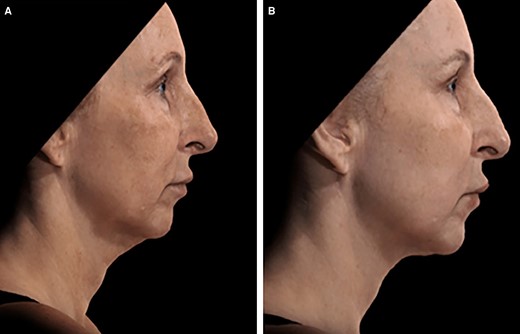
A 58-year-old female patient seeking improvement for lax tissue in the neck and submental region who underwent helium plasma treatment only. (A) Patient in standard anatomical position right lateral view preoperative image. (B) Patient in standard anatomical position right lateral view postoperative image taken at Day 180 follow-up visit.
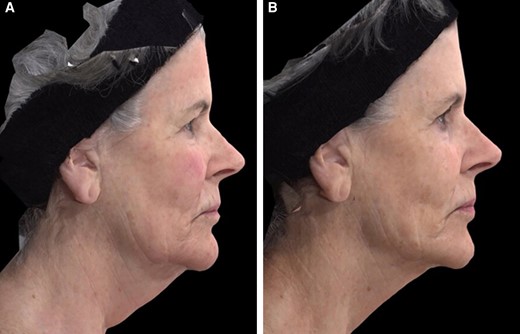
A 63-year-old female patient seeking improvement for lax tissue in the neck and submental region who underwent helium plasma treatment only. (A) Patient in standard anatomical position right lateral view preoperative image. (B) Patient in standard anatomical position right lateral view postoperative image taken at Day 180 follow-up visit.
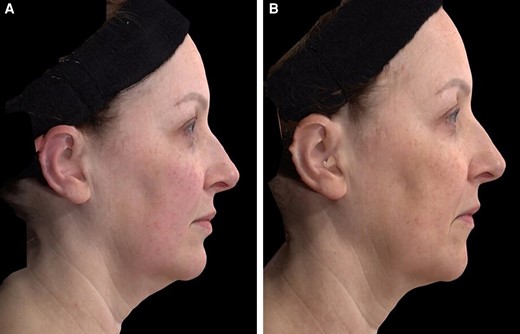
A 52-year-old female patient seeking improvement for lax tissue in the neck and submental region who underwent helium plasma treatment only. (A) Patient in standard anatomical position right lateral view preoperative image. (B) Patient in standard anatomical position right lateral view postoperative image taken at Day 180 follow-up visit.
Primary Effectiveness Endpoint and IPR Image Assessment at Day 180 and Day 90
| Follow-up time point . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| 180 days | 82.5% (52/63) | 55% | 70.9% | <.0001 | Pass |
| 90 days | 76.2% (48/63) | 55% | 63.8% | .0004 | Pass |
| Follow-up time point . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| 180 days | 82.5% (52/63) | 55% | 70.9% | <.0001 | Pass |
| 90 days | 76.2% (48/63) | 55% | 63.8% | .0004 | Pass |
IPR, independent photographic reviewer.
Primary Effectiveness Endpoint and IPR Image Assessment at Day 180 and Day 90
| Follow-up time point . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| 180 days | 82.5% (52/63) | 55% | 70.9% | <.0001 | Pass |
| 90 days | 76.2% (48/63) | 55% | 63.8% | .0004 | Pass |
| Follow-up time point . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| 180 days | 82.5% (52/63) | 55% | 70.9% | <.0001 | Pass |
| 90 days | 76.2% (48/63) | 55% | 63.8% | .0004 | Pass |
IPR, independent photographic reviewer.
The results for the additional effectiveness endpoints in the study at the Day 180 follow-up visit are summarized in Table 2. The quantitative assessment of overall lift of the neck and submental tissue resulted in an average reduction in area of 41 mm2 across all patients in the study, which exceeded the success criteria of 20 mm2. In addition, 68.3% of patients achieved a reduction in submental volume based on quantitative assessment of the 3D images with Canfield image analysis. The assessment of improvement in appearance with the GAIS showed that 85.5% of patients rated themselves improved, and study investigators noted improvement in 87.1% of patients. Patients also reported high satisfaction with the procedure, with 72.6% being happy with the results, 74.2% stating they would recommend the procedure to a friend, and 75.8% willing to have the procedure performed in another body area.
| Endpoint . | Result . |
|---|---|
| Quantitative improvement in lift of neck and submental tissue (average reduction in area across all patients) | 41 mm2 |
| Quantitative improvement in submental volume (% of patients) | 68.3% |
| Patient assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 85.5% |
| • No change | 11.3% |
| • Worse, much worse, or very much worse | 3.2% |
| Investigator assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 87.1% |
| • No change | 9.7% |
| • Worse, much worse, or very much worse | 3.2% |
| Patient satisfaction (% of patients) | |
| • Happy with the results | 72.6% |
| • Would recommend procedure to a friend | 74.2% |
| • Would have procedure performed in another body area | 75.8% |
| Endpoint . | Result . |
|---|---|
| Quantitative improvement in lift of neck and submental tissue (average reduction in area across all patients) | 41 mm2 |
| Quantitative improvement in submental volume (% of patients) | 68.3% |
| Patient assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 85.5% |
| • No change | 11.3% |
| • Worse, much worse, or very much worse | 3.2% |
| Investigator assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 87.1% |
| • No change | 9.7% |
| • Worse, much worse, or very much worse | 3.2% |
| Patient satisfaction (% of patients) | |
| • Happy with the results | 72.6% |
| • Would recommend procedure to a friend | 74.2% |
| • Would have procedure performed in another body area | 75.8% |
GAIS, Global Aesthetic Improvement Scale.
| Endpoint . | Result . |
|---|---|
| Quantitative improvement in lift of neck and submental tissue (average reduction in area across all patients) | 41 mm2 |
| Quantitative improvement in submental volume (% of patients) | 68.3% |
| Patient assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 85.5% |
| • No change | 11.3% |
| • Worse, much worse, or very much worse | 3.2% |
| Investigator assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 87.1% |
| • No change | 9.7% |
| • Worse, much worse, or very much worse | 3.2% |
| Patient satisfaction (% of patients) | |
| • Happy with the results | 72.6% |
| • Would recommend procedure to a friend | 74.2% |
| • Would have procedure performed in another body area | 75.8% |
| Endpoint . | Result . |
|---|---|
| Quantitative improvement in lift of neck and submental tissue (average reduction in area across all patients) | 41 mm2 |
| Quantitative improvement in submental volume (% of patients) | 68.3% |
| Patient assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 85.5% |
| • No change | 11.3% |
| • Worse, much worse, or very much worse | 3.2% |
| Investigator assessment of improvement on GAIS (% of patients) | |
| • Improved, much improved, or very much improved | 87.1% |
| • No change | 9.7% |
| • Worse, much worse, or very much worse | 3.2% |
| Patient satisfaction (% of patients) | |
| • Happy with the results | 72.6% |
| • Would recommend procedure to a friend | 74.2% |
| • Would have procedure performed in another body area | 75.8% |
GAIS, Global Aesthetic Improvement Scale.
Safety Endpoints
The primary safety endpoint in the study was met (Table 3). The observed proportion of patients who experienced moderate or less pain (NRS pain scale score of 7 or less) during the first 7 days postprocedure was 96.9% (97.5% 1-sided lower CL = 89.2%, P < .0001). The average NRS pain scores across all patients in the study were low throughout the entire follow-up period (Table 4). The highest average pain score of 2.3 ± 2.1 was reported on the first day following the procedure. The scores dropped to 0.6 ± 1.2 by Day 7, and all patients had returned to baseline levels by the Day 90 follow-up visit.
| Definition . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| NRS pain score of ≤7 through Day 7 (mild to moderate pain) | 96.9% (62/64) | 55% | 89.2% | <.0001 | Pass |
| Definition . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| NRS pain score of ≤7 through Day 7 (mild to moderate pain) | 96.9% (62/64) | 55% | 89.2% | <.0001 | Pass |
NRS, numeric rating scale.
| Definition . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| NRS pain score of ≤7 through Day 7 (mild to moderate pain) | 96.9% (62/64) | 55% | 89.2% | <.0001 | Pass |
| Definition . | Successful patients . | Performance goal . | 97.5% 1-sided lower bound . | Exact binomial P value . | Conclusion . |
|---|---|---|---|---|---|
| NRS pain score of ≤7 through Day 7 (mild to moderate pain) | 96.9% (62/64) | 55% | 89.2% | <.0001 | Pass |
NRS, numeric rating scale.
| Definition . | Baseline . | Postprocedure . | Day 1 . | Day 7 . | Day 14 . | Day 30 . | Day 90 . | Day 180 . |
|---|---|---|---|---|---|---|---|---|
| Average NRS pain score (0 = no pain, 10 = the most pain) | 0.0 ± 0.0 | 0.9 ± 1.5 | 2.3 ± 2.1 | 0.6 ± 1.2 | 0.3 ± 0.8 | 0.2 ± 0.5 | 0.0 ± 0.3 | 0.0 ± 0.0 |
| Definition . | Baseline . | Postprocedure . | Day 1 . | Day 7 . | Day 14 . | Day 30 . | Day 90 . | Day 180 . |
|---|---|---|---|---|---|---|---|---|
| Average NRS pain score (0 = no pain, 10 = the most pain) | 0.0 ± 0.0 | 0.9 ± 1.5 | 2.3 ± 2.1 | 0.6 ± 1.2 | 0.3 ± 0.8 | 0.2 ± 0.5 | 0.0 ± 0.3 | 0.0 ± 0.0 |
NRS, numeric rating scale.
| Definition . | Baseline . | Postprocedure . | Day 1 . | Day 7 . | Day 14 . | Day 30 . | Day 90 . | Day 180 . |
|---|---|---|---|---|---|---|---|---|
| Average NRS pain score (0 = no pain, 10 = the most pain) | 0.0 ± 0.0 | 0.9 ± 1.5 | 2.3 ± 2.1 | 0.6 ± 1.2 | 0.3 ± 0.8 | 0.2 ± 0.5 | 0.0 ± 0.3 | 0.0 ± 0.0 |
| Definition . | Baseline . | Postprocedure . | Day 1 . | Day 7 . | Day 14 . | Day 30 . | Day 90 . | Day 180 . |
|---|---|---|---|---|---|---|---|---|
| Average NRS pain score (0 = no pain, 10 = the most pain) | 0.0 ± 0.0 | 0.9 ± 1.5 | 2.3 ± 2.1 | 0.6 ± 1.2 | 0.3 ± 0.8 | 0.2 ± 0.5 | 0.0 ± 0.3 | 0.0 ± 0.0 |
NRS, numeric rating scale.
Summary of Expected Treatment Effects
The occurrence rates of the expected treatment effects resulting from the device or the procedure are summarized in Table 5. The majority of patients experienced some level of edema (92.3%), temporary changes to sensory nerve sensation (86.2%), and/or bruising (55.4%). To a lesser extent, patients also experienced the expected side effects of erythema, transient crepitus, subcutaneous nodules, and mild to moderate pain or tenderness following the procedure.
| Expected treatment effect . | % of patients . |
|---|---|
| Edema/swelling | 92.3% |
| Temporary sensory nerve changes including touch sensitivity, itching, temporary numbness/tingling | 86.2% |
| Ecchymosis/bruising | 55.4% |
| Erythema | 46.2% |
| Temporary or transient crepitus | 40.0% |
| Mild to moderate pain or tenderness | 24.6% |
| Nodules/subcutaneous lumps (migratory firmness) | 12.3% |
| Expected treatment effect . | % of patients . |
|---|---|
| Edema/swelling | 92.3% |
| Temporary sensory nerve changes including touch sensitivity, itching, temporary numbness/tingling | 86.2% |
| Ecchymosis/bruising | 55.4% |
| Erythema | 46.2% |
| Temporary or transient crepitus | 40.0% |
| Mild to moderate pain or tenderness | 24.6% |
| Nodules/subcutaneous lumps (migratory firmness) | 12.3% |
| Expected treatment effect . | % of patients . |
|---|---|
| Edema/swelling | 92.3% |
| Temporary sensory nerve changes including touch sensitivity, itching, temporary numbness/tingling | 86.2% |
| Ecchymosis/bruising | 55.4% |
| Erythema | 46.2% |
| Temporary or transient crepitus | 40.0% |
| Mild to moderate pain or tenderness | 24.6% |
| Nodules/subcutaneous lumps (migratory firmness) | 12.3% |
| Expected treatment effect . | % of patients . |
|---|---|
| Edema/swelling | 92.3% |
| Temporary sensory nerve changes including touch sensitivity, itching, temporary numbness/tingling | 86.2% |
| Ecchymosis/bruising | 55.4% |
| Erythema | 46.2% |
| Temporary or transient crepitus | 40.0% |
| Mild to moderate pain or tenderness | 24.6% |
| Nodules/subcutaneous lumps (migratory firmness) | 12.3% |
Sensory nerve sensation was assessed at baseline and throughout the follow-up period with the Ten Test originally published by Strauch et al in 1996.28 The test was performed by comparing the sensation of a normal, untreated area to that of the neck and submental area. With the fingers, the investigator lightly stroked the normal area, while simultaneously and with equal pressure stroking the test area. The patient was then asked to rate the sensation in the test area on a scale of 1 to 10, with 10 being the level of sensibility felt in the normal, untreated area. The Ten Test was repeated at each follow-up visit and the sensory nerve change was not considered to be fully resolved until the sensation in the test area returned to a score of 10. These events resolved in an average of 76.1 days (10.9 weeks), which aligns with the average durations of 6 to 10 weeks reported in the literature for liposuction and other procedures that involve undermining of the skin.29,30 No interventions were required for resolution.
Summary of Adverse Events
All other complications experienced in the study were classified as adverse events (AEs), and their rates of occurrence are summarized in Table 6. The most frequently occurring adverse events were temporary motor nerve weakness (6.2%), pruritus not related to nerve changes (6.2%), and hematoma (4.6%). The temporary motor nerve weakness AEs affected the marginal mandibular nerve and were characterized as slight asymmetry compared to baseline during follow-up visit assessments or by documentation of the event by the patient in a patient diary.
| Adverse event . | % of patients . |
|---|---|
| Temporary motor nerve changes including nerve weakness, muscle atrophy, twitching, and paralysis | 6.2% |
| Pruritus/itching not related to sensory nerve changes | 6.2% |
| Hematoma | 4.6% |
| Epidermolysis/blisters | 1.5% |
| Subcutaneous induration | 3.1% |
| Systemic events such as flulike symptoms | 3.1% |
| Abscess/infection | 1.5% |
| Gas buildup | 1.5% |
| Scarring | 1.5% |
| Other: | 9.2% |
| Earache | |
| Lower GI bleed/stomach ulcer/abdominal hernia | |
| Acute appendicitis | |
| Kidney stone | |
| Nerve paralysis to upper left eyelid and brow resulting from car accident Abrasions on left cheek and brow resulting from car accident |
| Adverse event . | % of patients . |
|---|---|
| Temporary motor nerve changes including nerve weakness, muscle atrophy, twitching, and paralysis | 6.2% |
| Pruritus/itching not related to sensory nerve changes | 6.2% |
| Hematoma | 4.6% |
| Epidermolysis/blisters | 1.5% |
| Subcutaneous induration | 3.1% |
| Systemic events such as flulike symptoms | 3.1% |
| Abscess/infection | 1.5% |
| Gas buildup | 1.5% |
| Scarring | 1.5% |
| Other: | 9.2% |
| Earache | |
| Lower GI bleed/stomach ulcer/abdominal hernia | |
| Acute appendicitis | |
| Kidney stone | |
| Nerve paralysis to upper left eyelid and brow resulting from car accident Abrasions on left cheek and brow resulting from car accident |
| Adverse event . | % of patients . |
|---|---|
| Temporary motor nerve changes including nerve weakness, muscle atrophy, twitching, and paralysis | 6.2% |
| Pruritus/itching not related to sensory nerve changes | 6.2% |
| Hematoma | 4.6% |
| Epidermolysis/blisters | 1.5% |
| Subcutaneous induration | 3.1% |
| Systemic events such as flulike symptoms | 3.1% |
| Abscess/infection | 1.5% |
| Gas buildup | 1.5% |
| Scarring | 1.5% |
| Other: | 9.2% |
| Earache | |
| Lower GI bleed/stomach ulcer/abdominal hernia | |
| Acute appendicitis | |
| Kidney stone | |
| Nerve paralysis to upper left eyelid and brow resulting from car accident Abrasions on left cheek and brow resulting from car accident |
| Adverse event . | % of patients . |
|---|---|
| Temporary motor nerve changes including nerve weakness, muscle atrophy, twitching, and paralysis | 6.2% |
| Pruritus/itching not related to sensory nerve changes | 6.2% |
| Hematoma | 4.6% |
| Epidermolysis/blisters | 1.5% |
| Subcutaneous induration | 3.1% |
| Systemic events such as flulike symptoms | 3.1% |
| Abscess/infection | 1.5% |
| Gas buildup | 1.5% |
| Scarring | 1.5% |
| Other: | 9.2% |
| Earache | |
| Lower GI bleed/stomach ulcer/abdominal hernia | |
| Acute appendicitis | |
| Kidney stone | |
| Nerve paralysis to upper left eyelid and brow resulting from car accident Abrasions on left cheek and brow resulting from car accident |
No intervention was required for resolution of these events. The pruritus and hematoma adverse events also resolved with no interventions required. There was 1 occurrence each (1.5%) of blister formation and scarring. The blister formation was related to rubbing from the compression garment, and the scarring involved small, thin hypertrophic scars at the perilobular incisions. There were no adverse events related to burns or overheating of the tissue.
There were 3 serious adverse events reported in the study that were not related to the study procedure or the study device. One patient experienced lower gastrointestinal bleeding associated with an abdominal hernia and stomach ulcer. One patient was admitted to the hospital due to acute appendicitis. A third patient reported a kidney stone. There were no serious adverse events related to the helium plasma treatment.
DISCUSSION
The study was designed to assess the effectiveness and safety of the minimally invasive helium plasma device for improving the appearance of lax skin in the neck and submentum. To confirm effectiveness, endpoints were designed for assessments from 3 different evaluators with different perspectives on the outcomes: a panel of 3 independent physician reviewers, the study investigators who performed the procedures, and the patients themselves. The primary effectiveness endpoint was based on assessment of before and after photographs by the independent reviewers. To avoid any potential of bias in this assessment, Canfield Imaging Services was contracted to identify and train the reviewers as well as conduct the image review, independent of the study sponsor or the investigators. The results of the photographic review demonstrated that individuals not involved in the study treatments were able to see improvement in the appearance of skin laxity in 82.5% of patients. The investigators and patients also noted improvement, as evidenced by their GAIS assessments. Investigators reported that 87.1% of patients had improved at the 180-day follow-up visit, and 85.5% of patients reported themselves as improved at the same time point. The majority of patients also reported satisfaction with their results on the patient satisfaction questionnaire. The effectiveness of the treatment was apparent to the independent reviewers, investigators, and patients across multiple different endpoints and was validated by quantitative measurements of improvement.
In addition to achieving the intended effectiveness results, the safety endpoints were also achieved. The primary safety endpoint was met and evidenced by almost all patients reporting moderate or less pain scores (scores of 7 or less on the NRS pain scale) through the 7-day follow-up period. Average NRS scores were 0.9, 2.3, and 0.6 immediately postprocedure and at Day 1 and Day 7 follow-up visits, respectively, indicating very little pain resulting from the procedure. All but 2 adverse events resolved before the 180-day follow-up visit, with the majority requiring no intervention for resolution. Of the 2 events that resolved after the 180-day follow-up visit, 1 was the small scars resulting from the perilobular incisions and the other was a subcutaneous induration that was treated with ultrasound and manual lymphatic drainage. Both were classified as mild in severity. All other reported adverse events were classified as either mild or moderate by the investigators, with the majority being in the mild category. There were no serious adverse events reported related to the procedure or the device. In addition, there were no new or unexpected risks identified in Phase 2 of the study. These results support the safe use of this device for improvement of the appearance of lax tissue in the neck and submental region.
Minimally invasive subdermal tissue heating devices have bridged the gap between excisional procedures and noninvasive energy devices by striking a balance between the benefits and the risks to the patient. All devices in this category, including laser, radiofrequency, and plasma-based devices, work under the same guiding principle of soft tissue contraction through the delivery of heat to the subcutaneous tissues and fibroseptal network. The devices are introduced percutaneously and deliver energy to the fibrous connective tissue, deep dermis, and muscle fascia. Collagen is the main protein that comprises these tissues. The contractile properties of heated collagen have been well established and shown to be beneficial in numerous applications, including ophthalmologic and orthopedic procedures and varicose vein ablation.31-33 The coagulation/denaturation temperature of collagen has been established to be 66.8°C, but this can vary slightly for different types of collagen and different locations in the body.34 Once denatured, collagen fibers rapidly contract through the transformation of their native helical structure into a more random coiled structure.19,34 Therefore, as the subcutaneous tissues are heated they contract and subsequently draw the skin closer to the muscle, resulting in a reduction of skin laxity. The effectiveness of these devices has advanced to the point that excisional procedures can be deferred or avoided completely with procedures involving less risk to the patient than the more invasive alternatives.35
Because the general principle of coagulation resulting in soft tissue contraction is understood by most physicians employing devices to address skin laxity, most of these devices have been cleared by the US FDA under a general intended use related to coagulation. The Smartlipo LAL device (Cynosure, Westford, MA) received clearance for the surgical incision, excision, vaporization, ablation, and coagulation of soft tissue.23 The BodyTite and FaceTite bipolar RF devices (InMode, Yokneam, Israel) received clearance for electrocoagulation and hemostasis, and the Renuvion helium plasma device received an initial clearance for cutting, coagulation, and ablation of soft tissue.17,24 These general intended uses are pursued as a first step for marketing by many companies because the submission requirements are not too burdensome and do not include the need to conduct clinical studies. The pathway to FDA clearance typically involves performing ex vivo tissue testing on porcine or bovine kidney, muscle, and liver to establish the coagulative tissue effects of a new product in comparison with an existing predicate device. This ex vivo tissue testing, and the other testing required to support clearance, is performed in accordance with guidance provided by the FDA to industry for 510(k) submissions involving electrosurgical devices.36
General intended use often causes confusion for physicians and patients about how a device can be utilized and for medical device companies about how their devices can be marketed. Products with a general intended use for the coagulation of soft tissue can be marketed as a surgical tool to produce the tissue effects associated with coagulation. However, the FDA prohibits claims associated with clinical outcomes produced by these tissue effects without the support of clinical study data. For example, although it is known that the minimally invasive subdermal tissue heating devices tighten the skin as a result of coagulating and contracting the subcutaneous soft tissue, the device companies cannot market their products for the specific clinical outcomes of skin tightening or reducing skin laxity under the general clearance. Clinical studies must be conducted to provide statistically significant evidence that a device produces those specific clinical outcomes. A recent example of marketplace confusion associated with general vs specific device indications occurred with the FDA's statement related to devices marketed for vaginal rejuvenation.37 Some of the named products had general clearances for electrocoagulation and hemostasis. FDA took issue with the devices being marketed for specific clinical outcomes such as the treatment of sexual dysfunction, vaginal rejuvenation, and urinary stress inconsistence.
The purpose of this clinical study was to generate valid scientific evidence that a helium plasma device reduces skin laxity in the neck to support US FDA clearance and allow marketing of the device for this specific clinical outcome. Before beginning the study, the protocol was reviewed and approved by the FDA. A first phase involving 17 patients was conducted to confirm the safety profile of the device before enrolling a larger number of patients. The safety data from Phase 1 was reviewed by the FDA, and approval was granted to continue to Phase 2 and the enrollment of 65 additional patients. The safety and efficacy data gathered from the day of the procedure to the 6-month follow-up time point were then submitted to the FDA. The review included assessment of the primary study endpoints and a review of the safety profile in comparison with energy-based and non-energy–based liposuction devices. This rigorous process ultimately resulted in FDA clearance for the Renuvion device “for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region.” This is the first such clearance for a minimally invasive subdermal tissue heating device.
The limitations of this study included a limited follow-up period and lack of a control arm in the study design. The patients were evaluated through the 180-day time point. Although this time frame was long enough to establish the effectiveness of the treatment and for any adverse events to fully resolve, future studies involving longer follow-up periods to determine if results continue to improve past 6 months postprocedure and how long the improvement can be expected to last will be helpful. In addition, the study design consisted of a single arm in which all patients received the study treatment. A study involving a direct comparison of safety and efficacy with a control group would be interesting.
CONCLUSIONS
This prospective, multicenter, evaluator-blinded, FDA investigational device exemption clinical trial was performed to evaluate the safety and effectiveness of the Renuvion helium plasma device for improving loose skin in the neck and submental region. The totality of the data collected in this study demonstrates benefit to the majority of patients treated, with 82.5% achieving success at the primary effectiveness endpoint. Additional quantitative and qualitative effectiveness endpoints involving Canfield image analysis and assessments by the investigators and the patients also demonstrated benefit of treatment with the study device. The primary safety endpoint was achieved in 96.9% of patients. There were no serious adverse events reported that were related to the study device or the study procedure. Further, the expected treatment effects and adverse events were within the range expected for minimally invasive subdermal treatments in the neck and submental area. These outcomes from the study resulted in FDA 510(k) clearance in July 2022 to expand the indications for the device to include subcutaneous dermatological and aesthetic procedures to improve the appearance of loose skin in the neck and submental region.38
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Disclosures
All physician study investigators are consultants for Apyx Medical (Clearwater, FL). Dr Ruff is a member of the medical advisory board for Apyx Medical, and Dr Gentile was a member of the medical advisory board for Apyx Medical during the trial. Drs Ruff and Gentile own stock options for Apyx Medical.
Funding
The study was funded by Apyx Medical Corporation (Clearwater, FL). Physicians received site payments for their participation in this clinical investigation.
REFERENCES
US Food and Drug Administration. 510(k) summary for InMode RF System. K151793. February 19, 2016. Accessed October 4, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf15/K151793.pdf
US Food and Drug Administration. 510(k) summary for SmartLipo ND:YAG Laser system. K062321. October 31, 2006. Accessed October 4, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf6/K062321.pdf
US Food and Drug Administration. 510(k) summary for Apyx Plasma/RF Handpiece. K191542. October 11, 2019. Accessed October 4, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K191542.pdf
US Food and Drug Administration. 510(k) summary for Renuvion APR handpiece. K220970. July 15, 2022. Accessed October 4, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf22/K220970.pdf
Author notes
Dr Ruff is a plastic surgeon in private practice in Washington, DC, USA.
Dr Bharti is plastic surgeon in private practice in Huntersville, NC, USA.
Dr Hunstad is plastic surgeon in private practice in Huntersville, NC, USA.
Dr Kortesis is plastic surgeon in private practice in Huntersville, NC, USA. Dr Kortesis is a Breast Surgery contributing editor for ASJ Open Forum.
Dr DiBernardo is a plastic surgeon in private practice in Montclair, NJ, USA.
Dr Gentile is a plastic surgeon in private practice in Youngstown, OH, USA.
Dr Cohen is a plastic surgeon in private practice in San Diego, CA, USA and is a clinical editor for Aesthetic Surgery Journal.
Ms Martinez is a freelance medical writer in Washington, DC, USA.
Dr Shridharani is an associate clinical professor, Department of Surgery, Division of Plastic and Reconstructive Surgery, Washington University–St.Louis, St.Louis, MO and is a Cosmetic Medicine section editor for Aesthetic Surgery Journal.



