-
PDF
- Split View
-
Views
-
Cite
Cite
Patrick Mallucci, Giovanni Bistoni, Experience and Indications for the Use of the P4HB Scaffold (GalaFLEX) in Aesthetic Breast Surgery: A 100-Case Experience, Aesthetic Surgery Journal, Volume 42, Issue 12, December 2022, Pages 1394–1405, https://doi.org/10.1093/asj/sjac198
Close - Share Icon Share
Abstract
The popularity of utilizing mesh in the breast has varied over the years. It is well described, and yet there has been poor uptake of its utilization in aesthetic breast surgery. Poly-4-huydroxybutarate (P4HB; GalaFLEX, Galatea, Lexington, MA) has recently been described as a useful adjunct in supporting poor tissue quality with positive early outcomes and low complication rates.
The authors sought to determine the outcome and range of indications of PH4B in aesthetic breast surgery to document its effectiveness in assisting with long-term outcomes in mastopexy.
Firstly, an observational study was undertaken standardizing mastopexy techniques with P4HB included in the procedure. Photographic measurements were taken to determine the extent of lower pole descent and lower pole stretch at 3 months and 1 year postoperative to observe change over time. Secondly, other indications were explored, including the correction of secondary defects such as inferior malposition and symmastia.
The results of the mastopexy study were highly encouraging and comparable with those previously published in the literature, confirming sustained stability of the lower pole over time for mastopexies and implant mastopexies. In addition, the GalaFLEX was successfully employed in correcting many secondary defects.
P4HB is extremely versatile and easy to utilize and has low complication rates. The results have led to a change in practice, with routine incorporation of GalaFLEX for all routine mastopexies. It has also replaced the utilization of acellular dermal matrices in aesthetic breast surgery.

The utilization of poly-4-huydroxybutarate (P4HB) in the breast is well documented, although large patient series and long-term outcome are lacking. In February 2011 the Galatea Corporation (Lexington, MA) introduced GalaFLEX for utilization in plastic and reconstructive surgery. The GalaFLEX scaffold is a biologically derived, macroporus, monofilament, long-term bioabsorbable implant made from P4HB. The polymer is biologically engineered and produced from E. coli.1 The monofilament design decreases the risk of infection and promotes wound healing, and the open pore scaffold acts as a lattice for tissue ingrowth and promotes remodeling over time. The scaffold is rapidly revascularized and becomes fully integrated with adjacent tissue. Resorption is usually complete within 18 to 24 months.2,3 The GalaFLEX provides strength to the soft tissues throughout the critical wound-healing phase and beyond, retaining 50% of its strength at 16 weeks, which compares more favorably than many alternative meshes on the market. Over time it is fully metabolized into carbon dioxide and water.4 Because of these advantages, P4HB scaffolds are now being utilized in a variety of both reconstructive and aesthetic breast procedures.2,4-6 In reconstructive surgery, it has been utilized as an alternative to acellular dermal matrix (ADM) or in combination with ADM both in delayed and immediate reconstruction.7-17 Surgical procedures on the ptotic breast are challenging by the very nature of the poor tissue quality in these patients, often related to pregnancy, significant weight fluctuation, the aging process as well as multiple previous surgeries.
The advent of a reliable and predictable scaffold as a better and more affordable alternative than ADM has been welcomed in aesthetic surgery. Early studies in the breast indicate ease of utilization of P4HB, with a variety of indications for both primary and secondary cases.4-6
Our initial 100-case experience echoes that of previous studies, also with very low complication rates. Unlike with ADMs, we have not observed red breast syndrome or postoperative seromas, obviating the need for drains or postoperative antibiotic cover. The study confirms previous reports showing evidence of lower pole support over time but in much larger numbers. This positive experience has consequently led the author to utilize GalaFLEX not only for complex secondary cases but as routine in all primary mastopexy cases as well as small to moderate reductions. Its ease of utilization, low complication rates, and relative affordability have made this the first-choice material for such cases and have rendered ADMs virtually obsolete in the author’s practice.
METHODS
A total 100 consecutive patients were included in the study from January 2018 until December 2021. All the patients were female. Patients in whom additional soft-tissue or implant support was deemed necessary or beneficial were offered P4HB (GalaFLEX) as part of their surgical treatment.
Age ranged from 20 to 79 years with a mean of 43.3 years. BMI ranged from 19.2 and 32.4 with a mean of 23.6. Follow-up was a minimum of 6 months postoperative with a maximum of 36 months postoperative, with median follow-up of 14 months. The most common indications were for mastopexy alone, augmentation/mastopexy, and secondary correction of implant malposition, including bottoming out, symmastia, and prevention of implant rotation.
Full informed consent was obtained for all patients undergoing surgery with the scaffold according to the Helsinki principle. This included detailed information about the scaffold itself, including its manufacture, mode of action, longevity, and potential complications.
Of the 100 cases, 79 involved a mastopexy (with or without an implant). The remaining 21 cases were an assortment of secondary indications, such as implant malposition, symmastia, and rotation. Of the 79 mastopexies, only 65 were included in the study. Fourteen were excluded either because there was insufficient data or they did not meet the criteria for postoperative analysis of 1 year after surgery (Figure 1).

Mastopexy/Reduction Technique
Our mastopexy technique has been modified to allow for the inclusion of GalaFLEX as a routine element of the procedure (Figure 2). The technique is based on the utilization of a variety of superiorly based pedicles, allowing exposure and mobilization of the lower pole of the breast such that it can be suspended and fixed with a horizontal strip of P4HB cut to size. The GalaFLEX was sutured along its inferior border to the chest wall deep fascia with interrupted 3/0 monocryl sutures and superiorly onto the breast itself. It can be utilized for inverted T or vertical scar techniques (Figure 3; Video).
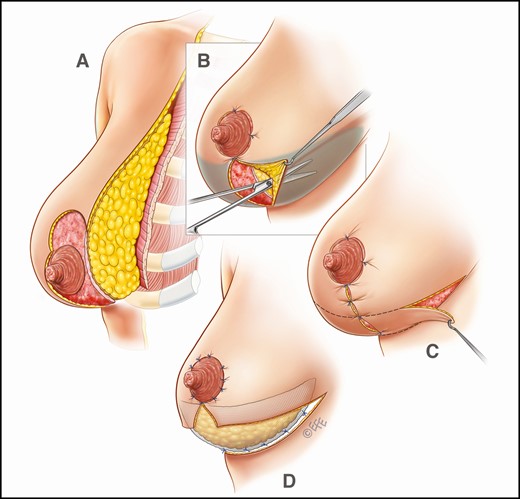
Operative sketches illustrating mastopexy technique (without implants), including insertion of GalaFLEX mesh.
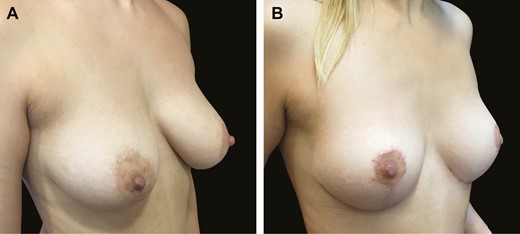
Typical mastopexy result in this 38-year-old female patient shown (A) before and (B) 6 months after surgery, illustrating extremely stable lower pole position.
Augmentation/Mastopexy Technique
For augmentation mastopexies GalaFLEX was employed in a hammock-style support of the implant by suturing the mesh from the lower border of the pectoralis over the implant to the chest wall fascia at the level of the inframammary fold (IMF) (Figure 4).
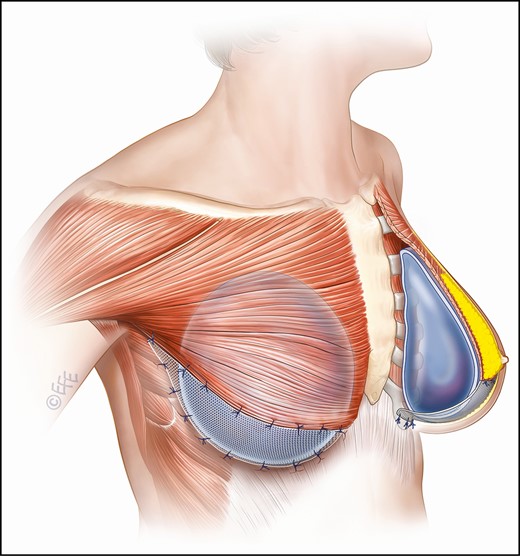
Diagram illustrating mesh placement when GalaFLEX is employed for augmentation/mastopexy. The thin black line represents the mesh supporting the implant, sutured form the lower border of the pectoralis muscle to the chest wall posteriorly.
Analysis of Lower Pole Stability
All cases involving mastopexy (with or without implants) were analyzed for lower pole stability employing photographic analysis of a three-quarter profile image of the breast. Regarding photography angles, it is always difficult to pick the perfect angle, but we felt the oblique view yielded the best exposure of the behavior of the lower pole. It is also consistent with previous work published by the author in assessing lower pole form.18
The lower pole stretch was determined by 2 separate measurements: (1) lower pole descent, defined as the percentage increase in vertical length between the nipple and the IMF between 2 postoperative time points: 6 weeks and 1 year; and (2) arc length increase, defined as the percentage increase in the curvilinear distance from the nipple to the IMF utilizing the same time points (Figure 5).
(A) Preoperative image of this 42-year-old female patient with a previous mastopexy without mesh. (B) The image on the left illustrates the same patient 3 months post revision, and the image on the right is one year postoperatively. The black rectangle represents nipple to fold height at 3 months, and the red rectangle represents the nipple to fold height at 1 year. The difference between these measurements equals the percentage increase in fold descent. The green line represents the curve length from nipple to IMF at 3 months and 1 year, minus the percentage increase between these time points equals the lower pole stretch over time.
The length measurements were carried out utilizing Autodesk Autocad 2022 photo editing software (San Rafael, CA). Photos were matched as accurately as possible for enlargement and orientation to obtain the best possible match.
Other Cases
The remaining 21 cases were a variety of cases in which GalaFLEX was employed to correct secondary malpositions. These were not subject to measurement as in the case of the mastopexies described above. In 12 cases the hammock arrangement from pectoralis major to chest wall fascia was also utilized for inferior implant malposition to support the implant and prevent further subluxation below the IMF.
Two cases of symmastia were also treated with GalaFLEX scaffold in a manner already described with ADMs, utilized a medially positioned section of scaffold to close the intercommunicating defect between the 2 breast cavities.19 In 7 cases GalaFLEX scaffold was utilized with anatomical implants in secondary cases by wrapping the scaffold around the implant to stabilize against rotation.20
RESULTS
Analysis of Lower Pole Stability in Mastopexies
Photographic analysis revealed that in all groups, ptosis had been fully corrected. A highly consistent maintenance of lower pole position was obtained throughout the time points defined; that is, there was very little change in lower pole stretch between 6 weeks and 1 year postoperative. Table 1 lists the measurements for the 45 mastopexies between the 2 time points described, and Table 2 lists the same for the augmentation mastopexy group.
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 1.30% | 5.66% |
| 2 | 0.52% | 0.82% |
| 3 | 6.93% | 5.98% |
| 4 | 8.92% | 0.85% |
| 5 | 12.63% | 10.57% |
| 6 | 5.76% | 8.26% |
| 7 | 5.20% | 4.59% |
| 8 | 0.78% | 0.95% |
| 9 | 5.80% | 4.54% |
| 10 | 8.55% | 8.64% |
| 11 | 1.75% | 3.36% |
| 12 | 6.87% | 26.85% |
| 13 | 9.78% | 8.99% |
| 14 | 7.43% | 6.75% |
| 15 | 11.88% | 19.87% |
| 16 | 1.01% | 2.51% |
| 17 | 4.78% | 6.84% |
| 18 | 5.89% | 7.78% |
| 19 | 0.68% | 0.79% |
| 20 | 1.44% | 2.57% |
| 21 | 9.74% | 8.68% |
| 22 | 6.67% | 7.77% |
| 23 | 5.81% | 4.87% |
| 24 | 8.75% | 7.91% |
| 25 | 12.56% | 13.83% |
| 26 | 3.78% | 4.66% |
| 27 | 5.90% | 4.90% |
| 28 | 7.67% | 8.56% |
| 29 | 8.45% | 7.10% |
| 30 | 4.89% | 5.72% |
| 31 | 6.98% | 7.99% |
| 32 | 8.43% | 11.75% |
| 33 | 1.03% | 2.84% |
| 34 | 2.46% | 3.87% |
| 35 | 4.88% | 6.35% |
| 36 | 10.65% | 14.24% |
| 37 | 7.88% | 8.91% |
| 38 | 6.53% | 7.18% |
| 39 | 1.67% | 2.09% |
| 40 | 8.76% | 9.32% |
| 41 | 7.55% | 9.56% |
| 42 | 1.98% | 3.78% |
| 43 | 9.51% | 11.42% |
| 44 | 2.05% | 3.67% |
| 45 | 5.32% | 6.18% |
| Total | 5.95% | 7.11% |
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 1.30% | 5.66% |
| 2 | 0.52% | 0.82% |
| 3 | 6.93% | 5.98% |
| 4 | 8.92% | 0.85% |
| 5 | 12.63% | 10.57% |
| 6 | 5.76% | 8.26% |
| 7 | 5.20% | 4.59% |
| 8 | 0.78% | 0.95% |
| 9 | 5.80% | 4.54% |
| 10 | 8.55% | 8.64% |
| 11 | 1.75% | 3.36% |
| 12 | 6.87% | 26.85% |
| 13 | 9.78% | 8.99% |
| 14 | 7.43% | 6.75% |
| 15 | 11.88% | 19.87% |
| 16 | 1.01% | 2.51% |
| 17 | 4.78% | 6.84% |
| 18 | 5.89% | 7.78% |
| 19 | 0.68% | 0.79% |
| 20 | 1.44% | 2.57% |
| 21 | 9.74% | 8.68% |
| 22 | 6.67% | 7.77% |
| 23 | 5.81% | 4.87% |
| 24 | 8.75% | 7.91% |
| 25 | 12.56% | 13.83% |
| 26 | 3.78% | 4.66% |
| 27 | 5.90% | 4.90% |
| 28 | 7.67% | 8.56% |
| 29 | 8.45% | 7.10% |
| 30 | 4.89% | 5.72% |
| 31 | 6.98% | 7.99% |
| 32 | 8.43% | 11.75% |
| 33 | 1.03% | 2.84% |
| 34 | 2.46% | 3.87% |
| 35 | 4.88% | 6.35% |
| 36 | 10.65% | 14.24% |
| 37 | 7.88% | 8.91% |
| 38 | 6.53% | 7.18% |
| 39 | 1.67% | 2.09% |
| 40 | 8.76% | 9.32% |
| 41 | 7.55% | 9.56% |
| 42 | 1.98% | 3.78% |
| 43 | 9.51% | 11.42% |
| 44 | 2.05% | 3.67% |
| 45 | 5.32% | 6.18% |
| Total | 5.95% | 7.11% |
aIMF, inframammary fold. Percentage calculated between 6 weeks and 1 year postoperative.
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 1.30% | 5.66% |
| 2 | 0.52% | 0.82% |
| 3 | 6.93% | 5.98% |
| 4 | 8.92% | 0.85% |
| 5 | 12.63% | 10.57% |
| 6 | 5.76% | 8.26% |
| 7 | 5.20% | 4.59% |
| 8 | 0.78% | 0.95% |
| 9 | 5.80% | 4.54% |
| 10 | 8.55% | 8.64% |
| 11 | 1.75% | 3.36% |
| 12 | 6.87% | 26.85% |
| 13 | 9.78% | 8.99% |
| 14 | 7.43% | 6.75% |
| 15 | 11.88% | 19.87% |
| 16 | 1.01% | 2.51% |
| 17 | 4.78% | 6.84% |
| 18 | 5.89% | 7.78% |
| 19 | 0.68% | 0.79% |
| 20 | 1.44% | 2.57% |
| 21 | 9.74% | 8.68% |
| 22 | 6.67% | 7.77% |
| 23 | 5.81% | 4.87% |
| 24 | 8.75% | 7.91% |
| 25 | 12.56% | 13.83% |
| 26 | 3.78% | 4.66% |
| 27 | 5.90% | 4.90% |
| 28 | 7.67% | 8.56% |
| 29 | 8.45% | 7.10% |
| 30 | 4.89% | 5.72% |
| 31 | 6.98% | 7.99% |
| 32 | 8.43% | 11.75% |
| 33 | 1.03% | 2.84% |
| 34 | 2.46% | 3.87% |
| 35 | 4.88% | 6.35% |
| 36 | 10.65% | 14.24% |
| 37 | 7.88% | 8.91% |
| 38 | 6.53% | 7.18% |
| 39 | 1.67% | 2.09% |
| 40 | 8.76% | 9.32% |
| 41 | 7.55% | 9.56% |
| 42 | 1.98% | 3.78% |
| 43 | 9.51% | 11.42% |
| 44 | 2.05% | 3.67% |
| 45 | 5.32% | 6.18% |
| Total | 5.95% | 7.11% |
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 1.30% | 5.66% |
| 2 | 0.52% | 0.82% |
| 3 | 6.93% | 5.98% |
| 4 | 8.92% | 0.85% |
| 5 | 12.63% | 10.57% |
| 6 | 5.76% | 8.26% |
| 7 | 5.20% | 4.59% |
| 8 | 0.78% | 0.95% |
| 9 | 5.80% | 4.54% |
| 10 | 8.55% | 8.64% |
| 11 | 1.75% | 3.36% |
| 12 | 6.87% | 26.85% |
| 13 | 9.78% | 8.99% |
| 14 | 7.43% | 6.75% |
| 15 | 11.88% | 19.87% |
| 16 | 1.01% | 2.51% |
| 17 | 4.78% | 6.84% |
| 18 | 5.89% | 7.78% |
| 19 | 0.68% | 0.79% |
| 20 | 1.44% | 2.57% |
| 21 | 9.74% | 8.68% |
| 22 | 6.67% | 7.77% |
| 23 | 5.81% | 4.87% |
| 24 | 8.75% | 7.91% |
| 25 | 12.56% | 13.83% |
| 26 | 3.78% | 4.66% |
| 27 | 5.90% | 4.90% |
| 28 | 7.67% | 8.56% |
| 29 | 8.45% | 7.10% |
| 30 | 4.89% | 5.72% |
| 31 | 6.98% | 7.99% |
| 32 | 8.43% | 11.75% |
| 33 | 1.03% | 2.84% |
| 34 | 2.46% | 3.87% |
| 35 | 4.88% | 6.35% |
| 36 | 10.65% | 14.24% |
| 37 | 7.88% | 8.91% |
| 38 | 6.53% | 7.18% |
| 39 | 1.67% | 2.09% |
| 40 | 8.76% | 9.32% |
| 41 | 7.55% | 9.56% |
| 42 | 1.98% | 3.78% |
| 43 | 9.51% | 11.42% |
| 44 | 2.05% | 3.67% |
| 45 | 5.32% | 6.18% |
| Total | 5.95% | 7.11% |
aIMF, inframammary fold. Percentage calculated between 6 weeks and 1 year postoperative.
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 7.22% | 8.56% |
| 2 | 3.55% | 4.82% |
| 3 | 7.89% | 8.88% |
| 4 | 6.91% | 7.05% |
| 5 | 6.73% | 8.57% |
| 6 | 15.46% | 18.26% |
| 7 | 7.21% | 8.59% |
| 8 | 1.98% | 2.75% |
| 9 | 6.11% | 7.14% |
| 10 | 7.95% | 8.90% |
| 11 | 2.25% | 3.75% |
| 12 | 8.85% | 9.13% |
| 13 | 4.78% | 5.59% |
| 14 | 7.44% | 8.25% |
| 15 | 10.38% | 11.85% |
| 16 | 5.87% | 6.31% |
| 17 | 6.98% | 7.94% |
| 18 | 8.11% | 9.34% |
| 19 | 3.69% | 4.19% |
| 20 | 3.97% | 5.02% |
| Total | 6.66% | 7.74% |
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 7.22% | 8.56% |
| 2 | 3.55% | 4.82% |
| 3 | 7.89% | 8.88% |
| 4 | 6.91% | 7.05% |
| 5 | 6.73% | 8.57% |
| 6 | 15.46% | 18.26% |
| 7 | 7.21% | 8.59% |
| 8 | 1.98% | 2.75% |
| 9 | 6.11% | 7.14% |
| 10 | 7.95% | 8.90% |
| 11 | 2.25% | 3.75% |
| 12 | 8.85% | 9.13% |
| 13 | 4.78% | 5.59% |
| 14 | 7.44% | 8.25% |
| 15 | 10.38% | 11.85% |
| 16 | 5.87% | 6.31% |
| 17 | 6.98% | 7.94% |
| 18 | 8.11% | 9.34% |
| 19 | 3.69% | 4.19% |
| 20 | 3.97% | 5.02% |
| Total | 6.66% | 7.74% |
aIMF, inframammary fold. Percentage calculated between 6 weeks and 1 year postoperative.
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 7.22% | 8.56% |
| 2 | 3.55% | 4.82% |
| 3 | 7.89% | 8.88% |
| 4 | 6.91% | 7.05% |
| 5 | 6.73% | 8.57% |
| 6 | 15.46% | 18.26% |
| 7 | 7.21% | 8.59% |
| 8 | 1.98% | 2.75% |
| 9 | 6.11% | 7.14% |
| 10 | 7.95% | 8.90% |
| 11 | 2.25% | 3.75% |
| 12 | 8.85% | 9.13% |
| 13 | 4.78% | 5.59% |
| 14 | 7.44% | 8.25% |
| 15 | 10.38% | 11.85% |
| 16 | 5.87% | 6.31% |
| 17 | 6.98% | 7.94% |
| 18 | 8.11% | 9.34% |
| 19 | 3.69% | 4.19% |
| 20 | 3.97% | 5.02% |
| Total | 6.66% | 7.74% |
| Patients . | Differential in descent of IMF . | Differential in lengthening of lower pole arch . |
|---|---|---|
| 1 | 7.22% | 8.56% |
| 2 | 3.55% | 4.82% |
| 3 | 7.89% | 8.88% |
| 4 | 6.91% | 7.05% |
| 5 | 6.73% | 8.57% |
| 6 | 15.46% | 18.26% |
| 7 | 7.21% | 8.59% |
| 8 | 1.98% | 2.75% |
| 9 | 6.11% | 7.14% |
| 10 | 7.95% | 8.90% |
| 11 | 2.25% | 3.75% |
| 12 | 8.85% | 9.13% |
| 13 | 4.78% | 5.59% |
| 14 | 7.44% | 8.25% |
| 15 | 10.38% | 11.85% |
| 16 | 5.87% | 6.31% |
| 17 | 6.98% | 7.94% |
| 18 | 8.11% | 9.34% |
| 19 | 3.69% | 4.19% |
| 20 | 3.97% | 5.02% |
| Total | 6.66% | 7.74% |
aIMF, inframammary fold. Percentage calculated between 6 weeks and 1 year postoperative.
Mastopexy Alone Group
The range in percentage IMF descent varied from 0.52% to 12.63% with a mean of 5.95% between 6 weeks and 1 year. The range in percentage arc length increase varied from 0.79% to 26.85%, with a mean arc length expansion of 7.11% (Table 1; Figures 6-8).
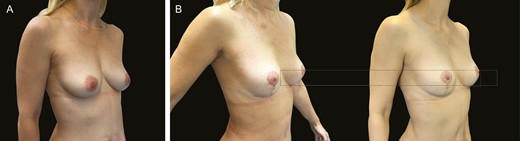
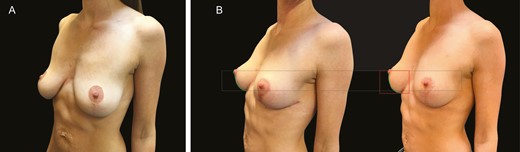
(A) Preoperative mastopexy image of this 39-year-old female patient. (B) The image on the left is the patient 3 months postoperatively, and the right is 1 year postoperatively.
(A) Preoperative mastopexy image of this 38-year-old female patient. (B) The image on the left is the patient 3 months postoperatively, and the right is 1 year postoperatively.
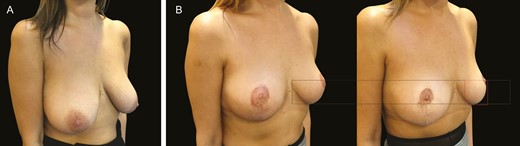
(A) Preoperative image of breast reduction in this 31-year-old female patient. (B) The image on the left is the patient 3 months after a moderate breast reduction (420 g) and the right is 1 year postoperatively.
Augmentation/Mastopexy Group
The range for IMF descent was from 1.98% to 15.46%, with a mean of 6.66%. The arc length differential ranged from 2.75% to 18.26%, with a mean of 7.74% (Table 2; Figure 9). As shown in Figures 3 and 5 to 13, The position of the IMF scar was highly consistent with almost no upward drift through recurrent ptosis or bottoming out. There were no reoperations for recurrent ptosis. Our longest follow-up of 3.5 years exhibited very little change in lower pole laxity even between 3 months and 3.5 years (Figure 10).
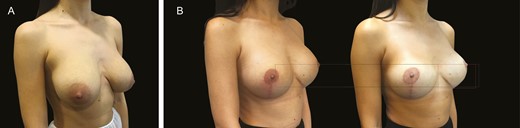
(A) Preoperative image of heavy breasts with implants in this 35-year-old female patient. (B) The image on the left is the patient 3 months after an implant exchange and mastopexy, and the right is 1 year postoperatively.
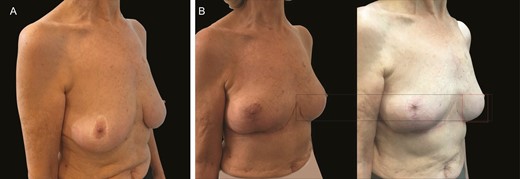
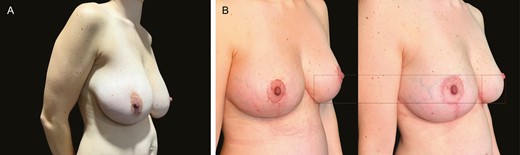
(A) Preoperative image of this 70-year-old female patient with previous mastopexy. (B) Postoperative images following revision mastopexy with mesh at 3 months and 36 months illustrate extreme stability at 36 months with virtually no descent of the lower pole over time.
Results for Other Indications
All cases of malposition were adequately corrected, with no incidence of recurrence or need for reoperation. These included symmastia cases, inferior malpositions as well as correction of rotation (Figures 11, 12).
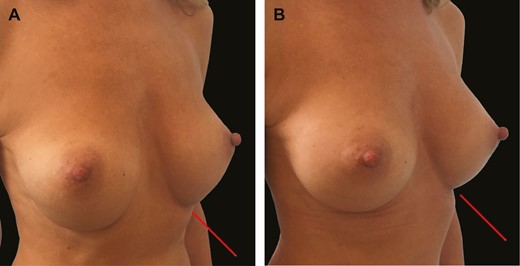
(A) Three-quarter view of this 29-year-old female patient shows bottoming out following breast augmentation with 275-cc round textured implants and failed capsulorrhaphy. (B) Six-month postoperative image illustrates stable fixation of inframammary fold with GalaFLEX reinforcement 1 year postoperatively.
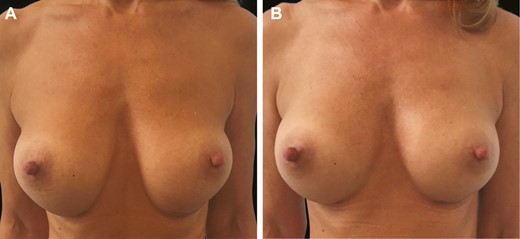
(A) Preoperative and (B) 6-month postoperative anterior view of the 29-year-old female patient shown in Figure 11.
Complications/Adverse Events
There were 2 adverse events: 1 was an infection in a 79-year-old woman presenting for secondary revision augmentation mastopexy. She developed an infection 14 days after surgery, and both implant and scaffold were removed. It was impossible to determine whether the implant was the main problem or the scaffold. Given the fact that this was already a revisional case, along with the age of the patient, the possibility of complications of healing and potential implant infection/loss were discussed in detail preoperatively with the patient.
The second was an acute seroma in a primary augmentation/mastopexy post gym activity, against advice, 5 days after her surgery. The pressure from her seroma had dislodged the lower sutures from her scaffold to the chest wall fascia, requiring resuturing. There were no cases of extrusion, no red breast, no pure scaffold infections, and no complaints about palpability of the scaffold.
DISCUSSION
The aim of this article has been twofold: firstly, to look at an extensive single-surgeon experience utilizing GalaFLEX scaffold in its effectiveness in mastopexy procedures and small reductions (<400 g) and secondly, to explore a host of other indications not adequately explored in previous literature regarding the utilization of GalaFLEX in aesthetic breast surgery. It has been employed for both primary and secondary procedures with and without implants.
Our first 100 cases presented illustrate the versatility of P4HB (GalaFLEX) across a wide variety of clinical situations and confirm its effectiveness in adding stability to the breast in mastopexies as previously reported by others.4-6 To our knowledge 3.5 years is the longest postoperative time point employing GalaFLEX published to date demonstrating excellent stability throughout.
The mean age of the cohort (43.3 years) represents many women seeking corrective surgery following pregnancy once they have finished their families. The effect of pregnancy on the anatomy, tissue quality, and position of the breasts is well documented.21 It would seem logical therefore that the utilization of an internal soft-tissue support would be of benefit in this challenging group. The same is true of patients in whom significant fluctuations in weight has occurred or in those who have had their tissues weakened by the presence of oversized implants over time.
The technique for mastopexy described in this study was adapted specifically to facilitate the incorporation of the scaffold into the procedure, and although it is the author’s preference to utilize an inverted T-scar pattern as routine, it is entirely suited for those who prefer to employ vertical scar techniques. The technique we describe utilizes fixation sutures securing the P4HB to the chest wall fascia. Other authors advocate the on-laying of the mesh into position without the need for fixation. We feel that given the observed descent in IMF position, it makes sense to fix the mesh to minimize this possibility over time.
There is a paucity of literature documenting changes in breast shape over time. Swanson has extensively documented changes to breast shape, projection, and many other parameters following differing breast procedures; however, changes in these parameters over time are less well documented.22,23 Goes et al have found success in employing biologically derived and mixed scaffold containing both absorbable and non-absorbable elements.24,25 However, none of the studies utilize measurable elements to identify change over time.
Although it is difficult to prove conclusively, and in quantitative terms, the efficacy of the utilization of mesh in the breast, Adams and Moses published preliminary data from a single-surgeon study of 11 patients that asserts the effectiveness of P4HB on lower pole stability in mastopexies through 3-dimensional imaging.26,27 They reported a lower pole stretch of approximately 5% at 1 year.5 In a subsequent multicenter study, they reached similar conclusions.4 Our data compare very favorably with their findings; our average lower pole descent equated to 5.95%, with an increase in arc length of 7.11%. Values for the implant/mastopexy group were slightly elevated at 6.66% and 7.74%, respectively.
We selected 2 slightly different parameters for analysis: lower pole descent and arc length increase. Lower pole descent assesses the changes at the IMF pertinent to original horizontal scar position at the time of surgery—this was kept very stable as verified photographically by almost no upward drift of the scar over time. Unstable IMFs allow for bottoming out with elevation of the scar onto the lower pole of the breast. The mesh was very effective at preventing this.
In all cases, the increase in arc length slightly exceeded the decent of the IMF. The arc is influenced by skin elasticity and breast weight. This element is not as controllable by the presence of the GalaFLEX scaffold. It is therefore perhaps not surprising that the arc length exceeds the fold descent. Given this observation, it would be possible to maintain stable fold descent in the face of much more significant arc length increase in those with very thin and inelastic skin.
One of the only other studies to measure changes in the breast over time reported a lower pole stretch of 20% over a 3-year period in a vertical scar breast reduction group (without the utilization of scaffold or other support), with most of the change occurring in the first year.28 The GalaFLEX studies demonstrate a significant improvement with respect to the latter and present the evidence basis for the utilization of soft-tissue support in such cases. As with previous studies, our data also demonstrate little change beyond the early postoperative period.4-6
However, it is also important to point out that these are not entirely comparable situations. A 20% lower pole stretch in a breast reduction group employing a vertical scar technique with heavier breasts is likely to have greater stretch than our mastopexies utilizing inverted T scars. Overall, we recognize the difficulty in trying to measure and quantify precisely the contribution made by the GalaFLEX (or by any other support structure) to the stability of the breast. However, to carry out a properly randomized controlled study comparing the effect of P4HB in 1 breast with an untouched control in the other, although ideal, would be virtually impossible. Even if the scaffold were placed on 1 side and not the other, the variables between the 2 breasts and the different surgery on each breast would not render fully conclusive or reliable results.
We have therefore endeavored to standardize our images as much as possible employing a simple photographic linear analysis tool to compare images. Our larger numbers than those of previously published studies help to reinforce our findings, although we acknowledge the shortcomings of such a study and the need to produce more data over longer time points.
We have also endeavored to include challenging clinical cases with extremely poor tissues to showcase the performance of the scaffold. Our massive weight loss patient typifies the problem: thin skin, hypermobility, poor elasticity. The scaffold not only gives stability over time, but it also acts as a vehicle for cohesion and unification of the lax breast, giving a beneficial rigidity to the gland (Figure 13).
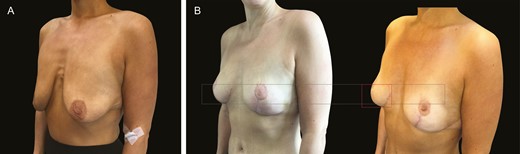
(A) Preoperative massive weight loss in this 52-year-old female patient (40-kg weight loss) wanting mastopexy but no implant. (B) Three-month and 1-year postoperative images illustrate stability throughout even in the absence of implants.
Our longest follow-up in a 68-year-old patient also demonstrated extreme stability over time, with very little change in lower pole stretch between 6 weeks, 18 months, and 36 months postoperative (Figure 10), reaffirming the properties described. Although much focus is placed on upper pole fullness in cosmetic breast surgery, the emphasis throughout this study has been on discussion around lower pole stability. Expectations must be managed in individuals with ptotic breasts and inelastic skin; maintenance of upper pole fullness (especially a “fake” look) is simply unrealistic, even when an implant is combined with mastopexy procedures. Lower pole stability, however, is the structural cornerstone that the breast depends on for its positional security and by which its aesthetic profile is most importantly determined.29 Once lower pole stability is lost, all shape and position is lost and the outcome is no longer satisfactory.
In relation to implants, our main indication for utilization has been in challenging implant/mastopexies as well as correction of implant malposition. For the implant mastopexies, the GalaFLEX was utilized in a hammock-style configuration supporting the implant in the lower pole of the breast to avoid inferior malposition. It is worth noting that the extra stretch in the lower pole observed with the implant group (7.11% vs 7.74%) is not surprising given the extra weight of the implant as well as the stretch of the tissues incurred by its presence.
The average implant volume was moderate (256 cc) and reflects both the author’s approach to implant mastopexy as well as the size preference of a more typical European population. It would seem likely that in countries where larger volumes are utilized with smooth devices, the role of GalaFLEX is likely to be even more significant in the prevention of inferior and lateral malposition.
Very little has been published about other uses of GalaFLEX in aesthetic surgery. Nair and Mills described 5 cases in which GalaFLEX was utilized to correct a variety of secondary problems, including poor tissue cover, pectoral muscle malposition, and capsular contracture.7 Williams et al described the preliminary utilization of GalaFLEX for ptosis, capsular reinforcement, and as a superficial musculo-aponeurotic system support in the face.6 However, these studies were preliminary in nature with very small numbers. We have utilized the scaffold diffusely across several secondary breast procedures (21 cases), including the correction of secondary malposition, such as bottoming out, symmastia, and as a means of treating rotation of anatomical implants in patients in whom plane change was not possible or sufficient. In all cases there has been total resolution of the clinical situation except in 1 implant rotation, where a subsequent rotation occurred following GalaFLEX placement.
Although the utilization of ADMs for treating similar complications is well described, we feel there are several advantages of P4HB over ADMs.14-20 The extremely rapid tissue integration and monofilament construction are highly protective against infection. Unlike with ADMs where postoperative seromas and red breast are not uncommon, we have not observed any red breast syndrome or routine postoperative seromas with GalaFLEX. As a consequence, we utilize neither drains nor postoperative antibiotics as routine.
Adding any extraneous material to the breast is naturally of concern, and sceptics may avoid the utilization of such materials in favor of local options, such as capsular flaps, capsulorrhaphy, and the like.30-33 These methods are unreliable, especially when inherent tissue is lacking or of poor quality. We believe that the versatility, ease of utilization, and extremely low complication rates presented here negate those concerns. The monofilament nature of the P4HB, the rapid integration into the tissues, and its tensile strength and biodegradation over a long period of time (18-24 months) are ideal properties for the indications described in soft-tissue support of the breast. The biodegradable element is also reassuring to those who are seeking to avoid the inclusion of a permanent foreign body as part of any procedure. Given the experience and the findings presented, it is the author’s routine practice to incorporate GalaFLEX into all mastopexies and small reductions.
CONCLUSIONS
Whereas previous preliminary studies have alluded to the indications for the utilization of GalaFLEX in the breast, the 100 cases presented here provide the strongest published evidence of the safety and versatility of GalaFLEX scaffold in aesthetic breast surgery. Its ease of utilization, unique physical properties, and extremely low complication rates render it a useful tool for the support and correction of ptosis as well as for the variety of other indications specified. Its relative affordability compared with ADMs has transformed its availability to a much wider public, especially in the self-pay sector of aesthetic surgery. Irrespective of cost, we believe the improved properties of the scaffold have rendered the utilization of ADM virtually obsolete in aesthetic breast surgery. It is not possible to comment or compare the performance of P4HB with other available meshes because the senior author has limited experience with such alternatives; clearly this is an area for further investigation. P4HB was selected for utilization based on the available biological data and the previously published data discussed. We welcome the development of further studies across different centers in the future to continue with careful data collection to be able to add to the experience and knowledge presented here.
Disclosures
Dr Mallucci is a minor shareholder for Polytech (Dieburg, Germany), unrelated to the utilization of P4HB in any way. Dr Bistoni declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.



