-
PDF
- Split View
-
Views
-
Cite
Cite
Alexandre Mendonça Munhoz, Ary de Azevedo Marques Neto, João Maximiliano, Subfascial Ergonomic Axillary Hybrid (SEAH) Breast Augmentation: A Surgical Approach Combining the Advantages of Incision, Pocket, Silicone Gel, and Fat Grafting in Primary and Revision Breast Augmentation Surgery, Aesthetic Surgery Journal, Volume 41, Issue 6, June 2021, Pages NP364–NP384, https://doi.org/10.1093/asj/sjab029
Close - Share Icon Share
Abstract
Simultaneous application of the axillary approach (AA) with the subfascial pocket (SF) has been proposed for breast augmentation (BA) surgery. New silicone implant technology and recent improvements in autologous fat grafting (AFG) have ushered in a new era for BA.
The aim of this study was to present the combined subfascial ergonomic axillary hybrid (SEAH) method and evaluate its aesthetic benefits after primary/secondary BA.
In total, 42 patients (84 breasts) underwent BA with the SEAH technique; this approach was indicated when the overlying tissue was insufficient to adequately cover the implant.
Mean patient age was 34.6 years (range, 28-56 years), mean BMI was 18.8 kg/m2 (range, 14.4-26.1 kg/m2). The most common implant (Motiva SmoothSilk surface Ergonomix style) volume was 255 cc (range, 175-355 cc), patients received a mean fat volume of 96 mL (range, 60-145 mL) per breast in the subcutaneous tissue. The average lower pole stretch value was 40.5% (21.75 mm) and 13.1% (9.9 mm) for preoperative to 10 days postprocedure and 10 days to 18 months postprocedure, respectively. Postoperative complications included subcutaneous banding in the axilla (n = 3, 7.1%), small wound dehiscence (n = 1, 2.3%), and hypertrophic scarring (n = 1, 2.3%). No rippling, implant malposition, infection, or fat necrosis was observed during a mean follow-up of 18 months (range, 6-32 months).
SEAH is a useful and versatile technique combining the benefits of AFG and implant-based augmentation, particularly with regard to soft tissue coverage, and avoids the limitations of the submuscular position. The combination of ergonomic gel implants and a SF pocket can yield satisfactory aesthetic outcomes.

Over time, advances in breast augmentation (BA) techniques and new implant designs have led to improvements in safety with better aesthetic outcomes.1-4 Among the main techniques, combining a subfascial (SF) pocket5-13 with an axillary approach (AA)14-19 has gained popularity because no incisions are made on the breast and no distortion appears when the pectoral muscle is contracted.17-19
Silicone gel breast implants have improved and advanced in recent decades with the introduction of new surfaces and viscoelastic gel properties.3,4,20-25 One example of this progress is the Motiva SmoothSilk Ergonomix style implant (SSE; Motiva, Establishment Labs, Alajuela, Costa Rica), which is one of the first generation of breast implants with very low roughness to avoid tissue ingrowth and minimize bacterial adhesion.24-30 In addition to their surface topography, these implants also offer enhanced rheological properties, simulating the natural dynamics of breast tissue and allowing a natural and shaped contour.26-30
The past decade has seen a resurgence in the use of autologous fat grafting (AFG) in aesthetic breast surgery.13,31-40 Simultaneous use of this technique with silicone implants, defined as composite or hybrid breast augmentation (HBA), has recently been evaluated as a valuable technique for BA.13,32,34,37,38,40 Combining these 2 techniques offers natural cleavage and camouflages the implant edges.13,38,40
Although BA is a well-studied procedure, previous reports concerning the SF technique are limited, and all series describe past generations of silicone implants.5-13 Furthermore, few detailed clinical reports specifically address operative planning, outcomes, and complications following simultaneous AA, SF, and AFG.34,36,38-40 Along similar lines, no study involving SSE implants has analyzed the combination of these techniques in a single procedure. This article consequently is the first detailed description of the technique, known as the SEAH approach (subfascial ergonomic implants with an axillary approach in a hybrid surgery) for patients undergoing primary and secondary BA.
METHODS
Charts of all consecutive primary and secondary BA procedures performed by the axillary approach were reviewed retrospectively. All study participants provided written informed consent, and the study was conducted in accordance with the provisions of the Declaration of Helsinki. All procedures were conducted at an outpatient facility by 1 surgeon (A.M.M.) over a 32-month period (June 2017-February 2019). All patients were candidates for primary BA, defined as having bilateral hypomastia with no ptosis or previous BA with complications/complaints such as capsular contracture (CC), asymmetry, rippling, implant rupture, or volume/shape dissatisfaction; the degree of CC was determined according to the Baker scale. Data on patient age, BMI, and pinch test were also collected for each patient. Complications were classified as minor or major and divided into 2 types: implant-related and tissue-related complications. Periods selected for analysis included less than 10 days, 1, 3, 6, and 12 months.
Surgical Planning/Technique
Preoperative Markings/Patient and Implant Selection
This retrospective study comprised female patients who met the inclusion criteria (Tables 1 and 2). When making the markings, it is convenient to determine as accurately as possible the pocket dimensions for the chosen implant. This aspect will assist pocket dissection and avoid overdissection, which can lead to implant displacement. For this reason, the region of the inframammary fold (IMF) is defined along with the height of the selected implant. Skin marks are drawn: current inframammary fold (CIMF), lateral limit of the pocket represented by the anterior axillary line (AAL), and midsternal line (MSL). Parasternal lines (PSLs) are also usually drawn, maintaining 2 to 3 cm between the breasts. The base diameter of the selected implant is centered under the breast, in this manner defining the vertical and horizontal limits of the pocket location. Thus, the lower and upper limits of the pocket, representing the future IMF (FIMF) and superior breast line (SBL), are planned according to implant volume (Figures 1 and 2), which allows accurate centering of the implant and maintains precise pocket dimensions based on implant size. The deepest axillary fold is chosen as the incision location (Video 1). Implant volume is selected together with the patient, considering factors such as height, weight, and thoracic cage. Breast and thoracic asymmetries are identified and corrected as much as possible with AFG and different implants, if necessary (Figure 2A-C).
Inclusion Criteria for Primary Subfascial, Ergonomix, Axillary, Hybrid (SEAH) Breast Augmentation
| Patient selection/inclusion criteria: primary augmentation . |
|---|
| • Severe hypomastia/amastia |
| • Poorly defined inframammary fold |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
| • Pinch test result of <2 cm |
| Patient selection/inclusion criteria: primary augmentation . |
|---|
| • Severe hypomastia/amastia |
| • Poorly defined inframammary fold |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
| • Pinch test result of <2 cm |
Inclusion Criteria for Primary Subfascial, Ergonomix, Axillary, Hybrid (SEAH) Breast Augmentation
| Patient selection/inclusion criteria: primary augmentation . |
|---|
| • Severe hypomastia/amastia |
| • Poorly defined inframammary fold |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
| • Pinch test result of <2 cm |
| Patient selection/inclusion criteria: primary augmentation . |
|---|
| • Severe hypomastia/amastia |
| • Poorly defined inframammary fold |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
| • Pinch test result of <2 cm |
Inclusion Criteria for Secondary Subfascial, Ergonomix, Axillary, Hybrid (SEAH) Breast Augmentation
| Patient selection/inclusion criteria: secondary augmentation . |
|---|
| • Previous axillary breast augmentation |
| • Previous subfascial/subglandular pocket with pinch test <2 cm |
| • Previous submuscular pocket with pinch test <2 cm |
| • Visible implant contours/rippling |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
| Patient selection/inclusion criteria: secondary augmentation . |
|---|
| • Previous axillary breast augmentation |
| • Previous subfascial/subglandular pocket with pinch test <2 cm |
| • Previous submuscular pocket with pinch test <2 cm |
| • Visible implant contours/rippling |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
Inclusion Criteria for Secondary Subfascial, Ergonomix, Axillary, Hybrid (SEAH) Breast Augmentation
| Patient selection/inclusion criteria: secondary augmentation . |
|---|
| • Previous axillary breast augmentation |
| • Previous subfascial/subglandular pocket with pinch test <2 cm |
| • Previous submuscular pocket with pinch test <2 cm |
| • Visible implant contours/rippling |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
| Patient selection/inclusion criteria: secondary augmentation . |
|---|
| • Previous axillary breast augmentation |
| • Previous subfascial/subglandular pocket with pinch test <2 cm |
| • Previous submuscular pocket with pinch test <2 cm |
| • Visible implant contours/rippling |
| • A wide intermammary distance susceptible to reduction |
| • Thin patients (BMI < 20 kg/m2) |
| • Less upper pole coverage |
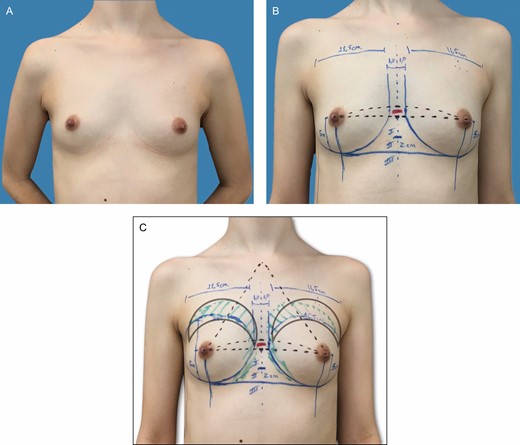
(A-C) Preoperative markings on a 31-year-old patient with bilateral symmetric hypomastia and pinch test of 1.0 cm. These skin marks include current symmetric inframammary fold, lateral limit of the pocket, represented by the anterior axillary line, midsternal line, and parasternal lines, with an average distance of 2 to 3 cm between the breasts and the area of fat grafting in the upper pole.
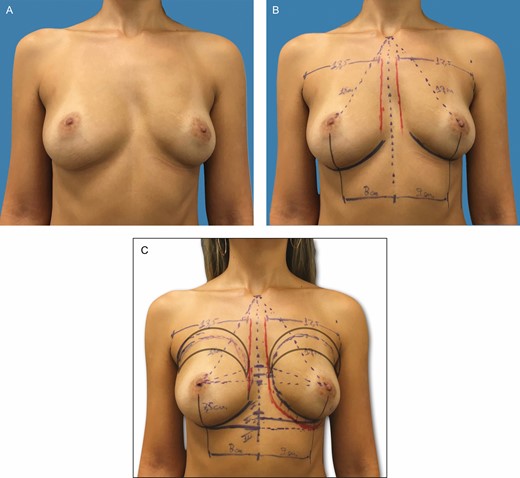
(A-C) Preoperative markings on a 37-year-old patient with bilateral asymmetric hypomastia and pinch test of 1.0 cm. These skin marks include current inframammary fold, 2.0-cm difference between the right and left current inframammary fold, lateral limit of the pocket, represented by the anterior axillary line, midsternal line, and parasternal lines, with an average distance of 2 to 3 cm between the breasts and the area of fat grafting in the upper pole and parasternal region.
SF Pocket Dissection
With the patient under general anesthesia, dissection is performed until the lateral border of the pectoralis muscle is reached. The fascia is incised perpendicular to the skin incision (Video 1). Using customized retractors and electrocautery, we dissect in the upper-medial pole and progressively move toward the intermediate pole, laterally up to the lateral-inferior quadrants (Video 2). The gland is lifted away from the chest, and residual attachments between the fascia and muscle are preserved at the level of the FIMF. The limits of the pocket and the FIMF are checked against the markings with a customized dissector (Video 2). In cases where the FIMF must be lowered, the base of the selected implant serves as the template that dictates where the IMF will be placed. By measuring down from the center of the breast the equivalent to one-half of the base diameter of the implant, the expected location of the FIMF can be determined. During the surgery, the exact placing of the FIMF is adapted from this point, depending on lower pole skin elasticity and implant projection. Lateral dissection should be limited to the AAL to avoid lateral implant displacement. Before implant insertion, the pockets are irrigated with antibiotic solution (80 mg gentamicin, 500 mg cephazolin, 500 mL saline). The implants are then placed in the SF pockets, and the patient is positioned upright to assess implant position and identify areas for AFG (Figure 3A-C; Video 3).
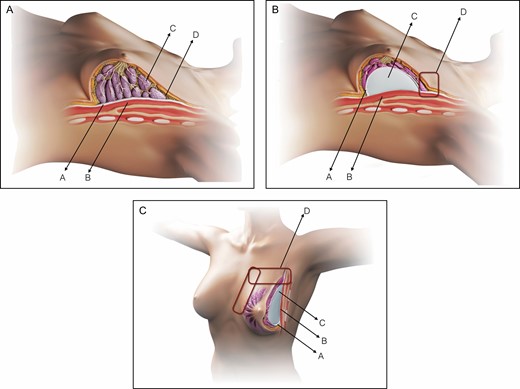
Schematic representation of the SEAH procedure. (A) Normal anatomy of the left breast, preoperative view: pectoralis fascia (A), pectoralis major muscle (B), glandular tissue (C) and upper pole subcutaneous tissue (D). (B) Normal anatomy of the left breast, intraoperative view following subfascial breast augmentation by the axillary approach: pectoralis fascia (A), pectoralis major muscle (B), silicone gel implant (C), and upper pole transition with abrupt limit (D). (C) Normal anatomy of the left breast, intraoperative left oblique view following subfascial breast augmentation by the axillary approach: pectoralis fascia (A), pectoralis major muscle (B), silicone gel implant (C), and upper, upper-medial, and medial pole transition with abrupt limit (D).
AFG
With the patient seated at a 90° angle, the medial/superior edge of the breast is marked, and then both implants are pushed in a medial/upward direction to simulate the cleavage limits. Usually, AFG is harvested from the abdomen or inner/outer thighs by utilizing a 3.0-mm cannula with beveled 1.5-mm ports (Faga Medical, Bauru, Brazil) connected to a 60-mL Luer-Lok syringe (BD Medical, Curitiba, Brazil) (Video 4). The harvested fat is then washed and filtrated with lactated Ringer solution through a closed system (PureGraft, Solana Beach, CA) and transferred to 3-mL syringes (BD Medical, Curitiba, Brazil) for injection. Following the technique described by Coleman,31 AFG is injected with a 15-cm, 1.9- to 2.1-mm-diameter cannula (Faga Medical, Bauru, Brazil), with retrograde strings (Figure 4A). The graft is spread superficially in the subcutaneous tissue from the upper pole toward the lateral and medial upper quadrants to simulate a homogeneous transition between the implant and surrounding areas (Figure 4B). If necessary, a second puncture is performed to crisscross the injections (Figure 4C,D; Video 4). The wound is closed in layers without suction drains, and an elastic band is placed over the superior breast poles.
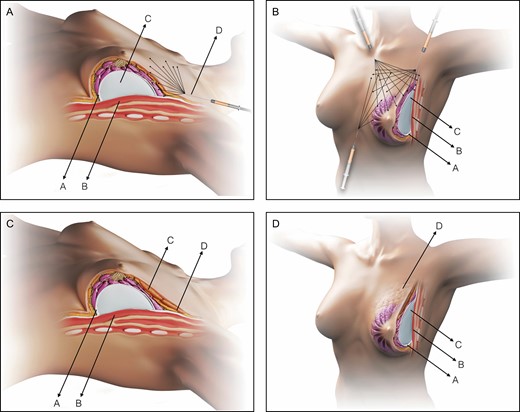
Schematic representation of the SEAH procedure. (A) Normal anatomy of the left breast, intraoperative view following subfascial breast augmentation with the axillary approach and during fat graft into the upper pole transition: pectoralis fascia (A), pectoralis major muscle (B), silicone gel implant (C), and fat grafting into the upper pole performed with a 3 mL syringe and a 1.9-mm cannula (D). (B) Normal anatomy of the left breast, intraoperative left oblique view following subfascial breast augmentation with the axillary approach and during fat graft into the upper, upper-medial, and medial pole transition: pectoralis fascia (A), pectoralis major muscle (B), and silicone gel implant (C). (C) Normal anatomy of the left breast, postoperative view following SEAH procedure: pectoralis fascia (A), pectoralis major muscle (B), fat graft over the area of the silicone gel implant (C), and fat graft over the area of the transition between the area with the silicone gel implant and the upper pole (D). (D) Normal anatomy of the left breast, postoperative left oblique view following SEAH procedure: pectoralis fascia (A), pectoralis major muscle (B), silicone gel implant (C), and fat graft over the area of the transition between the area with the silicone gel implant and the upper pole (D).
Postoperative Care
All patients initially receive intravenous antibiotics, and oral antibiotics are continued for 48 hours. The elastic band is kept in place for 4 weeks; early massage/mobilization of the breasts is avoided for at least 2 to 3 months, and physical activities are delayed for 6 to 8 weeks.
Three-Dimensional Images
Three-dimensional (3D) images were obtained with a Divina 3D scanner system (AX3 Technologies, Miami, FL) to assess breast position, inferior pole stretching, and breast volume. The following parameters were used: nipple-IMF distance (N-IMF), breast volume (BV), and lower pole stretch (LPS), as described by Adams and Moses.41 Imaging was only obtained from patients who underwent primary BA and were followed within the established periods for at least 18 months. These measurements were compared prior to surgery and again at 10 days, 3 months, 6 months, 1 year, and 18 months postoperatively. Scans were obtained from all patients standing with both arms at 30° abduction with respect to shoulder height in order to avoid displacement when overlaying images.
RESULTS
Between 2017 and 2019, 42 patients (84 breasts) with a mean age of 34.6 years (range, 28-56 years) and mean BMI of 18.8 kg/m2 (range, 14.4-26.1 kg/m2) underwent SEAH breast augmentation (Figures 5-7; Supplemental Figure 1A-F). Twenty-three patients (54.7%) underwent primary BA and 19 (45.2%) secondary BA (Table 3). Full-projection style SSE silicone gel implants (Motiva, Establishment Labs, Alajuela, Costa Rica) were used, with an average volume of 255 cc (range, 175-355 cc) (Table 4). Fat was obtained from the abdomen and thighs (inner and outer) in 70% of cases, followed by the hip (20%) and knee (10%), with an average of 380 mL (250-650 mL) harvested in each patient. Patients received a mean of 96 mL of fat (range, 60-145 mL) on each side; the volume between breasts differed (>15% of volume) in 6 patients (14.2%). Fat grafting was only performed in the upper cleavage area in 30 patients (71.4%), in the upper and medial area in 10 patients (23.8%), and in areas associated with asymmetric ribs in 2 patients (4.7%). Mean operating time was 150 minutes (range, 80-200 minutes).
| Characteristic . | Value (%) . |
|---|---|
| No of patients/breasts | 42/84 |
| Age (years) | |
| Median | 34.6 |
| Range | 28-56 |
| BMI | |
| Median | 18.8 |
| Underweight (<18.5 kg/m2) | 5 (11.9) |
| Normal weight (18.5-24.9 kg/m2) | 29 (69) |
| Overweight (25-29.9 kg/m2) | 8 (19) |
| Obese (>30 kg/m2) | 0 (0.0) |
| Reasons for secondary SEAH BAa | |
| Capsular contracture | 7 (36.8) |
| Size/shape change | 6 (31.5) |
| Implant rupture | 3 (15.7) |
| Dynamic breast | 2 (10.5) |
| Rippling | 1 (5.2) |
| Characteristic . | Value (%) . |
|---|---|
| No of patients/breasts | 42/84 |
| Age (years) | |
| Median | 34.6 |
| Range | 28-56 |
| BMI | |
| Median | 18.8 |
| Underweight (<18.5 kg/m2) | 5 (11.9) |
| Normal weight (18.5-24.9 kg/m2) | 29 (69) |
| Overweight (25-29.9 kg/m2) | 8 (19) |
| Obese (>30 kg/m2) | 0 (0.0) |
| Reasons for secondary SEAH BAa | |
| Capsular contracture | 7 (36.8) |
| Size/shape change | 6 (31.5) |
| Implant rupture | 3 (15.7) |
| Dynamic breast | 2 (10.5) |
| Rippling | 1 (5.2) |
BA, breast augmentation; SEAH, subfascial ergonomic axillary hybrid. an = 19 patients. Some patients presented more than one event.
| Characteristic . | Value (%) . |
|---|---|
| No of patients/breasts | 42/84 |
| Age (years) | |
| Median | 34.6 |
| Range | 28-56 |
| BMI | |
| Median | 18.8 |
| Underweight (<18.5 kg/m2) | 5 (11.9) |
| Normal weight (18.5-24.9 kg/m2) | 29 (69) |
| Overweight (25-29.9 kg/m2) | 8 (19) |
| Obese (>30 kg/m2) | 0 (0.0) |
| Reasons for secondary SEAH BAa | |
| Capsular contracture | 7 (36.8) |
| Size/shape change | 6 (31.5) |
| Implant rupture | 3 (15.7) |
| Dynamic breast | 2 (10.5) |
| Rippling | 1 (5.2) |
| Characteristic . | Value (%) . |
|---|---|
| No of patients/breasts | 42/84 |
| Age (years) | |
| Median | 34.6 |
| Range | 28-56 |
| BMI | |
| Median | 18.8 |
| Underweight (<18.5 kg/m2) | 5 (11.9) |
| Normal weight (18.5-24.9 kg/m2) | 29 (69) |
| Overweight (25-29.9 kg/m2) | 8 (19) |
| Obese (>30 kg/m2) | 0 (0.0) |
| Reasons for secondary SEAH BAa | |
| Capsular contracture | 7 (36.8) |
| Size/shape change | 6 (31.5) |
| Implant rupture | 3 (15.7) |
| Dynamic breast | 2 (10.5) |
| Rippling | 1 (5.2) |
BA, breast augmentation; SEAH, subfascial ergonomic axillary hybrid. an = 19 patients. Some patients presented more than one event.
SmoothSilk Surface Implants, Ergonomix Full Projection Style Characteristics
| Implant volume (cc)/base diameter (cm) . | Total . |
|---|---|
| <205/9.5 | 2 (4.7%) |
| 205-255/9.5-10.25 | 15 (35.7%) |
| 255-295/10.25-10.75 | 19 (45.2%) |
| 295-335/10.75-11.25 | 4 (9.5%) |
| >335/11.25 | 2 (4.7%) |
| Total | 42 (100%) |
| Implant volume (cc)/base diameter (cm) . | Total . |
|---|---|
| <205/9.5 | 2 (4.7%) |
| 205-255/9.5-10.25 | 15 (35.7%) |
| 255-295/10.25-10.75 | 19 (45.2%) |
| 295-335/10.75-11.25 | 4 (9.5%) |
| >335/11.25 | 2 (4.7%) |
| Total | 42 (100%) |
SmoothSilk Surface Implants, Ergonomix Full Projection Style Characteristics
| Implant volume (cc)/base diameter (cm) . | Total . |
|---|---|
| <205/9.5 | 2 (4.7%) |
| 205-255/9.5-10.25 | 15 (35.7%) |
| 255-295/10.25-10.75 | 19 (45.2%) |
| 295-335/10.75-11.25 | 4 (9.5%) |
| >335/11.25 | 2 (4.7%) |
| Total | 42 (100%) |
| Implant volume (cc)/base diameter (cm) . | Total . |
|---|---|
| <205/9.5 | 2 (4.7%) |
| 205-255/9.5-10.25 | 15 (35.7%) |
| 255-295/10.25-10.75 | 19 (45.2%) |
| 295-335/10.75-11.25 | 4 (9.5%) |
| >335/11.25 | 2 (4.7%) |
| Total | 42 (100%) |
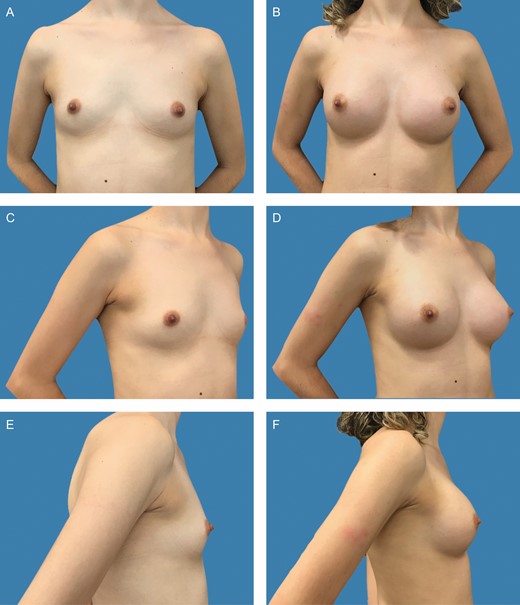
Preoperative frontal (A), left oblique (C), and lateral (E) views of a 31-year-old female patient with bilateral symmetric hypomastia. Preoperative markings and surgical planning are shown in detail in Figure 1A-C. Presence of symmetric inframammary folds, with pinch test of 1 cm. (B, D, F) Appearance 2 years postprocedure, showing very good outcome with bilateral 255-cc Motiva SmoothSilk surface Ergonomix implants, combined with 85 and 95 mL of autologous fat graft on the upper and upper-medial poles on the left and right sides, respectively.
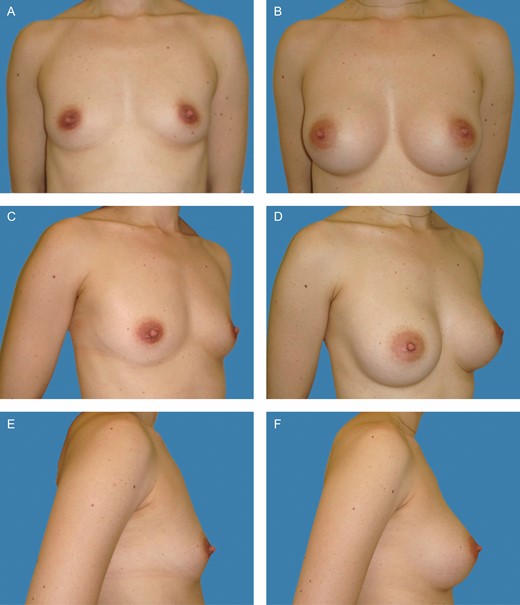
Preoperative frontal (A), left oblique (C), and lateral views (E) of a 26-year-old female patient with bilateral symmetric hypomastia. Presence of symmetric inframammary folds, with pinch test of 1.5 cm. (B, D, F) Appearance 2 years postprocedure, showing very good outcome with bilateral 275-cc Motiva SmoothSilk surface Ergonomix implants, combined with with 75 and 25 mL of autologous fat graft on the upper and upper-medial poles on the left and right sides, respectively.
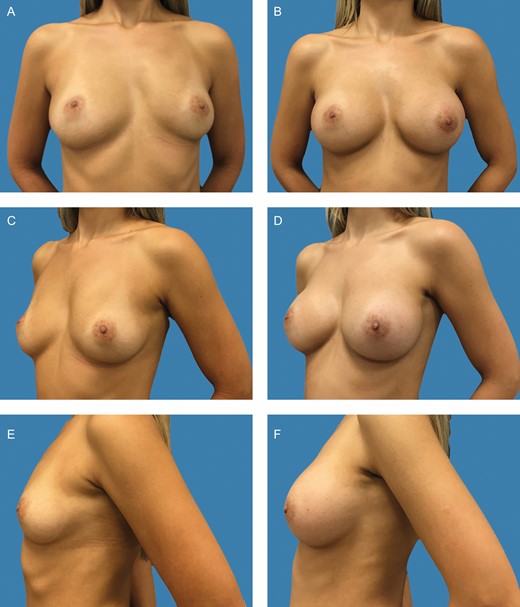
Preoperative frontal (A), left oblique (C), and lateral views (E) of a 37-year-old female patient with bilateral asymmetric hypomastia. Preoperative markings and surgical planning were shown in detail in Figure 2A-C. Presence of asymmetric inframammary folds, with pinch test of 1 cm. (B, D, F) Appearance 1 year postoperative, showing very good outcome with 375-cc and 275-cc Motiva SmoothSilk surface Ergonomix implants on the left and right sides respectively, combined with 115 and 75 mL of autologous fat graft on the upper and upper-medial poles on the left and right sides, respectively.
Outcome/Complications
Seven cases (16.6%) of complications were observed in 5 patients (11.9%); they included subcutaneous banding in the axilla (n = 3, 7.1%), a small wound dehiscence (n = 1, 2.3%), hypertrophic scarring at the axilla incision (n = 1, 2.3%), hematoma (n = 1, 2.3%), and CC contracture (Baker II/III) secondary to hematoma (n = 1, 1.7%). Two patients (4.7%) presented more than 1 complication (Table 5). No cases of rippling, implant malposition, infection, fat necrosis, or donor site complications were observed during a mean follow-up of 18 months (range, 6-30 months). The implant edges were not perceptible, even in the largest implant (335 cc). No patients developed visible rippling when in the upright position and leaning forward 90° from the waist. One (2.3%) underwent revision surgery with capsulotomy and implant repositioning due to CC, and obtained a satisfactory outcome.
| Complications . | Total . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 3 (7.1%) |
| Hypertrophic scars | 1 (2.3%) |
| Wound dehiscence | 1 (2.3%) |
| Implant | |
| Hematoma | 1 (2.3%) |
| Capsular contracture | 1 (2.3%) |
| Infection | 0 (0.0%) |
| Rippling | 0 (0.0%) |
| Displacement | 0 (0.0%) |
| Totala | 7 (16.6%) |
| Complications . | Total . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 3 (7.1%) |
| Hypertrophic scars | 1 (2.3%) |
| Wound dehiscence | 1 (2.3%) |
| Implant | |
| Hematoma | 1 (2.3%) |
| Capsular contracture | 1 (2.3%) |
| Infection | 0 (0.0%) |
| Rippling | 0 (0.0%) |
| Displacement | 0 (0.0%) |
| Totala | 7 (16.6%) |
aSome patients presented more than 1 complication, eg, hypertrophic scar and wound dehiscence, and hematoma and capsular contracture.
| Complications . | Total . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 3 (7.1%) |
| Hypertrophic scars | 1 (2.3%) |
| Wound dehiscence | 1 (2.3%) |
| Implant | |
| Hematoma | 1 (2.3%) |
| Capsular contracture | 1 (2.3%) |
| Infection | 0 (0.0%) |
| Rippling | 0 (0.0%) |
| Displacement | 0 (0.0%) |
| Totala | 7 (16.6%) |
| Complications . | Total . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 3 (7.1%) |
| Hypertrophic scars | 1 (2.3%) |
| Wound dehiscence | 1 (2.3%) |
| Implant | |
| Hematoma | 1 (2.3%) |
| Capsular contracture | 1 (2.3%) |
| Infection | 0 (0.0%) |
| Rippling | 0 (0.0%) |
| Displacement | 0 (0.0%) |
| Totala | 7 (16.6%) |
aSome patients presented more than 1 complication, eg, hypertrophic scar and wound dehiscence, and hematoma and capsular contracture.
Patient Satisfaction
Patient satisfaction was analyzed at the most recent follow-up by administering an acquired informal questionnaire to grade the patient’s level of satisfaction (Appendix). The questionnaire was anonymous and distributed by the clinic secretaries in paper format. The patients classified their level of satisfaction as very satisfied, satisfied, disappointed, or regretting their decision to have surgery. Patient satisfaction was evaluated at least 6 months postprocedure (range, 6-8 months). Forty patients (95.2%) were either very satisfied or satisfied with the aesthetic result. Two patients (4.7%) were partially disappointed; 1 patient (2.3%) thought the implants were small, and 1 (2.3%) complained of mild breast asymmetry due to CC. No patients regretted the surgery. Satisfactory aesthetic results were obtained, maintaining a natural breast shape and soft transition between the subcutaneous tissue and implant edge in the upper and medial pole (Figures 5-7; Supplemental Figure 1A-F). In all cases except one (due to slight dehiscence and hypertrophic scarring), the axillar incision healed with imperceptible scarring.
3D Imaging Measurements
Seventeen patients (40.4%) underwent prospective evaluation via 3D image analysis. Preoperative average measurements (average of right and left sides) for N-IMF were 53.65 mm (range, 45-68 mm), and for BV 272.3 mL (range, 180-350 mL). At 18 months postprocedure, the average (right and left sides) value for N-IMF was 85.3 mm (range, 76-97 mm) (Figure 8A). The postoperative average BV value for right and left sides (at 18 months) was 599.7 mL (range, 440-725 mL) (Figure 8B). Average LPS value for right and left sides was 40.57% (21.75 mm, range 19.33-22.48 mm) and 13.1% (9.9 mm, range 8.65-10.67 mm) for preoperative to 10 days postprocedure and 10 days to 18 months postprocedure, respectively. For 3 to 12 months after surgery, LPS was 2.95% (2.45 mm, range 1.99-2.85 mm). Comparing 6 months (85.5 mm) and 18 months (85.3 mm) after surgery shows no statistical difference in N-IMF distance (P = 0.585) (Figure 8A). Average values for BV prior to surgery and 18 months postprocedure were 272.3 (range, 180-350 mL) and 722.5 mL (range, 440-725 mL), respectively. Comparison of these values 3 months (583.35 mL) and 18 months (599.7 mL) postprocedure shows no statistical difference (P = 0.475) (Figure 8B).
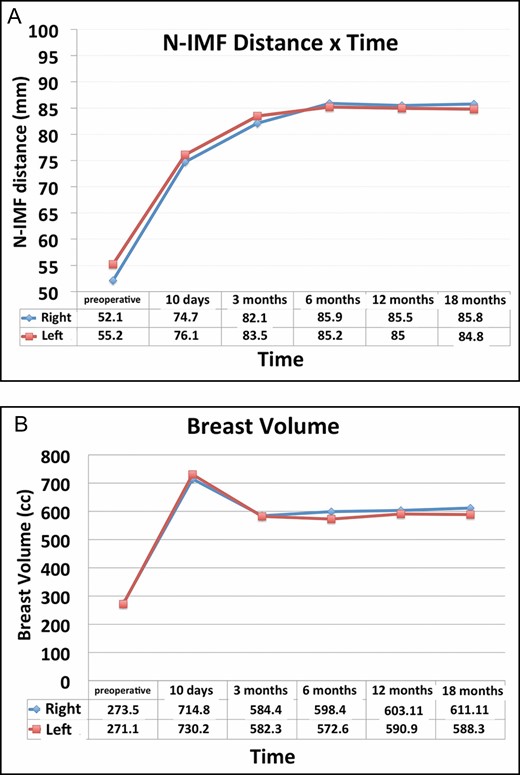
(A) Divina 3D scanner system evaluation of the inferior pole stretching through nipple to inframammary fold (N-IMF) distance (mm) × postoperative period (preoperative, 10 days, 3, 6, 12, and 18 months), right and left breasts. (B) Divina 3D scanner system evaluation of breast volume (cc) × postoperative period (preoperative, 10 days, 3, 6, 12, and 18 months), right and left breasts.
Radiologic Findings
The minimum period for performing imaging exams was 6 months after surgery, with an average of 8 months (range, 6-14 months). Mammograms, breast ultrasounds, and MRI were performed in a group of 29 patients (69%) and all exams were classified as BI-RADS 2 according to the American College of Radiology (ACR) classification. In 3 breasts (5.17%), small and localized oil cysts were observed. Two mammograms (3.4%) showed radiographic signs of architectural distortion, characterized by well-circumscribed and nonconfluent areas of variable tissue density. Benign calcifications were observed in 7 breasts (12%). No cases of suspicious calcifications, fat necrosis, or lumps were observed (Figure 9).
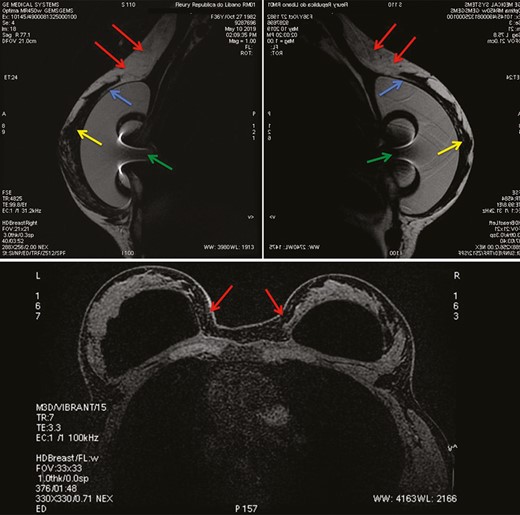
Sagittal plane of subtracted T1-weighted images from left (upper-left) and right (upper right) breast MRI in a 37-year-old woman 1 year after a subfascial ergonomic axillary hybrid procedure due to severe hypomastia and breast asymmetry showing the position of the silicone implants in the subfascial pocket. Axial plane (below) of subtracted T2-weighted images from breast MRI of the same patient. In the sagittal images (above, left and right) it is possible to observe the fat graft integrated in the upper region of the breast, promoting a homogeneous transition between the region with and without the silicone implant (red arrows). The blue and yellow arrows show the pectoral fascia and the breast parenchyma, respectively. The green arrow represents the radiofrequency identification system artifact inside the silicone gel. The axial image (below) shows the proper position of the implant and the fat graft integrated in the parasternal region. No cases of suspicious calcifications, fat necrosis, or lumps were observed.
DISCUSSION
There are a wide variety of surgical approaches to BA, which have evolved over the years.1-4 Since its introduction in the 1970s,42,43 the AA has become an attractive technique because the incision is hidden outside the breast area in an aesthetically acceptable region.44,45 Knowledge of axillary and surgical breast anatomy allows surgeons to dissect adjacent to the axillary area and enter the pocket locations while minimizing risks of accidental injury to anatomic structures.45
Since its first description, the AA has undergone technical modifications. The submuscular (SM) pocket was first introduced to provide adequate implant coverage,17,18,44,45 but was limited by morbidity and muscular dynamics.17,18,45-47 More superficial placement of the silicone implant may also present limitations. Amid this scenario, the SF pocket technique introduced in the 1990s presents an alternative because the fascia can provide supplementary tissue coverage and avoid the limitations of the SM pocket.5-8,12 This technique offers faster postoperative recovery than the total SM pocket, without breast animation when the pectoral muscle is contracted.5-13,17 Furthermore, the implant is not subjected to muscular pressure and tissue dynamics from the overlying pectoralis muscle. Because of this latter aspect, silicone gel can provide direct fill and pressure to the breast shape, especially in the upper and medial aspects of the breast.45
Because of progress in BA techniques, the SF pocket has come to be associated with the AA.15-19 In our study, the AA resembled the approach described by Graf et al.15 The pectoral fascia is incised and the dissection continued through the SF plane under direct view (Video 1). Despite controversy related to inadequate soft tissue coverage of the fascia thickness,45,46 some studies have demonstrated satisfactory outcomes in selected patients.15-19 In our experience, the pectoral fascia is a well-defined structure that is valuable in minimizing the appearance of the implant limits.17,18 In circumstances where the fascia cannot provide adequate coverage, complementing with AFG can be useful, particularly in slim patients with limited soft tissue coverage.13,34,36,37
Over the past decade, there has been renewed interest in AFG for aesthetic breast surgery, especially in association with BA.31-40 Defined as composite or hybrid techniques,13,32,34,36,38-40 simultaneous use of AFG and silicone implants has been recently evaluated as useful. In fact, recent improvements in the AFG technique have reduced the incidence of local complications secondary to poor AFG intake.13,31,48-52 Despite debate over the AFG technique, it might be assumed that when large volumes of fat are grafted, local complications and fat absorption may increase.53-55 Meanwhile, grafting small volumes of AFG increases the surface area between the graft and recipient site and improves intake.53 In our sample, AFG is harvested with a 3.0-mm cannula with beveled 1.5-mm ports. The purified AFG is then grafted with a 15-cm, 1.9- to 2.1-mm-diameter cannula via retrograde strings into the subcutaneous tissue to simulate a homogeneous transition between the implant and surrounding areas (Video 4). In our experience, and according to previous studies,53-55 AFG intake depends on the diffusion of nutrients from recipient tissues, and for this reason fat grafts must be kept small to improve viability.53
In our experience, HBA permits core volume projection and natural cleavage while camouflaging implant edges.13 In our series, the ideal candidates for the SEAH technique have pinch test results of <2 cm and significant hypomastia (Video 4). In cases where pinch test results exceed 3 to 4 cm, in our experience SF and AFG are unnecessary and instead conventional SF BA is indicated. In secondary BA, the main indications are soft tissue deficiency and the presence of visible implant contours/rippling.52 SEAH is mainly indicated to avoid the SM pocket and in cases where the overlying soft tissue is limited. In our sample, almost one-third of patients underwent secondary BA, and the majority had 1 previous BA with various implant types and sizes, mostly performed elsewhere.
Patients seeking BA frequently have inadequate soft tissue and excessively wide cleavage gaps between breasts. As other authors have observed,38,40 AFG is also useful in reducing intermammary distance and achieving a smoother transition between the 2 breasts. Other indications for HBA also include patients who want breast implants larger than their soft tissue footprints would otherwise accommodate.50 The SEAH technique offers the combined advantages of fat grafting with the subfascial plane, the rheologic properties of the gel, and a lack of scarring on the breast. For this reason, identifying a donor area for fat harvesting is an essential part of planning the procedure. In very thin patients with low pinch test results, or those who do not want liposuction, the concepts of SEAH can be implemented by creating a submuscular pocket, but this brings the disadvantages of a muscular pocket.
Concerning the donor areas, we usually begin by identifying the best donor sites, which are marked prior to surgery with the patient in an upright position; these include the abdomen, flanks, inner thigh, trochanteric region, and the medial aspect of the knee, depending on where fat is located in each patient. In our series, an average of 380 mL of fat was harvested in each patient. For most very thin patients, the lateral/medial regions of the thighs serve as the donor site, followed by the flank and abdomen. The average BMI of patients in our series was 18.8 kg/m2, but most of them (88%) were normal or overweight, with adequate quantities of fat to harvest from the abdomen. Five patients (12%) were thinner (BMI < 18 kg/m2), which can be a limiting factor for this technique and bring a higher risk of contour deformities. In this group, the donor area should be carefully selected in order to avoid contour deformities. In this group, we suggest selecting all available donor areas, which allows collection of small volumes (40-60 mL) from each area and consequently involves less risk of overharvesting. Fortunately, a large volume of AFG is not needed for HBA, which permits adequate control of the donor area without contour deformities.
In our technique, the harvested fat is washed and filtrated through a closed system (similar to the technique proposed by Sforza et al56) and transferred to 3-mL syringes for injection. This system avoids contamination of the fat and prolonged exposure to air throughout the entire process.57 One advantage of this system is that the free lipid and red blood cells are removed in a less traumatic way than in other AFG preparation systems. Some authors have advocated overharvesting an additional 30% of the planned volume in order to obtain 70% to 75% AFG intake.56,57 The AFG is usually spread superficially in the subcutaneous tissue from the upper pole toward the lateral and medial upper breast areas to simulate a homogeneous transition between the implant and surrounding areas.
In our view, the best time to perform the AFG is once the implants have been placed, with the patient seated at a 90° angle to evaluate the cleavage areas (Video 4). The volume of fat inserted over the upper part of the implant routinely approaches one-quarter or one-third of the volume of the implant itself, but this ratio is not precise and depends on previous soft tissue coverage and implant projection. In contrast, other authors have stated that the volume of AFG inserted over a breast implant routinely approaches the implant volume.50 We believe that the ideal shape is achieved in the SEAH technique by converting a round implant, adding AFG in a pattern resembling an anatomic shape on the upper aspect of the implant (and not necessarily throughout the whole implant). In our previous study involving upper pole transition, a geometric figure led us to a mathematical formula for accurately estimating the necessary volume of AFG.51 In this analysis, a direct relation was observed between implant volume and AFG volume needed in the upper pole to simulate a conical and homogeneous shape; greater implant projection (high and extra-high style) led to greater fat volumes, with extra-high projection requiring approximately 30% more AFG volume than a moderate projection implant of similar volume to produce a anatomic shape.51
In our experience, there is no maximum AFG volume, and each case may have different fat needs. Aspects such as skin elasticity, presence of glandular tissue, previous surgery and fibrosis, and implant volume can affect the recipient area’s capacity to accept AFG. We always avoid overfilling in AFG to limit ischemia and necrosis. With this in mind, care must be taken with larger implants that result in noncompliant soft tissues with limited distensibility. In cases that involve large-volume implant, the tissue becomes less compliant as the fat is grafted, and small volumes of AFG can lead to significant increases in pressure and fat ischemia.57 In patients who have previously undergone BA and exhibit CC, breast tissue is generally much less compliant and much less tolerant of large volumes of AFG, particularly when larger implants are used. For this reason, in order to improve vascularization and AFG intake we recommend that the grafted fat be spread carefully as a fine mist of small droplets by the sprinkler principle, avoiding larger AFG volumes and higher pressure in the tissue. According to Auclair et al, in order to obtain substantial full-surface implant cover, the volume of AFG must approach that of the implants themselves.34 In cases where only specific areas of the breast require preferential fill, such as the upper and medial quadrants, smaller volumes may be adequate. In our series, patients received an average volume of 96 mL of AFG on each side, and the volume between breasts differed (>15% of volume) in almost 15% of patients.
Silicone gel implants have evolved in recent years with the introduction of new surfaces and rheologic properties.3,4,20-30 The SSE style is one of the first generation of breast implants to incorporate surface topography with a 3D inverted imprinting technology.21,22,24,25 These round base implants also offer higher viscoelastic properties with a 100% gel filling, simulating a natural shape with less fullness at the upper pole compared to traditional round implants.26-28 Because of its rheologic properties, this specific silicone gel presents a low viscosity but much higher elasticity, shifting the maximum point of projection to the lower pole when the patient is standing.28 In 2018, a prospective US FDA trial was started to analyze the safety and effectiveness of SSE implants in US patients undergoing primary BA, reconstruction, and revision surgeries.58 This multicenter prospective core study involves 750 patients in 4 cohorts and an MRI subpopulation of 250 patients to evaluate rupture rates. Like previous FDA premarket clinical trials of other established brands of silicone implants in the United States, clinical data and outcome are expected after at least 3 years of follow-up and potential approval of these implants for the US market.
In our sample, this implant was specifically advantageous for patients desiring a natural upper pole contour who had a short areola-to-IMF distance with insufficient lower breast poles. The location of the axillary incision is crucial to minimize scar visibility; the most favorable location is in the hair-bearing skin inside the deepest axillary fold, with the frontmost part of the incision 2 to 3 cm posterior to the lateral edge of the pectoralis muscle (Video 1). Axillary scar quality and minimizing damage to implants during insertion are far more important than incision length. Incision length generally varies from 4.5 to 6 cm with high-cohesive/form-stable implants to accommodate different retractors.17,18 Because of the viscoelastic properties of the ergonomic implant and lower roughness surface, a short incision of approximately 3 cm is sufficient for placement. For small SSE implants (<200 cc), 2 to 2.5 cm is adequate, whereas larger implants (>350 cc) may require an incision of 3.0 to 4.0 cm.
SmoothSilk implants have no pores, which limits the number of sites for tissue ingrowth and consequently reduces tissue adherence.20-25 Because tissue ingrowth does not occur, the pocket dimensions must be planned carefully in order to avoid implant displacement.28 For this reason, prior to the procedure it is crucial to determine the pocket dimensions for the chosen implant as precisely as possible, and calculate the region of the FIMF along with the height of the selected implant. The implant/sizer diameter can be centered under the breast, thus defining the vertical and horizontal limits of the pocket. Depending on lower pole skin elasticity and implant projection, the exact placing of the FIMF can be determined from the preoperative markings. One advantage of the SF pocket is better implant stability due to the strong adherence of the pectoral fascia to the pectoralis muscle. It is our impression that even when the FIMF must be lowered, the location of the implant and adherence of the fascia in the adjacent areas keep the implant in its proper place, thus avoiding lateral or inferior displacement. Furthermore, because the entire dissection is performed through the axilla, the superficial fascia (superficial and deep layers)59 in the IMF region are preserved, with no need for fixation or reinforcement after implant implacement. In revision cases, with a previous submuscular pocket, we changed to the SF pocket in order to achieve an adequate implant position and more stability. In specific cases, the use of sutures can be an option and is feasible with endoscopy assistance.
We strongly recommend limiting pocket dissection to the previous skin marks in order to create a precise, snug pocket that maintains implant stability. The ideal is to be conservative in the first dissection of the pocket and use instruments (customized dissector) to check the dimensions of the pocket and, if necessary, increase this space in localized regions (Video 2).17 Also essential is the use of an elastic band over the superior breast poles to prevent upward migration and implant displacement. For larger implants, and if it is necessary to lower the IMF, we suggest placing the abdominal band close to the FMIF for better stability. Early massage, mobilization of the implants, and physical activities should be avoided to reduce the risk of implant rotation.17,18 Our outcomes demonstrate that implant stability at the IMF and AAL are not significantly affected, and that this present technique maintains the implant in proper position with no lateralization. SSE implants also incorporate a radiofrequency identification (RFID-M) system; like any metallic foreign body, a small and predictable imaging void artifact is created during MRI sequences, which permits more precise diagnosis of implant position60 (Figure 9; Video 3).
Despite the rheologic advantages and viscoelastic properties of the gel, previous studies on ergonomic implants are restricted and mostly based on the expertise of a few authors.26-30,51,52 As a result, data on the exact period of time necessary for the implant to reach its final position in the lower pole are inaccurate. In our study, in addition to the physical examination/photographs, an objective evaluation was performed for patients who were followed for at least 18 months to evaluate the final position of the implant and lower pole stretch, assessing the latter through pre-established landmarks.30,41 In the primary augmentation group, almost 74% of patients were assessed prospectively via 3D image analysis. The remaining cases of this group were not included in the prospective assessment because complete imaging follow-up performed during specific periods was not available. In our sample, the average LPS was almost 40% (21.75 mm) and 13% (9.9 mm), from preoperative to 10 days after surgery and 10 days to 18 months postprocedure, respectively. This evaluation indicated that most LPS occurred early in the first 6 months and remained more or less steady over the last months of follow-up (Figure 8A). Furthermore, because of the greater projection of the ergonomic implant in the region below the NAC with the patient in a standing position, AFG adequately maintains projection of the upper pole after the implant reaches its final position in the lower pole (Figures 10A,B, 11A,B; Supplemental Figure 2). In our sample, all patients were young with good skin quality, and medium-volume implants were used. Because the vast majority of implants reached their final position within 6 months of surgery and breast volume and shape did not continue to change, we selected a minimum 6-month period to evaluate results and patient satisfaction. Nevertheless, in patients with poor skin quality who receive large-volume implants, a longer follow-up period may be required.30 In our study we did not include the secondary cases in the 3D evaluation, because there were no significant changes in volume or expansion of the lower pole (such as those that occurred in the primary augmentation group). Furthermore, because none of the cases involved grade III/IV CC, total capsulectomy was not performed in any of the secondary cases, and the presence of the previous capsule makes it difficult to correctly assess the behavior of the silicone gel and the stretching of the lower pole of the breast. Thus, additional studies will be needed to assess the behavior of the lower pole in patients undergoing secondary BA and preservation of the previous capsule.
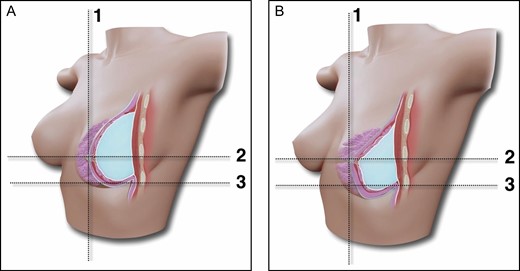
(A,B) Schematic representation of the conventional breast augmentation technique outcome and the relation of the lower pole stretching and upper pole projection. Breast augmentation by the axillary approach, subfascial pocket, and ergonomic implant with no fat grafting. Due to the viscoelastic properties of the gel, greater projection tends to occur in the lower pole, with consequent loss of projection in the upper pole. Lines 1 and 2 mark the position of the nipple-areola complex; line 3 is the position of the inframammary fold.
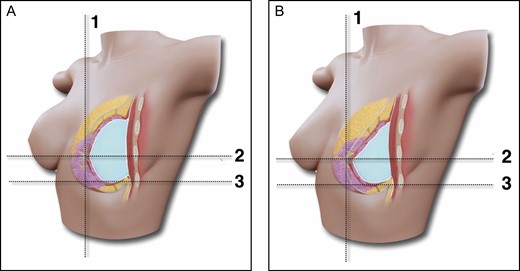
(A,B) Schematic representation of the subfascial ergonomic axillary hybrid technique outcome and the relation of the lower pole stretching and upper pole projection. Breast augmentation through the axillary approach, subfascial pocket, ergonomic implant and fat grafting. The fat graft in the upper pole provides better balance between the upper and lower poles and consequently maintains projection after the implant reaches its final position. Lines 1 and 2 mark the position of the nipple-areola complex; line 3 is the position of the inframammary fold.
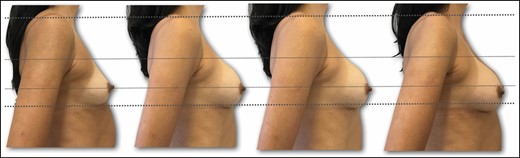
Preoperative right lateral view of a 36-year-old female patient with bilateral symmetric hypomastia. Outcome for implant position and lower pole stretching during follow-up period (preoperative, 10 days, 3 months, 18 months).
In our sample, at 6 months after surgery nearly 94% of the patients were either very satisfied or satisfied with their results. None regretted the surgery, and all liked the shape, cleavage, and soft transition between the subcutaneous tissue and implant edge. Besides satisfaction, reoperation rates are among the most objective measures of surgical outcome,61 and early reoperation rates are especially relevant because many reoperations are directly related to surgical planning and technique. In our sample, the reoperation rate was 1.7%, compared with 15% to 24% for pre-market approval studies.62 Patients continue to be followed up with the aim of evaluating long-term reoperation rates and comparing them with previous studies.
In our study, most complications were minor and occurred in the initial postoperative period, including subcutaneous banding in the axilla, minor wound dehiscence, and hypertrophic scarring. Based on our previous experience,17,18,51,52 patients with axillary banding were directed to perform a local massage, with satisfactory outcome and complete resolution of symptoms. In these cases, we do not recommend early manipulation or arm-stretching maneuvers in order to avoid implant displacement.52 No cases of infection, rotation, hematoma, seroma, or fat necrosis were observed during a mean follow-up of 18 months. Sim et al, in a series of 232 cases of endoscopic AA BA, observed occurrence of 0.8% seroma and 2.16% CC (mostly unilateral and Baker III).62 In one of the largest AA BA series, Huang et al observed complication rates of 2.9% for implant malposition and 1.9% for CC.63 Giordano et al64 observed a 1.63% incidence for CC, whereas Namnoum et al65 found a relative risk of 2.42% following SM augmentation with the AA. Despite our satisfactory outcome and lower incidence of complications, surgeons must be aware that BA procedures by the AA require greater expertise with regard to the relevant anatomy in the axilla, and more consideration of operative planning and technique than other approaches. Although only 1 case of CC secondary to a hematoma was observed in our series, more patients and longer follow-up are necessary to draw significant conclusions.
Our experience corroborates the observation by Kerfant et al37 that implant volume is directly associated with the risk of complications. In addition, some authors have stated that large volumes of AFG associated with large implants can increase interstitial pressure, resulting in decreased tissue perfusion and less AFG intake.48,53,55 On the other hand, we believe that in the HBA the use of fat allows smaller implants, potentially reducing long-term complications. In our sample the average implant volume was 255 cc, which can partially explain the low complication rate. The same rationale can also be concluded for the AFG volume, because in our sample patients received a mean of 96 mL of fat per breast. By placing lower volumes of fat and avoiding high pressure on the subcutaneous tissue, better AFG intake can be achieved with fewer fat-related complications. These findings would appear to confirm that the HBA technique may be restricted by the volume of fat that can be grafted because there is an inverse relation between AFG volume and fat integration.48-50,53
Some studies demonstrate that AFG may result in varying degrees of nodule formation and calcifications, which could potentially interfere with breast cancer screening or even result in additional biopsies and unnecessary costs.53,66,67 For this reason, all patients who are candidates for SEAH surgery must undergo breast imaging exam prior to the procedure in order to assess the presence of nodules, cysts, or calcifications and to establish a baseline for comparison during the postoperative follow-up. Our guidelines require ultrasound imaging for women under age 40 and ultrasound and mammograms for women over age 40. In our series, all image results from our study were classified as BI-RADS 2 (Figure 9). Cases of architectural distortion observed in 3.4% of the breasts analyzed did not complicate radiographic examination. Oil cysts and benign microcalcifications were observed in 5.1% and 12% of the breasts, respectively, and no cases of suspicious calcifications or nodules were noted. According to Veber et al, AFG is occasionally associated with the presence of brighter areas on mammograms, but these low-density areas do not affect the ACR rating.67 In a series analyzing mammograms from patients who underwent AFG, the same group found no difference in BI-RADS status before and after AFG. Furthermore, the postprocedure mammograms were interpreted effectively and unambiguously, and the rating remained stable during the observation period (both before and after AFG).67
In the present study as well as our previous experience,17-19 all cases were performed under direct view, without the use of endoscopy in primary cases, with customized retractors (Videos 1 and 2). This is important; most studies emphasize the role of direct view, with or without endoscopic assistance, to obtain better visualization of the muscle fascia and perforator vessels.14,15,17,45-47,62,68-72 Despite the clear advantages, some surgeons are satisfied with their own nonendoscopic techniques or partial endoscopic assistance. Solz described performing blunt pocket dissection with the fingers and a customized breast dissector (H Solz, personal communication, 2005). Similarly, Nordquist utilized endoscopy only to release the inferior-medial muscular fibers of the pectoralis muscle (J Nordquist, personal communication, 2019), whereas Benito-Ruiz and Hidalgo noted that the endoscope is not always necessary and may be used only to determine the correct dissection plane.73,74 However, we believe that the blind technique does not guarantee adequate hemostasis, accurate pocket dissection, or implant placement, and we have also found that considerable soft tissue and rib trauma resulting from blunt dissection can contribute to postoperative pain and immobility.44 In a large series of AA procedures, Sim and Park actively utilized direct view instead of blunt dissection.62,70
Compared to other incisions, AA presents some disadvantages. Because the incision is located in a remote region, revision surgeries, capsulectomy, and implant replacement may theoretically be complex.45 Despite these drawbacks, almost 45% of our patients underwent secondary BA involving capsulotomy, pocket change (SM to SF), and AFG with satisfactory outcomes. In a previous publication we assessed the results of secondary BA procedures by the AA and provided an algorithm for reoperative technique selection.52 In this study, 62 patients underwent secondary BA by the AA, which was indicated for CC in nearly half of the sample. These findings indicate that AA can be useful in secondary BA cases, with acceptable complication rates and the added bonus of avoiding additional scarring on the breast.
This study has some strengths; all cases were operated by a single surgeon with over 20 years’ experience with the AA SF technique who applied the same preoperative planning and surgical technique. Even with adequate pocket dissection and precise hemostasis, the AA is not reproducible like the IMF approach, and previous training and surgical skills are necessary.17,52 Longer operative time and extra costs can also be expected, but the additional operative time becomes less significant with experience. Although our mean operating time was 150 minutes, which is still acceptable, we had the additional benefits of liposuction on the donor sites. In our sample, 4 patients underwent liposuction for aesthetic purposes to improve body contouring, which extended operative time. In the remaining patients, liposuction was limited and exclusively collected fat for grafting, resulted in an approximate surgical time of 110 minutes.
This study also has some limitations. First, our sample was observational and nonrandomized, and may consequently have been prone to selection bias. We continue to collect prospective data on this subject and describe future outcomes. Second, AA is not suitable for patients with breast ptosis, and provides limited exposure to correct tubular breasts with constricted lower poles; as a result, our findings cannot be easily generalized to all BA candidates. Third, although there are different ways to evaluate satisfactory results, our objective was to describe the technique and assess short- and long-term complications. Future investigations should consequently evaluate the aesthetic results by administering validated patient questionnaires and assess AFG intake through objective analysis. Fourth, because of patient anatomy and preferences, our sample was restricted to only SSE implants (high projection) with volumes ranging from 175 to 355 cc. Additional study is therefore required to determine whether these results extend to other types of implants; our technique could be validated for saline-filled, nonergonomic gel implants and moderate/extra projection styles, for example.
Although BA has been thoroughly studied, previous reports on the AA and SF technique are limited, and no previous studies have evaluated the behavior of recent generations of implants such as the SSE. Similarly, few detailed clinical reports have specifically described operative planning, outcomes, and complications following simultaneous AA, SF, and AFG. This present article is consequently the first detailed description of the SEAH approach for patients who are candidates for primary and secondary BA.
CONCLUSIONS
The SEAH approach, combined with recent progress in surgical techniques and new-generation implants, can improve aesthetic outcomes following BA, and our results demonstrate it to be a consistent procedure. Even so, important technical steps must be planned prior to surgery. Preoperative patient evaluation is crucial to evaluate the indications, select proper volumes for the implant and AFG, and define pocket dimensions, as is careful intraoperative management. When combined with clinical expertise, this evidence will help surgeons achieve safer aesthetic outcomes from BA.
Disclosures
Dr Munhoz serves as a consultant/board member for Establishment Labs Holdings, Inc. (Alajuela, Costa Rica) and has shares of stocks in the company, but has received no financial support or assistance in the preparation of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES



