-
PDF
- Split View
-
Views
-
Cite
Cite
Alexandre Mendonça Munhoz, Reoperative Transaxillary Approach Algorithm: Extending the Surgical Alternatives for Secondary Breast Augmentation in the Era of Scarless Surgery, Aesthetic Surgery Journal, Volume 40, Issue 11, November 2020, Pages 1179–1192, https://doi.org/10.1093/asj/sjz339
Close - Share Icon Share
Abstract
Although the transaxillary approach (TAA) is useful in primary breast augmentation (BA) surgery, drawbacks of this technique include the need to correct complications arising from reuse of the axillary incision.
The purpose of this study was to assess the outcomes of secondary BA procedures performed via the TAA in a cohort of patients operated on by a single surgeon and to provide an algorithm for reoperative TAA technique selection.
Sixty-two patients (122 breasts) underwent secondary TAA BA, which was indicated for capsular contracture (CC) in 35 patients (56.4%). Periods for analysis included less than 10 days, 1, 3, 6, and 12 months, and then at 2-year intervals postprocedure.
Forty-three patients (69.3%) had a previous premuscular (PM) pocket; in 35 (81.3%) of these patients the new pocket was kept in the same position. Nineteen patients (30.7%) had a previous submuscular pocket, and 15 patients (78.9%) had the new pocket transferred to the PM plane. Ten cases of complications were observed in 8 patients (16.1%), Baker grade II/III CC in 3 (4.8%), and axillary banding in 2 (3.2%), during a mean follow-up of 72 months (range, 6-170 months). Fifty-nine patients (95.1%) were either very satisfied or satisfied with their aesthetic result.
Recent progress in surgical techniques has led to significant improvements in aesthetic outcomes following BA. The TAA can play a useful role in secondary BA cases and our results show this procedure to be useful, with acceptable complication rates, and the added bonus of avoiding additional scarring on the breast.

Breast augmentation (BA) is among the most common aesthetic surgical procedures, with more than 300,000 performed in 2018, a 48% increase since the year 2000.1 Despite good aesthetic results and high levels of patient satisfaction, reoperations are to be expected; in fact, the 10-year Core Study found risk rates for reoperation in subjects undergoing primary and secondary BA with round implants of 36.1% and 46%, respectively.2 A prospective 10-year follow-up study, which assessed shaped form-stable implants, showed similar results, with reoperation in 29.7% of primary and 43.4% of secondary BA subjects.3 As the number of patients undergoing BA has risen, the number of long-term reoperations is also expected to have increased.
The transaxillary approach (TAA) for primary BA has been amply described; 4-17 its principal advantages are lack of incisions on the breast and the ability to access the submuscular (SM), subfascial (SF), and subglandular (SG) pockets. This technique is currently the most popular approach in East Asia, with round or shaped implants placed via blunt dissection or the endoscopic technique.16,17 Although the TAA has been available for decades, critics have pointed out its limits in achieving accurate positioning of the implant in the pocket, especially in the reoperation scenario.18-21 However, in the author’s experience of performing primary BA,12,19 the use of customized retractors and endoscopic assistance enhances the TAA by permitting better placement of the implant inferiorly and improved management of the inframammary fold (IMF).
Recent advancements in surgical techniques have led to improved outcomes related to safety and aesthetics.21-23 However, drawbacks persist, and include difficulties correcting BA complications via the same axillary incision and the inevitable additional scar in the periareolar (PA) area or IMF after reoperation. The fact that future techniques may vary and that extra scars may be required could influence the choice of surgical access in primary surgery. This, along with the need for training on specific instruments and the associated learning curve, may be associated with the less frequent use of the TAA in primary BA procedures in Western countries.
Although the TAA has been widely studied, the literature is sparse on its use in correcting previous BA,24,25 with most of the clinical series addressing primary BA.6-16,19 Furthermore, few detailed clinical reports specifically address operative planning, outcomes, and complications following secondary BA via the TAA.17,24,25 Because the current evidence base regarding the TAA in reoperative BA has its limitations, this study describes the technique and assesses outcomes in patients who underwent a secondary TAA procedure. In addition, an algorithm is proposed to assist with the selection of techniques suitable for BA patients with previous axillary incision.
METHODS
A retrospective chart review of all consecutive secondary TAA BA procedures was performed. All study participants gave written informed consent, and the study was conducted in accordance with the provisions of the Declaration of Helsinki. All procedures were conducted at a single outpatient facility by 1 surgeon (A.M.M.) over a 20-year period (January 1999 to February 2019). Patients were supplied with detailed information regarding the surgical procedure and provided written informed consent. All patients were candidates for secondary BA, defined as having previous BA complications/complaints such as capsular contracture (CC), implant rupture, asymmetry, rippling, or volume/shape dissatisfaction. The degree of CC was determined according to the Baker scale. Data on patient age, body mass index (BMI), type of previous BA surgery (TAA, PA, IMF), and implant-related data (surface, shape, volume, and position) were also collected for each patient. Complications were classified as minor or major and divided into 2 types: implant-related and tissue-related complications. Implant malposition was defined as displacement of an implant that was initially placed correctly, and was graded as having occurred or not occurred, partially rotated, or totally rotated. Periods selected for analysis included less than 10 days, 1, 3, 6, and 12 months, and then at 2-year intervals postprocedure. Patient satisfaction was evaluated from the chart at the latest follow-up. For this objective, an acquired-informal questionnaire was used to grade the patient’s level of satisfaction with the aesthetic results. The patients classified their level of satisfaction as very satisfied, satisfied, disappointed, or regretted their decision. Postoperative photographs were obtained at follow-up appointments and compared with the preoperative photographs.
Instruments
The surgical instruments used for reoperative TAA BA (Supplemental Figure 1, available online at www.aestheticsurgeryjournal.com) include a needle tip electrocautery and a J-shaped rod, and 3-cm fiberoptic retractors (15 and 23 cm in length). For the pocket check we used 2 tennis-racket-shaped instruments, and we placed the implant into the pocket with a blunt dissector. The endoscopic equipment includes a 10-mm, 10° and 30° angled endoscope with matching endoscopic retractor associated with a suction cautery handle. A customized endoscopic retractor, which includes the camera system in a metal sleeve, was also utilized in order to allow adequate control of the retractor, camera, and suction via a single handheld device (Supplemental Figure 1). We usually worked with a standard endoscopic tower equipped with a xenon light source and incorporating a high-definition or wide-view endoscopic monitor.
Patient Selection, Planning, and Markings
All patients are marked preoperatively, and the previous axillary scar is always used. The current IMF (CIMF) and lateral limit of the pocket represented by the anterior axillary line (AAL) and the midsternal line (MSL) are marked on the skin. The parasternal lines (PSLs) are also marked, maintaining a distance of 2 to 3 cm between the breasts (Figure 1). It is crucial to evaluate the lateral/medial extent of the previous breast pocket, and to do so the implant is manually manipulated medially and laterally. The extension of the new pocket is marked according to the size of the new implant; in some situations this is an intraoperative decision. In order to avoid unnecessary expansion of the pocket, preoperative markings are always conservative in nature. The upper and lower limits of the pocket represented by the new IMF (NIMF) and superior breast line (SBL) are generally planned according to implant volume, which allows accurate centering of the implant and maintains precise pocket dimensions based on the implant size. Implant volume is always selected via joint decision with the patient, considering factors such as height, weight, and thoracic cage. Breast and thoracic asymmetries are also identified at this time, and corrected as much as possible with autogenous fat grafting (AFG) and different implants if necessary (Figure 2).
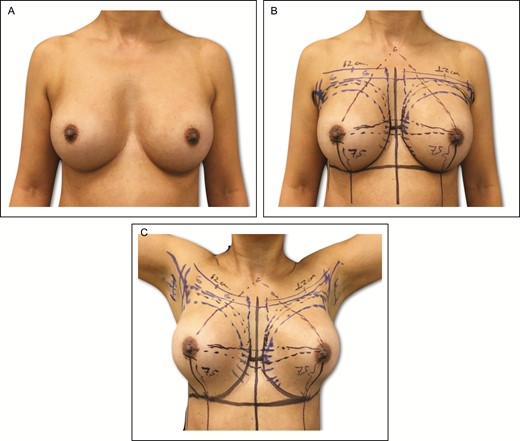
(A-C) Preoperative markings on 45-year-old female patient with a previous history of subfascial breast augmentation via the transaxillary approach 12 years previously. In this same patient, these skin marks include current symmetric inframammary fold, lateral limit of the pocket, represented by the anterior axillary line, midsternal line, and parasternal lines, with an average distance of 2 to 3 cm between the breasts.
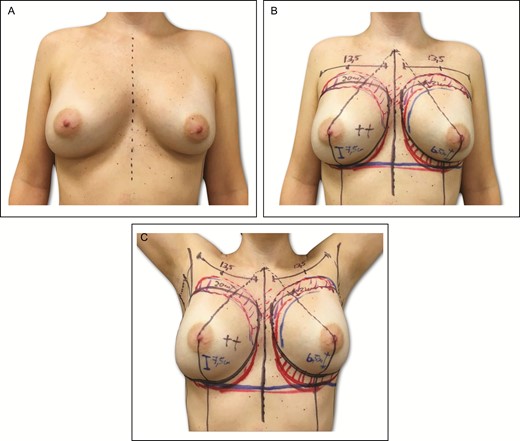
(A-C) Preoperative markings on 34-year-old female patient with previous history of submuscular breast augmentation via the transaxillary approach 15 years previously. In this same patient, similar markings associated with the preliminary area of autogenous fat grafting located on the upper and middle breast areas and intramammary fold asymmetry.
Surgical Planning/Technique
Skin Incision, Axillary Dissection
With the patient under general anesthesia, dissection begins in the previous axillary incision up to the subcutaneous plane. This plane shows a clear presence of fibrosis and scar tissue which may be deeper or more superficial depending on the previous technique. At this stage, it is crucial that the dissection be more superficial and closer to the skin flap in order to avoid invading the posterior triangle of the axilla and injuring important anatomic structures within the fibrous tissue (Video 1, available online at www.aestheticsurgery.com). The flap thickness generally ranges from 3 to 5 mm in slimmer patients to 1 cm in obese patients. The dissection continues until the lateral border of the pectoralis muscle. In implants located in the SM pocket, the dissection is performed under direct view for approximately 2 to 5 cm until the fibrous capsule of the implant is identified. Most often, besides the fibrous tissue this dissection is performed in an almost avascular plane with direct cauterization of small vessels. For implants located in the premuscular (PM) pocket, the pectoralis fascia is incised, and we use customized retractors and electrocautery to dissect in the upper-medial pole and progressively move laterally toward the intermediate pole up to the lateral-inferior quadrants, as we normally do in primary BA (Video 2, available online at www.aestheticsurgeryjournal.com). The previous implant and gland are lifted away from the pectoralis muscle, and the residual fibrous attachments between the capsule and muscle are sectioned at lateral and medial parts and at the IMF level. The limits of the pocket and the NIMF are determined with the dissector and verified according to the markings. As mentioned earlier, it is important not to expand this dissection beyond the limits of the previous implant in order avoid creating a large and loose SF pocket for the new implant. In patients with Baker grade II/III CC, we perform a capsulotomy under direct view through the endoscope. In cases of Baker grade III/IV CC, we recommend partial capsulectomy for SM pockets or even total capsulectomy for implants located in the PM planes (Video 3, available online at www.aestheticsurgeryjournal.com). Once the volume of the new implant has been determined through the use of measurements or sizers, the new pocket is expanded to contain the new implant in tight compression. Lateral dissection should be limited to the AAL to avoid lateral implant displacement. Before implant insertion, the pocket is irrigated with antibiotic solution (80 mg gentamicin + 500 mg cephazolin + 500 mL saline). The new implants are placed in the new location, and the patient is seated at a 90° angle to assess implant position, correct location, symmetry of the NIMF, and extension of the lateral and medial dissection. In cases involving a PM pocket and inadequate soft-tissue coverage, AFG can be performed as a complement in the upper and middle areas. To do so, the medial/superior edge of the breast is marked, and both implants are pushed in a medial/upward direction to simulate the cleavage limits. Based on the Coleman principles, AFG is injected via a 15-cm cannula, 1.9 to 2.1 mm in diameter (Faga Medical, Bauru, Brazil), to produce retrograde strings, and spread superficially in the subcutaneous tissue from the upper pole toward the lateral and medial pole to simulate a homogeneous transition between the areas with and without implant. A layered wound closure is performed without the use of suction drains, and an elastic band is placed over the superior breast poles.
Postoperative Care
All patients receive intravenous antibiotics, and oral antibiotics are continued for 48 hours. The elastic band is maintained for 4 weeks, and patients avoid early massaging/mobilization of the breasts for at least 2 to 3 months and physical activities for a period of 6 to 8 weeks.
RESULTS
Between January 2004 and February 2019, 62 patients (122 breasts) with a mean age of 38.6 years (range, 25-58 years) and mean BMI of 23.8 kg/m2 (range, 18.8-27.7 kg/m2) underwent secondary BA via the TAA, as seen in Table 1, Figures 3–5, and Supplemental Figure 2, available online at www.aestheticsurgeryjournal.com. Fifty-three (85.4%) patients underwent the primary BA procedure elsewhere, and 9 (14.5%) patients were previously operated in our practice. The main causes for secondary BA via the TAA were CC (35 patients, 56.4%), size/shape change (15 patients, 24.1%), implant rupture (10 patients, 16.2%), rippling (9 patients, 14.5%), asymmetry (8 patients, 12.9%), and dynamic breast in a previous SM implant (3 patients, 4.8%). Sixty patients (96.7%) had a previous TAA from their primary BA, 1 (1.6%) had a previous PA incision, and 1 (1.6%) had an IMF incision (Table 1). Forty-three patients (69.3%) had a previous PM pocket; in 35 (81.3%) of these patients, the new pocket remained in the PM position, whereas in 8 (18.6%) the new pocket was converted to an SM pocket due to insufficient soft-tissue coverage. Nineteen patients (30.7%) had a previous SM pocket, and 9 (47.3%) had lateralization of the implant and signs of dynamic breast due to muscle contraction. In this group, 15 patients (78.9%) had the new pocket transferred to the SF plane, and in 4 patients (21%) the new pocket was kept in the SM position. In 60 patients (96.7%), a new pair of silicone gel implants was selected: Inamed (Santa Barbara, CA, USA) 120 Style in 5 patients (8%), Allergan (Irvine, CA, USA) Inspira Style in 32 patients (51.6%), Allergan 410 Style in 10 patients (16.1%), and Motiva (Establishment Labs, Coyol Free Zone, Alajuela, Costa Rica) SmoothSilk Ergonomix Style in 14 patients (22.5%). The average new implant volume was 295 cc (range, 215-425 cc). In 2 patients (3.3%) patients, a unilateral secondary TAA was performed and the previous implants were not replaced. AFG was indicated in 18 (36%) of the 50 cases in which the PM pocket was used, with a mean volume of 95 mL (range, 65-125 mL) added to each breast (Table 2). Fat was obtained from the abdomen and thighs (inner and outer) in 80% of cases, followed by the hips (15%) and knees (5%). An average of 380 mL (range, 250-650 mL) of fat was harvested in each patient. The mean operating time was 190 minutes (range, 90-240 minutes).
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Age, years | |
| Median | 38.6 |
| Range | 25–58 |
| BMI, kg/m2 | |
| Median | 23.8 |
| Underweight (<18.5 kg/m2) | 5 (8) |
| Normal weight (18.5-24.9 kg/m2) | 39 (62.9) |
| Overweight (25-29.9 kg/m2) | 17 (27.4) |
| Obese (>30 kg/m2) | 1 (1.6) |
| Reasons for secondary BAa | |
| Capsular contracture | 35 (56.4) |
| Size/shape change | 15 (24.1) |
| Implant rupture | 10 (16.2) |
| Rippling | 9 (14.5) |
| Asymmetry | 8 (12.9) |
| Dynamic breast | 3 (4.8) |
| Previous BA incision | |
| TAA | 60 (96.7) |
| IMF | 1 (1.6) |
| PA | 1 (1.6) |
| Previous breast implant | |
| Macrotexturized | 36 (58) |
| Microtexturized | 21 (33.8) |
| Smooth | 5 (8) |
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Age, years | |
| Median | 38.6 |
| Range | 25–58 |
| BMI, kg/m2 | |
| Median | 23.8 |
| Underweight (<18.5 kg/m2) | 5 (8) |
| Normal weight (18.5-24.9 kg/m2) | 39 (62.9) |
| Overweight (25-29.9 kg/m2) | 17 (27.4) |
| Obese (>30 kg/m2) | 1 (1.6) |
| Reasons for secondary BAa | |
| Capsular contracture | 35 (56.4) |
| Size/shape change | 15 (24.1) |
| Implant rupture | 10 (16.2) |
| Rippling | 9 (14.5) |
| Asymmetry | 8 (12.9) |
| Dynamic breast | 3 (4.8) |
| Previous BA incision | |
| TAA | 60 (96.7) |
| IMF | 1 (1.6) |
| PA | 1 (1.6) |
| Previous breast implant | |
| Macrotexturized | 36 (58) |
| Microtexturized | 21 (33.8) |
| Smooth | 5 (8) |
BA, breast augmentation; BMI, body mass index; IMF, inframammary incision; PA, periareolar incision; TAA, transaxillary approach. aSome patients presented with more than 1 event.
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Age, years | |
| Median | 38.6 |
| Range | 25–58 |
| BMI, kg/m2 | |
| Median | 23.8 |
| Underweight (<18.5 kg/m2) | 5 (8) |
| Normal weight (18.5-24.9 kg/m2) | 39 (62.9) |
| Overweight (25-29.9 kg/m2) | 17 (27.4) |
| Obese (>30 kg/m2) | 1 (1.6) |
| Reasons for secondary BAa | |
| Capsular contracture | 35 (56.4) |
| Size/shape change | 15 (24.1) |
| Implant rupture | 10 (16.2) |
| Rippling | 9 (14.5) |
| Asymmetry | 8 (12.9) |
| Dynamic breast | 3 (4.8) |
| Previous BA incision | |
| TAA | 60 (96.7) |
| IMF | 1 (1.6) |
| PA | 1 (1.6) |
| Previous breast implant | |
| Macrotexturized | 36 (58) |
| Microtexturized | 21 (33.8) |
| Smooth | 5 (8) |
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Age, years | |
| Median | 38.6 |
| Range | 25–58 |
| BMI, kg/m2 | |
| Median | 23.8 |
| Underweight (<18.5 kg/m2) | 5 (8) |
| Normal weight (18.5-24.9 kg/m2) | 39 (62.9) |
| Overweight (25-29.9 kg/m2) | 17 (27.4) |
| Obese (>30 kg/m2) | 1 (1.6) |
| Reasons for secondary BAa | |
| Capsular contracture | 35 (56.4) |
| Size/shape change | 15 (24.1) |
| Implant rupture | 10 (16.2) |
| Rippling | 9 (14.5) |
| Asymmetry | 8 (12.9) |
| Dynamic breast | 3 (4.8) |
| Previous BA incision | |
| TAA | 60 (96.7) |
| IMF | 1 (1.6) |
| PA | 1 (1.6) |
| Previous breast implant | |
| Macrotexturized | 36 (58) |
| Microtexturized | 21 (33.8) |
| Smooth | 5 (8) |
BA, breast augmentation; BMI, body mass index; IMF, inframammary incision; PA, periareolar incision; TAA, transaxillary approach. aSome patients presented with more than 1 event.
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Previous breast implant pocket | |
| PM | 43 (69.3) |
| SM | 19 (30.7) |
| New breast implant pocket | |
| SG | 0 (0) |
| SF | 50 (80.6) |
| SM | 12 (19.3) |
| New breast implant choice | |
| Inamed 120 style | 5 (8) |
| Allergan Inspira style | 32 (51.6) |
| Allergan 410 style | 10 (16.1) |
| Motiva SmoothSilk SurfaceErgonomix | 14 (22.5) |
| Autogenous fat grafting | |
| PM pocket | 50 (80.6) |
| Yes | 18 (36) |
| No | 32 (64) |
| SM pocket | 12 (19.3) |
| Yes | 0 (0) |
| No | 12 (100) |
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Previous breast implant pocket | |
| PM | 43 (69.3) |
| SM | 19 (30.7) |
| New breast implant pocket | |
| SG | 0 (0) |
| SF | 50 (80.6) |
| SM | 12 (19.3) |
| New breast implant choice | |
| Inamed 120 style | 5 (8) |
| Allergan Inspira style | 32 (51.6) |
| Allergan 410 style | 10 (16.1) |
| Motiva SmoothSilk SurfaceErgonomix | 14 (22.5) |
| Autogenous fat grafting | |
| PM pocket | 50 (80.6) |
| Yes | 18 (36) |
| No | 32 (64) |
| SM pocket | 12 (19.3) |
| Yes | 0 (0) |
| No | 12 (100) |
PM, premuscular; SM, submuscular; SG, subglandular; SF, subfascial; SM, submuscular.
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Previous breast implant pocket | |
| PM | 43 (69.3) |
| SM | 19 (30.7) |
| New breast implant pocket | |
| SG | 0 (0) |
| SF | 50 (80.6) |
| SM | 12 (19.3) |
| New breast implant choice | |
| Inamed 120 style | 5 (8) |
| Allergan Inspira style | 32 (51.6) |
| Allergan 410 style | 10 (16.1) |
| Motiva SmoothSilk SurfaceErgonomix | 14 (22.5) |
| Autogenous fat grafting | |
| PM pocket | 50 (80.6) |
| Yes | 18 (36) |
| No | 32 (64) |
| SM pocket | 12 (19.3) |
| Yes | 0 (0) |
| No | 12 (100) |
| Characteristic . | Value (%) . |
|---|---|
| No. of patients | 62 |
| Previous breast implant pocket | |
| PM | 43 (69.3) |
| SM | 19 (30.7) |
| New breast implant pocket | |
| SG | 0 (0) |
| SF | 50 (80.6) |
| SM | 12 (19.3) |
| New breast implant choice | |
| Inamed 120 style | 5 (8) |
| Allergan Inspira style | 32 (51.6) |
| Allergan 410 style | 10 (16.1) |
| Motiva SmoothSilk SurfaceErgonomix | 14 (22.5) |
| Autogenous fat grafting | |
| PM pocket | 50 (80.6) |
| Yes | 18 (36) |
| No | 32 (64) |
| SM pocket | 12 (19.3) |
| Yes | 0 (0) |
| No | 12 (100) |
PM, premuscular; SM, submuscular; SG, subglandular; SF, subfascial; SM, submuscular.
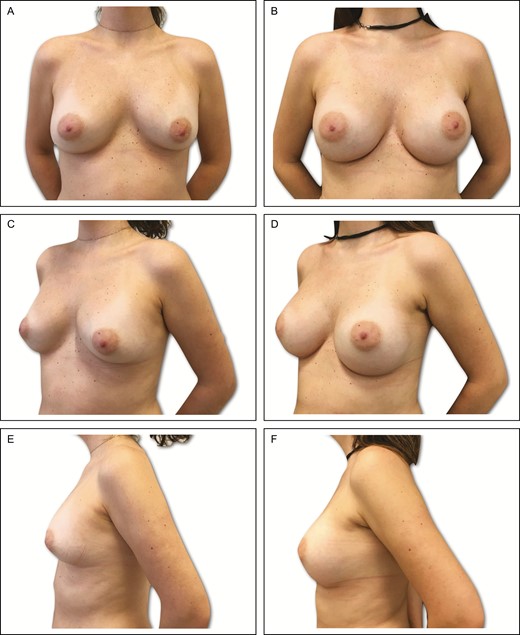
Preoperative (A) frontal, (C) left oblique, and (E) lateral views of a 34-year-old female patient with previous history of submuscular breast augmentation via the transaxillary approach 15 years previously. Presence of asymmetric intramammary folds, with a pinch test of 2 cm. (B, D, F) Appearance 2 years postprocedure in this patient, showing very good outcome with bilateral 335-cc Motiva SmoothSilk Surface Ergonomix implants, associated with 95 and 105 mL of autogenous fat grafting on the upper and upper-medial poles.
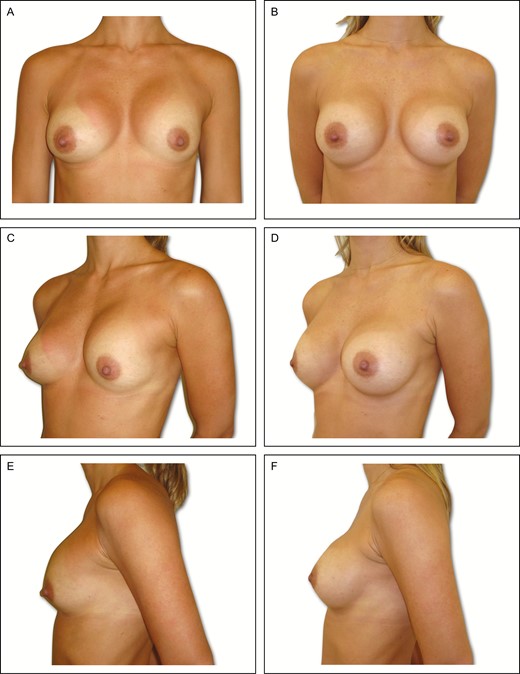
Preoperative (A) frontal, (C) left oblique, and (E) lateral views of a 34-year-old female patient with previous history of subglandular breast augmentation via the transaxillary approach 9 years previously. Presence of asymmetric intramammary folds, with Baker grade IV capsular contracture on the left side and Baker grade II on the right side, with a pinch test of 3 cm. (B, D, F) Appearance 6 years postoperative in this patient, showing very good outcome with bilateral 295-cc Allergan 410 implants.
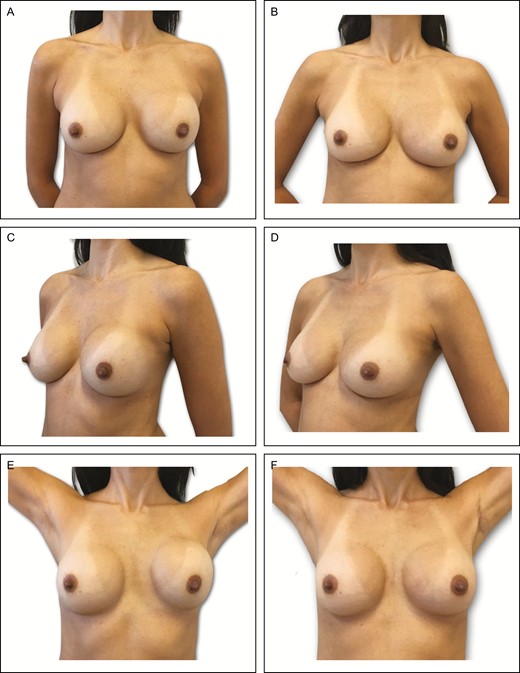
Preoperative (A) frontal, (C) left oblique, and (E) lateral views of a 36-year-old female patient with previous history of subfascial breast augmentation via the transaxillary approach 1 year before. The patient presented a left-side hematoma followed by Baker grade III capsular contracture on the same side. Presence of asymmetric intramammary folds and pinch test of 3 cm. (B, D, F) Appearance 1 year postprocedure showing very good outcome in this patient, with the same left implant (295-cc Motiva SmoothSilk Surface Ergonomix).
Outcome/Complications
Ten cases of complications were observed in 8 patients (16.1%): Baker grade II/III CC [n = 3 (4.8%)], subcutaneous banding in the axilla [n = 2 (3.2%)], seroma [n = 1 (1.6%)], minor wound dehiscence and hypertrophic scar at the axilla incision [n = 1 (1.6%)], malposition [n = 1 (1.6%)], and hematoma [n = 1 (1.6%)]. No cases of infection, rotation, or fat necrosis were observed during a mean follow-up of 72 months (range, 6-170 months), and no patients developed visible rippling (Table 3). Two patients (3.2%) underwent revision surgery due to hematoma and malposition, with satisfactory outcomes. The level of satisfaction was evaluated after a period of at least 6 months (range, 6-8 months). Fifty-nine patients (95.1%) were either very satisfied or satisfied with their aesthetic results. Three patients (4.8%) were partially disappointed: 2 patients (3.2%) thought the implants were small, and 1 (1.6%) complained of minor breast asymmetry due to previous CC. None regretted the surgery. A satisfactory aesthetic result was obtained, maintaining a natural breast shape (Figures 3-5 and Supplemental Figure 2).
| Complication . | Total no. (%) . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 2 (3.2) |
| Hypertrophic scars | 1 (1.6) |
| Wound dehiscence | 1 (1.6) |
| Implant | |
| Capsular contracture | 3 (4.8) |
| Hematoma | 1 (1.6) |
| Seroma | 1 (1.6) |
| Malposition/displacement | 1 (1.6) |
| Rippling | 0 (0.0) |
| Infection | 0 (0.0) |
| Totala | 10 (16.1) |
| Complication . | Total no. (%) . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 2 (3.2) |
| Hypertrophic scars | 1 (1.6) |
| Wound dehiscence | 1 (1.6) |
| Implant | |
| Capsular contracture | 3 (4.8) |
| Hematoma | 1 (1.6) |
| Seroma | 1 (1.6) |
| Malposition/displacement | 1 (1.6) |
| Rippling | 0 (0.0) |
| Infection | 0 (0.0) |
| Totala | 10 (16.1) |
aTwo patients presented more than 1 complication (hypertrophic scar and wound dehiscence) and (hematoma and capsular contracture).
| Complication . | Total no. (%) . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 2 (3.2) |
| Hypertrophic scars | 1 (1.6) |
| Wound dehiscence | 1 (1.6) |
| Implant | |
| Capsular contracture | 3 (4.8) |
| Hematoma | 1 (1.6) |
| Seroma | 1 (1.6) |
| Malposition/displacement | 1 (1.6) |
| Rippling | 0 (0.0) |
| Infection | 0 (0.0) |
| Totala | 10 (16.1) |
| Complication . | Total no. (%) . |
|---|---|
| Incision | |
| Axillary subcutaneous banding | 2 (3.2) |
| Hypertrophic scars | 1 (1.6) |
| Wound dehiscence | 1 (1.6) |
| Implant | |
| Capsular contracture | 3 (4.8) |
| Hematoma | 1 (1.6) |
| Seroma | 1 (1.6) |
| Malposition/displacement | 1 (1.6) |
| Rippling | 0 (0.0) |
| Infection | 0 (0.0) |
| Totala | 10 (16.1) |
aTwo patients presented more than 1 complication (hypertrophic scar and wound dehiscence) and (hematoma and capsular contracture).
DISCUSSION
Reoperative BA is frequently observed in private practice, and in addition to CC and asymmetry, size changes are the most frequent causes of surgical revision.26,27 Revision BA can be a complex procedure due to previous fibrosis, scars, and anatomic distortion caused by the primary operation,27 factors that markedly increase the incidence of complications. In fact, the prospective FDA Core Study reported in 2014 that reoperative BA is associated with a 28.7% risk of CC, compared with an 18.7% risk rate for primary BA.2 This indicates that even surgical experience and good knowledge of normal anatomic variations may not be sufficient in cases where breast anatomy is distorted as a result of previous procedures.
Despite ample literature existing on the outcome of primary BA, reports of reinterventions associated with BA are lacking. Because BA involving the TAA is relatively infrequent compared with other surgical approaches to augmentation, this study describes 1 surgeon’s experience over a 20-year period; even during this long-term evaluation, this approach was only used in 62 of the nearly 800 women who underwent BA.
Most revisionist interventions in patients who have previously undergone BA via the TAA are performed via new incisions such as the IMF or PA, which leave multiple and unnecessary scars. For some patients who wish to avoid additional breast scarring, reutilization of the same axillary incision is attractive.16 This argument is more relevant in Asian countries where patients frequently prefer to avoid additional scars, and surgeons more frequently consider utilizing the same axillary incision in secondary BA.16,23 In the era of “scarless principles” disseminated in most surgical fields as well as social media and among patients, this discussion is pertinent and current.28 It is consequently not surprising that minimally invasive surgery has spread rapidly after its introduction in the late 1990s. Patients have fewer, smaller incisions and higher satisfaction, and surgeons have promptly responded to patient demand by training and practicing these “scarless” techniques.28,29
In the analysis of our series, the majority of patients had undergone 1 previous BA operation with variable implant types and sizes, for the most part performed elsewhere. Almost 97% of patients had had their primary BA performed via the TAA. In all cases, the same scar was marked, and the incision length was increased, depending on the reason for reoperation. In cases where no CC was present and a small implant size was planned, the same length of the previous axillary incision was used. In cases with Baker grade III/IV CC which involved capsulotomy and a new, larger implant, the previous incision was increased but maintained in the posterior region in order to obtain a less visible scar, as described by Basile et al.24 In all cases, the measurement of incision length was assessed before and after reoperation. In the Baker grade III/IV CC group, the average incision increase was approximately 0.5 cm, and remained within the axilla limits with satisfactory results. In our series, 2 patients had previous a PA/IMF incision; in these cases, previous complications with IMF incision dehiscence in 1 patient with very thin skin and the unpredictable evolution of the new scar in the second patient who refused to reutilize the PA access were the reasons for secondary TAA.
Since its introduction in the 1970s,4,5 the TAA in BA has generated interest because the incision is hidden in an aesthetically acceptable region. The technique has undergone some technical adaptations since it was first reported; the SM pocket was initially introduced to provide adequate implant coverage,4-6,10 but its limitations included morbidity and breast animation.9,12,15,18,19 Some of these complications were related to technique, including traumatic dissection and restraints on creating an adequate pocket.30-32 In our sample, nearly 30% of patients had a previous SM pocket, and almost half of the this group presented implant distortion secondary to pectoralis muscle contraction. Most of this group had the new pocket transferred to the SF plane, with adequate correction of breast animation disorders in all cases (Supplemental Figure 3, available at www.aestheticsurgeryjournal.com).
Introduced in the 1990s,33,34 the SF pocket technique can be an option in selected patients because the fascia can provide additional tissue coverage and avoid the drawbacks of the SM plane.12,17,33-42 In our previous experience in primary BA, the SF technique can contribute to a faster postoperative recovery with less pain than the total SM pocket, without breast animation when the pectoral muscle is contracted.12,19 In our sample, and specifically when we change the pocket from SM to SF, the procedure was performed as described by Graf et al.9 The pectoral fascia is incised and the dissection continued through the SF plane under direct view employing customized retractors or endoscopic techniques. In order to avoid implant instability and the risk of displacement, we recommend limiting the new SF pocket dissection to the previous skin marks and the dimensions of the new implant. In this situation, the lateral aspect of the new pocket can be controlled more precisely through the novel SF pocket dissection, which is tailored to contain the new implant size.
Despite controversy related to inadequate soft-tissue coverage of fascia thickness,43 some studies have demonstrated satisfactory outcomes in selected patients.33,36,38,42 In our experience, the pectoral fascia is a well-defined structure in the upper thorax which is valuable in minimizing the appearance of the implant edges.37,39,41 In situations where the fascia could not provide adequate coverage for the implant, AFG supplementation proved an interesting technical alternative. This aspect was particularly evident in slim patients with limited soft-tissue coverage. In our series based on primary hybrid BA experience,41 we used a pinch test result of <2 cm as the main indication for AFG (Supplemental Figure 3). Furthermore, partial soft-tissue deficiency and presence of visible implant contours/rippling are also the main indications for hybrid BA surgery. In our sample, AFG was required in almost a quarter of the implants located in the SF plane because of insufficient pinch test results or implant edge visibility. In this group, a mean volume of 95 mL of fat was grafted to each breast, the majority in the superior, superior-medial, and medial areas. We have found that the best time to perform the AFG is after the new implants have been inserted, with the patient seated at a 90° angle to evaluate cleavage areas and symmetry.
The most frequent motives for secondary BA were CC (56.4%), size/shape change (24.1%), and implant rupture (16.2%). Sim et al44 evaluated the use of endoscopic and shaped implants associated with TAA BA in a series of 116 Asian patients. According to the authors, endoscopic BA was associated with shortened recovery times and satisfactory outcomes with a natural-appearing result. In this study, 3 of 116 patients (2.6%) experienced Baker grade III unilateral CC and 1 patient developed a unilateral hematoma. In one of the largest surveys of SM TAA, including 225 cases of secondary BA performed via the TAA, Huang et al25 observed that size change and implant malposition were the most common reasons for reoperations. According to the authors, 60% of cases of displaced implants were treated through the TAA without recurrence; CC was observed in 1.9% of patients and corrected via the TAA in 37.5% of cases, with only 1 relapse. In a large series with the longest follow-up of patients undergoing primary TAA BA involving saline and silicone gel implants, Gryskiewicz et al45 observed a 6.8% incidence of revision in the axillary cohort (136 of 2000). Overall, deflation of the saline implant was the most common reason for reoperations, accounting for almost 3.6% of all patients. These authors also described other motives such as size change, malposition, change from saline to silicone, and CC with incidences of 1.1%, 0.9%, 0.8%, and 0.6%, respectively.
In our series, breast asymmetry as a reason for secondary BA was observed in almost 13% (8 patients) of our sample. In this group, 5 had implant displacement due to previous overdissection (2 patients with bottoming out and 3 patients with lateral displacement). In these cases, we changed the pocket in order to achieve an adequate implant position and more stability. In our sample, the use of sutures can be an option and is feasible with endoscopy assistance; however, this maneuver was not necessary and the pocket change was satisfactory in all displaced cases.
There is no consensus about the best approach for cases where CC is present; some authors advocate capsulotomy, capsulectomy, pocket change, or implant replacement.27 Although pocket change and implant replacement are associated with lower CC relapse rates, the data related to capsulotomy/capsulectomy are less conclusive.23,27 In our sample, all cases of CC were treated with capsulotomy (mild degrees) or partial capsulectomy (severe cases associated with pocket change and implant replacement). Because severe cases of CC were frequently associated with asymmetry or implant malposition, pocket change was a more predictable procedure to control the new implant position. In our experience, this treatment decision must be weighed against the increased morbidity associated with large dissection and local trauma. To improve preoperative planning, to provide a clear surgical guideline, and to possibly facilitate consultation with and involvement of a plastic surgeon, the present TAA algorithm for secondary BA was developed (Supplemental Figure 3). During our follow-up, 3 patients developed Baker grade II/III CC (no cases of Baker grade IV were seen). The motive for reoperation in all of these cases was previous CC. Although these cases of CC were identified during follow-up, a larger patient sample and longer follow-up are necessary to draw significant conclusions.
Saline implants were not available in Brazil and all ruptured implants in our series were silicone gel, of lower and higher cohesivity, which represented almost 16% of the indications for secondary BA. All cases of rupture were intracapsular, ie, free silicone was contained with the surrounding fibrous capsule. In these cases explantation involved removal of the implant and any free silicone, capsulectomy, and replacement of the implant according to our algorithm. In order to optimize silicone gel removal and avoid further contamination of the pathway to the axillary incision, we use a larger-sized liposuction hose connected to a vacuum to remove the maximum volume of silicone gel located in the pocket (Video 2). After this maneuver, we performed the usual cleaning of the pocket with multiple saline washes and compresses to remove residues of silicone gel.
In our sample, almost 77% of implants used were macrotexturized (both round and shaped), and 22% were SmoothSilk surface (round base ergonomic style). Both types of implants perform differently in terms of tissue ingrowth and pocket adhesion.46,47 The SmoothSilk surface has no pores, which limits the number of sites for tissue ingrowth and consequently reduces the possibility of tissue adherence.47-50 Because of this lack of adherence, the pocket dimension must be planned carefully in order to avoid postoperative pocket expansion and implant displacement.50 We therefore advocate precise and tight pocket dissection and the use of an elastic band over the superior breast poles to prevent upward migration of the implants.19 Along similar lines, early massage, mobilization of the implants, and physical activities should be avoided to reduce the risk of implant displacement, rotation, and seroma.19,50
In this study, most complications were minor and occurred in the initial postoperative period, except CC. In our sample, 10 cases of complications were observed in 8 patients: the most common were Baker grade II/III CC, axillary banding, and seroma. No cases of infection, rotation, or fat necrosis were observed during a mean follow-up of 72 months. Two patients underwent revision surgery due to hematoma and malposition secondary to CC, and achieved satisfactory outcomes. The results from our study have verified that some complications were directly related to the axillary incision. In our sample, axillary upper inner arm subcutaneous banding was observed in 25% of the patients and represented almost 20% of all complications. In our study, the patients were instructed to perform a local massage after the second week, with satisfactory outcome and complete resolution of symptoms. In these cases, we do not advocate early arm-stretching maneuvers in order to avoid implant displacement.
Satisfaction was evaluated after at least 6 months of follow-up, because breast volume and shape continue to change. At the time of this writing, almost 95% of the sample was either very satisfied or satisfied with their result, and none regretted the surgery. Alongside aesthetic benefits related to the scar location, TAA associated with the SF pocket can also be considered a natural “no-touch” procedure, because it does not involve manipulation of the breast tissue and the implant is introduced at a significant distance from the nipple-areola complex.25 In our opinion, these aspects are responsible for the low infection and CC rates seen in our sample. However, in order to draw more definitive conclusions, prospective controlled studies are necessary with larger samples and longer follow-up comparing the TAA with other incisions.
The TAA has some disadvantages compared to other incisions. In theory, because this incision is located in a remote region, revision surgeries may be complex for inexperienced surgeons. Despite this theoretical limitation, our long experience with this technique in primary BA as well as customized retractors and endoscopy allowed us to perform capsulotomy/capsulectomy and pocket change in all cases with satisfactory outcomes. Any secondary BA surgery typically involves implant replacement, capsulotomy, capsulectomy, and readjustment of the old pocket to the dimensions of the new implant or the creation of a new pocket in another plane. It is clear to the vast majority of surgeons that the PA and IMF incisions provide direct access to the previous pocket and implant. In our 20-year experience, the TAA provides indirect access, which is compensated by the use of endoscopy and customized retractors. Even with these advantages, endoscopic surgery involves additional costs and requires special training, surgical skills, and a learning curve. A longer operative time can also be expected, but it seems clear that experience reduces any additional time. Secondary TAA BA is generally not recommended for women with glandular ptosis or severe asymmetry due to skin laxity.
Our study has some limitations. First, CC and size change accounted for nearly 80% of the indications for secondary BA via the TAA, which makes it difficult to generalize these findings to all patients with other complications from previous TAA procedures and candidates for revision BA. Second, because patients with previous BA are extremely heterogeneous with regard to prior implant type and pocket, reasons for secondary BA, and soft-tissue thickness, it is impossible to adjust risk, and we consequently did not attempt to perform a case-control study between primary and secondary BA via the TAA. Fourth and finally, because this present study was retrospective, observational, and nonrandomized, it may have been prone to selection bias, including a single center with a surgeon with 20 years’ experience performing the described technique. We continue to collect prospective data in this subject to report on our outcomes in the future.
Although BA is a well-studied procedure, previous reports concerning revision TAA are limited, and no previous studies have evaluated the performance of recent generations of implants such as the SmoothSilk surface. Additionally, very few detailed clinical reports specifically describe operative planning, outcomes, and complications specifically related to revision procedures. This present article is the first detailed description of the author’s approach to reoperative augmentation performed via the TAA for patients who are candidates for secondary BA. The concept of “scarless” surgery is engaging, particularly because a significant group of BA patients are young/middle-aged women who may be equally concerned with scars, aesthetic outcome, and treatment results.
CONCLUSIONS
We propose our updated algorithm and clinical experience with TAA BA in reoperations performed via the same previous incision. Based on our previously published studies,12,13,19,41 we have demonstrated reproducible outcomes with acceptable complications in a specific cohort of patients. The TAA can play a useful role in secondary BA cases and our results demonstrate that is a useful procedure; nevertheless, important technical steps must be planned previously. Preoperative patient evaluation is crucial to determine the indications, the pocket dimensions, and postoperative management. When combined with clinical expertise, this evidence will help the plastic surgeon provide patients with safer aesthetic outcomes following secondary BA with previous TAA. Future research should focus on comparing different surgical techniques and improving individualized indications to best serve patients undergoing secondary BA procedures.
Disclosures
Dr Munhoz serves as a consultant/board member for Establishment Labs, Holdings, Inc. and has shares of stocks in the company, but has received no financial support or assistance in the preparation of this article.
Funding
The author received no financial support for the research, authorship, and publication of this article.
REFERENCES



