-
PDF
- Split View
-
Views
-
Cite
Cite
Fang Wen Tseng, Kanthi Bommareddy, Konstantin Frank, Claudio DeLorenzi, Jeremy B Green, Neil Sadick, Rebecca Fitzgerald, Emy C Onishi, Arthur Swift, Sebastian Cotofana, Descriptive Analysis of 213 Positive Blood Aspiration Cases When Injecting Facial Soft Tissue Fillers, Aesthetic Surgery Journal, Volume 41, Issue 5, May 2021, Pages 616–624, https://doi.org/10.1093/asj/sjaa075
Close - Share Icon Share
Abstract
Pre-injection aspiration procedures could increase safety during soft tissue filler injections. However, various influencing factors have been detected in vitro that could result in false negative aspiration results.
A case series was retrospectively investigated to identify factors contributing to positive blood aspiration procedures in vivo.
This study evaluated 213 clinical cases positive for blood aspiration documented in an Asian population: 208 females (43.8 ± 7.2 years old) and 5 males (46.8 ± 7.8 years old) during soft tissue filler injections. Injection location, layer (depth) of injection, product injected, size of utilized needle (gauge), length of needle (inch), priming of needle (yes/no), injection angle (degree), and time until blood was visible in the needle hub (seconds) were evaluated.
The most frequent location where a positive aspiration was observed was the pyriform fossa (n = 56; 26.3%), the most frequent plane was the supra-periosteal plane (n = 195; 91.5%), and the most frequent needle utilized was a 27G needle (n = 125; 58.7%). Statistically significantly more positive cases were identified when the needle was primed compared with an unprimed needle (P < 0.001, which was independent of the product). The estimated incidence rate was 0.04% to 0.9% for having positive aspiration procedures per total performed injection procedures.
Pre-injection aspiration could be a valuable tool to prevent accidental intravascular injection of soft tissue filler. The results of the present investigation show that aspiration can be performed with an acceptable aspiration time, that is, less than 2 seconds, if a suitable product/needle combination is chosen.

According to the annual statistical report released by The Aesthetic Society, a total of 810,240 soft tissue filler injections were performed in 2018 in the United States.1 Concomitantly, the number of reported adverse events, of which tissue loss and intravascular related visual compromises are considered to be the most catastrophic, increased by 130% between 2015 and 2019.2,3 The current accepted pathophysiological mechanism behind intravascular related visual compromises is the inadvertent injection of filler material into the arterial vascular circulation with subsequent transportation to the ophthalmic artery vascular territory. There, either the central retinal artery itself or the multiple posterior ciliary arteries can be embolized by the filler material, resulting in occlusion and subsequent ischemia of the retina.4
Safety recommendations include low-pressure injections,5 utilization of a small bolus per pass,6 utilization (if possible) of larger sized cannulas (22 gauge),5,7,8 the selection of safer injection planes,9 and the implementation of a pre-injection aspiration procedure.10 The latter, however, is to date not universally accepted as mandatory in all facial areas due to the potential for false negative results. A false negative result would be defined as a negative aspiration occurring despite intravascular location of the needle tip. This effect could be due to collapse of the arterial wall by the applied negative pressure during the aspiration process11 or by a through and through phenomenon,10 where the needle tip is located intramurally or by an impaired combination of filler viscosity and needle characteristics (size and length). The latter false negative aspiration scenario could result from the filler material being too viscous to allow retrograde migration and thus aspiration of blood in a needle with a small diameter and/or increased length.
Previous studies investigated in vitro factors that could influence the outcome of performed aspiration procedures, which included the viscoelastic properties of the product,11-13 the plunger withdrawal time,12 the priming of the needle,11 and the pullback volume.13 However, all these studies were conducted in a laboratory setting, which had obvious shortcomings when equated to real-life conditions.
We present a retrospective study summarizing data collected from 213 positive aspirations obtained in true clinical settings over a period of 4 years. The analysis of this largest-to-date case series will hopefully increase the understanding of the role of positive blood aspirations and reduce the incidence of adverse vascular events and ultimately increase patient safety during soft tissue filler injections. Although no predictive statistical inferences can be generated, descriptive data still have practical value.
METHODS
Study Sample
This study is a retrospective analysis of 213 positive blood aspiration procedures obtained during facial soft tissue filler injection in Asian patients. All documented cases were consecutive patients injected between September 2015 and August 2019 (48 months) by the first author (F.W.T. has 12 years of experience with facial soft-tissue filler injections at the time of submission) in various facial regions utilizing a variety of facial soft tissue fillers (Tables 1 and 2). All patients were injected by the same specialist (F.W.T.) according to their individual aesthetic indications. Patient records were retrospectively analyzed and anonymized for further statistical processing. Written information and verbal explanations about the scope and goals of this retrospective analysis were given to participants before inclusion in this study. Patient records were not analyzed if willingness to participate was declined. Following the Declaration of Helsinki, written informed consent and signed picture and photo release agreement were obtained from every patient included. Patients were treated according to regional laws and good clinical practice independent of their willingness to share their data for this retrospective analysis. It is estimated that approximately 5760 to 7200 patients (ie, 120-150 patients per month) were injected during the 48-month observational period.
Number of Observed Positive Blood Aspirations at Respective Location and Depth of Needle Injection
| . | Subdermal/superficial fat . | Submucosal . | Supraperiosteal . | Total count . |
|---|---|---|---|---|
| Anterior inferior temple | 0 | 0 | 2 | 2 |
| Anterior superior temple | 0 | 0 | 42 | 42 |
| Chin | 0 | 0 | 15 | 15 |
| Deep midfacial fat compartments | 0 | 0 | 54 | 54 |
| Forehead | 0 | 0 | 8 | 8 |
| Lateral midface (zygomaticomaxillary fissure) | 0 | 0 | 5 | 5 |
| Lips | 2 | 1 | 0 | 3 |
| Nasal dorsum | 0 | 0 | 7 | 7 |
| Nasal spine | 0 | 0 | 3 | 3 |
| Nasolabial sulcus | 2 | 0 | 0 | 2 |
| Posterior inferior temple | 0 | 0 | 0 | 0 |
| Posterior superior temple | 0 | 0 | 5 | 5 |
| Pyriform fossa | 2 | 0 | 54 | 56 |
| Superficial medial cheek fat compartment | 11 | 0 | 0 | 11 |
| Total count | 17 | 1 | 195 | 213 |
| . | Subdermal/superficial fat . | Submucosal . | Supraperiosteal . | Total count . |
|---|---|---|---|---|
| Anterior inferior temple | 0 | 0 | 2 | 2 |
| Anterior superior temple | 0 | 0 | 42 | 42 |
| Chin | 0 | 0 | 15 | 15 |
| Deep midfacial fat compartments | 0 | 0 | 54 | 54 |
| Forehead | 0 | 0 | 8 | 8 |
| Lateral midface (zygomaticomaxillary fissure) | 0 | 0 | 5 | 5 |
| Lips | 2 | 1 | 0 | 3 |
| Nasal dorsum | 0 | 0 | 7 | 7 |
| Nasal spine | 0 | 0 | 3 | 3 |
| Nasolabial sulcus | 2 | 0 | 0 | 2 |
| Posterior inferior temple | 0 | 0 | 0 | 0 |
| Posterior superior temple | 0 | 0 | 5 | 5 |
| Pyriform fossa | 2 | 0 | 54 | 56 |
| Superficial medial cheek fat compartment | 11 | 0 | 0 | 11 |
| Total count | 17 | 1 | 195 | 213 |
Number of Observed Positive Blood Aspirations at Respective Location and Depth of Needle Injection
| . | Subdermal/superficial fat . | Submucosal . | Supraperiosteal . | Total count . |
|---|---|---|---|---|
| Anterior inferior temple | 0 | 0 | 2 | 2 |
| Anterior superior temple | 0 | 0 | 42 | 42 |
| Chin | 0 | 0 | 15 | 15 |
| Deep midfacial fat compartments | 0 | 0 | 54 | 54 |
| Forehead | 0 | 0 | 8 | 8 |
| Lateral midface (zygomaticomaxillary fissure) | 0 | 0 | 5 | 5 |
| Lips | 2 | 1 | 0 | 3 |
| Nasal dorsum | 0 | 0 | 7 | 7 |
| Nasal spine | 0 | 0 | 3 | 3 |
| Nasolabial sulcus | 2 | 0 | 0 | 2 |
| Posterior inferior temple | 0 | 0 | 0 | 0 |
| Posterior superior temple | 0 | 0 | 5 | 5 |
| Pyriform fossa | 2 | 0 | 54 | 56 |
| Superficial medial cheek fat compartment | 11 | 0 | 0 | 11 |
| Total count | 17 | 1 | 195 | 213 |
| . | Subdermal/superficial fat . | Submucosal . | Supraperiosteal . | Total count . |
|---|---|---|---|---|
| Anterior inferior temple | 0 | 0 | 2 | 2 |
| Anterior superior temple | 0 | 0 | 42 | 42 |
| Chin | 0 | 0 | 15 | 15 |
| Deep midfacial fat compartments | 0 | 0 | 54 | 54 |
| Forehead | 0 | 0 | 8 | 8 |
| Lateral midface (zygomaticomaxillary fissure) | 0 | 0 | 5 | 5 |
| Lips | 2 | 1 | 0 | 3 |
| Nasal dorsum | 0 | 0 | 7 | 7 |
| Nasal spine | 0 | 0 | 3 | 3 |
| Nasolabial sulcus | 2 | 0 | 0 | 2 |
| Posterior inferior temple | 0 | 0 | 0 | 0 |
| Posterior superior temple | 0 | 0 | 5 | 5 |
| Pyriform fossa | 2 | 0 | 54 | 56 |
| Superficial medial cheek fat compartment | 11 | 0 | 0 | 11 |
| Total count | 17 | 1 | 195 | 213 |
Number of Observed Positive Blood Aspirations for Products Contained in Syringe and Their Respective Dilution With Lidocaine
| . | 0.05 cc . | 0.1 cc . | Other cc . | No lidocaine added . | Total count . |
|---|---|---|---|---|---|
| Artecoll | 3 | 6 | 0 | 0 | 9 |
| Belotero Balance | 1 | 1 | 0 | 0 | 2 |
| Elravie Deep Line | 5 | 0 | 0 | 0 | 5 |
| Hyadermis Smile | 1 | 0 | 0 | 1 | 2 |
| Juvederm Ultra Plus | 15 | 1 | 0 | 0 | 16 |
| Juvederm Volift | 0 | 0 | 0 | 4 | 4 |
| Juvederm Voluma | 0 | 0 | 0 | 24 | 24 |
| Princess Volume | 1 | 0 | 0 | 0 | 1 |
| Restylane | 53 | 39 | 0 | 3 | 95 |
| Restylane Lyft (Perlane) | 2 | 0 | 0 | 12 | 14 |
| Restylane Vital | 0 | 1 | 0 | 0 | 1 |
| Sculptra | 0 | 0 | 38 | 0 | 38 |
| Sunmax Collagen Implant | 1 | 0 | 0 | 0 | 1 |
| Unknown | 0 | 1 | 0 | 0 | 1 |
| Total count | 82 | 49 | 38 | 44 | 213 |
| . | 0.05 cc . | 0.1 cc . | Other cc . | No lidocaine added . | Total count . |
|---|---|---|---|---|---|
| Artecoll | 3 | 6 | 0 | 0 | 9 |
| Belotero Balance | 1 | 1 | 0 | 0 | 2 |
| Elravie Deep Line | 5 | 0 | 0 | 0 | 5 |
| Hyadermis Smile | 1 | 0 | 0 | 1 | 2 |
| Juvederm Ultra Plus | 15 | 1 | 0 | 0 | 16 |
| Juvederm Volift | 0 | 0 | 0 | 4 | 4 |
| Juvederm Voluma | 0 | 0 | 0 | 24 | 24 |
| Princess Volume | 1 | 0 | 0 | 0 | 1 |
| Restylane | 53 | 39 | 0 | 3 | 95 |
| Restylane Lyft (Perlane) | 2 | 0 | 0 | 12 | 14 |
| Restylane Vital | 0 | 1 | 0 | 0 | 1 |
| Sculptra | 0 | 0 | 38 | 0 | 38 |
| Sunmax Collagen Implant | 1 | 0 | 0 | 0 | 1 |
| Unknown | 0 | 1 | 0 | 0 | 1 |
| Total count | 82 | 49 | 38 | 44 | 213 |
Number of Observed Positive Blood Aspirations for Products Contained in Syringe and Their Respective Dilution With Lidocaine
| . | 0.05 cc . | 0.1 cc . | Other cc . | No lidocaine added . | Total count . |
|---|---|---|---|---|---|
| Artecoll | 3 | 6 | 0 | 0 | 9 |
| Belotero Balance | 1 | 1 | 0 | 0 | 2 |
| Elravie Deep Line | 5 | 0 | 0 | 0 | 5 |
| Hyadermis Smile | 1 | 0 | 0 | 1 | 2 |
| Juvederm Ultra Plus | 15 | 1 | 0 | 0 | 16 |
| Juvederm Volift | 0 | 0 | 0 | 4 | 4 |
| Juvederm Voluma | 0 | 0 | 0 | 24 | 24 |
| Princess Volume | 1 | 0 | 0 | 0 | 1 |
| Restylane | 53 | 39 | 0 | 3 | 95 |
| Restylane Lyft (Perlane) | 2 | 0 | 0 | 12 | 14 |
| Restylane Vital | 0 | 1 | 0 | 0 | 1 |
| Sculptra | 0 | 0 | 38 | 0 | 38 |
| Sunmax Collagen Implant | 1 | 0 | 0 | 0 | 1 |
| Unknown | 0 | 1 | 0 | 0 | 1 |
| Total count | 82 | 49 | 38 | 44 | 213 |
| . | 0.05 cc . | 0.1 cc . | Other cc . | No lidocaine added . | Total count . |
|---|---|---|---|---|---|
| Artecoll | 3 | 6 | 0 | 0 | 9 |
| Belotero Balance | 1 | 1 | 0 | 0 | 2 |
| Elravie Deep Line | 5 | 0 | 0 | 0 | 5 |
| Hyadermis Smile | 1 | 0 | 0 | 1 | 2 |
| Juvederm Ultra Plus | 15 | 1 | 0 | 0 | 16 |
| Juvederm Volift | 0 | 0 | 0 | 4 | 4 |
| Juvederm Voluma | 0 | 0 | 0 | 24 | 24 |
| Princess Volume | 1 | 0 | 0 | 0 | 1 |
| Restylane | 53 | 39 | 0 | 3 | 95 |
| Restylane Lyft (Perlane) | 2 | 0 | 0 | 12 | 14 |
| Restylane Vital | 0 | 1 | 0 | 0 | 1 |
| Sculptra | 0 | 0 | 38 | 0 | 38 |
| Sunmax Collagen Implant | 1 | 0 | 0 | 0 | 1 |
| Unknown | 0 | 1 | 0 | 0 | 1 |
| Total count | 82 | 49 | 38 | 44 | 213 |
Data Collected
The following data were collected on a routine basis and documented by the first author of the study during his daily clinical practice treating consecutive patients: age, gender, facial region injected, layer (depth) of injection, product injected, whether lidocaine was added to the injected product, needle gauge and length, whether needle priming was performed, injection angle, and time until blood was visible in the needle hub (Tables 1-4). Data collection was conducted in various clinics (F.W.T. practices in multiple clinics) and during international travels of the first author. Data analysis (not data collection) is the subject of this study.
Number of Observed Positive Blood Aspirations for Differently Used Needle Sizes (23G-30G) and Whether Needle Was Already Filled With Product on Injection (Primed) or Not (Nonprimed)
| . | Primed . | Nonprimed . | Total count . |
|---|---|---|---|
| 23G, 1” | 1 | 0 | 1 |
| 24G, 1” | 1 | 0 | 1 |
| 25G, 1.5”, 1”, 5/8”, ½” | 36 | 8 | 44 |
| 26G ½” | 1 | 0 | 1 |
| 27G, ½” | 102 | 23 | 125 |
| 29G, ½” | 29 | 7 | 36 |
| 30G, ½” | 5 | 0 | 5 |
| Total count | 175 | 38 | 213 |
| . | Primed . | Nonprimed . | Total count . |
|---|---|---|---|
| 23G, 1” | 1 | 0 | 1 |
| 24G, 1” | 1 | 0 | 1 |
| 25G, 1.5”, 1”, 5/8”, ½” | 36 | 8 | 44 |
| 26G ½” | 1 | 0 | 1 |
| 27G, ½” | 102 | 23 | 125 |
| 29G, ½” | 29 | 7 | 36 |
| 30G, ½” | 5 | 0 | 5 |
| Total count | 175 | 38 | 213 |
Number of Observed Positive Blood Aspirations for Differently Used Needle Sizes (23G-30G) and Whether Needle Was Already Filled With Product on Injection (Primed) or Not (Nonprimed)
| . | Primed . | Nonprimed . | Total count . |
|---|---|---|---|
| 23G, 1” | 1 | 0 | 1 |
| 24G, 1” | 1 | 0 | 1 |
| 25G, 1.5”, 1”, 5/8”, ½” | 36 | 8 | 44 |
| 26G ½” | 1 | 0 | 1 |
| 27G, ½” | 102 | 23 | 125 |
| 29G, ½” | 29 | 7 | 36 |
| 30G, ½” | 5 | 0 | 5 |
| Total count | 175 | 38 | 213 |
| . | Primed . | Nonprimed . | Total count . |
|---|---|---|---|
| 23G, 1” | 1 | 0 | 1 |
| 24G, 1” | 1 | 0 | 1 |
| 25G, 1.5”, 1”, 5/8”, ½” | 36 | 8 | 44 |
| 26G ½” | 1 | 0 | 1 |
| 27G, ½” | 102 | 23 | 125 |
| 29G, ½” | 29 | 7 | 36 |
| 30G, ½” | 5 | 0 | 5 |
| Total count | 175 | 38 | 213 |
Cross-Tabulation Between Injected Product and Utilized Needle Sizes and Lengths of 213 Positive Aspiration Events
| . | 30 G, 1/2” . | 29 G, 1/2” . | 27 G, 1/2” . | 26 G, 1/2” . | 25 G, 1.5” . | 25 G, 1” . | 25 G, 5/8” . | 25 G, 1/2” . | 24 G, 1” . | 23 G, 1” . | Total count . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artecoll | 1 | 8 | 9 | ||||||||
| Belotero Balance | 2 | 2 | |||||||||
| Elravie Deep Line | 5 | 5 | |||||||||
| Hyadermis Smile | 2 | 2 | |||||||||
| Juvederm Ultra Plus | 16 | 16 | |||||||||
| Juvederm Volift | 4 | 4 | |||||||||
| Juvederm Voluma | 24 | 24 | |||||||||
| Princess Volume | 1 | 1 | |||||||||
| Restylane | 23 | 72 | 95 | ||||||||
| Restylane Lyft (Perlane) | 12 | 2 | 14 | ||||||||
| Restylane Vital | 1 | 1 | |||||||||
| Sculptra | 9 | 4 | 23 | 1 | 1 | 38 | |||||
| Sunmax Collagen Implant | 1 | 1 | |||||||||
| Unknown | 1 | 1 | |||||||||
| Total count | 5 | 36 | 125 | 1 | 9 | 4 | 23 | 8 | 1 | 1 | 213 |
| . | 30 G, 1/2” . | 29 G, 1/2” . | 27 G, 1/2” . | 26 G, 1/2” . | 25 G, 1.5” . | 25 G, 1” . | 25 G, 5/8” . | 25 G, 1/2” . | 24 G, 1” . | 23 G, 1” . | Total count . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artecoll | 1 | 8 | 9 | ||||||||
| Belotero Balance | 2 | 2 | |||||||||
| Elravie Deep Line | 5 | 5 | |||||||||
| Hyadermis Smile | 2 | 2 | |||||||||
| Juvederm Ultra Plus | 16 | 16 | |||||||||
| Juvederm Volift | 4 | 4 | |||||||||
| Juvederm Voluma | 24 | 24 | |||||||||
| Princess Volume | 1 | 1 | |||||||||
| Restylane | 23 | 72 | 95 | ||||||||
| Restylane Lyft (Perlane) | 12 | 2 | 14 | ||||||||
| Restylane Vital | 1 | 1 | |||||||||
| Sculptra | 9 | 4 | 23 | 1 | 1 | 38 | |||||
| Sunmax Collagen Implant | 1 | 1 | |||||||||
| Unknown | 1 | 1 | |||||||||
| Total count | 5 | 36 | 125 | 1 | 9 | 4 | 23 | 8 | 1 | 1 | 213 |
Cross-Tabulation Between Injected Product and Utilized Needle Sizes and Lengths of 213 Positive Aspiration Events
| . | 30 G, 1/2” . | 29 G, 1/2” . | 27 G, 1/2” . | 26 G, 1/2” . | 25 G, 1.5” . | 25 G, 1” . | 25 G, 5/8” . | 25 G, 1/2” . | 24 G, 1” . | 23 G, 1” . | Total count . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artecoll | 1 | 8 | 9 | ||||||||
| Belotero Balance | 2 | 2 | |||||||||
| Elravie Deep Line | 5 | 5 | |||||||||
| Hyadermis Smile | 2 | 2 | |||||||||
| Juvederm Ultra Plus | 16 | 16 | |||||||||
| Juvederm Volift | 4 | 4 | |||||||||
| Juvederm Voluma | 24 | 24 | |||||||||
| Princess Volume | 1 | 1 | |||||||||
| Restylane | 23 | 72 | 95 | ||||||||
| Restylane Lyft (Perlane) | 12 | 2 | 14 | ||||||||
| Restylane Vital | 1 | 1 | |||||||||
| Sculptra | 9 | 4 | 23 | 1 | 1 | 38 | |||||
| Sunmax Collagen Implant | 1 | 1 | |||||||||
| Unknown | 1 | 1 | |||||||||
| Total count | 5 | 36 | 125 | 1 | 9 | 4 | 23 | 8 | 1 | 1 | 213 |
| . | 30 G, 1/2” . | 29 G, 1/2” . | 27 G, 1/2” . | 26 G, 1/2” . | 25 G, 1.5” . | 25 G, 1” . | 25 G, 5/8” . | 25 G, 1/2” . | 24 G, 1” . | 23 G, 1” . | Total count . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artecoll | 1 | 8 | 9 | ||||||||
| Belotero Balance | 2 | 2 | |||||||||
| Elravie Deep Line | 5 | 5 | |||||||||
| Hyadermis Smile | 2 | 2 | |||||||||
| Juvederm Ultra Plus | 16 | 16 | |||||||||
| Juvederm Volift | 4 | 4 | |||||||||
| Juvederm Voluma | 24 | 24 | |||||||||
| Princess Volume | 1 | 1 | |||||||||
| Restylane | 23 | 72 | 95 | ||||||||
| Restylane Lyft (Perlane) | 12 | 2 | 14 | ||||||||
| Restylane Vital | 1 | 1 | |||||||||
| Sculptra | 9 | 4 | 23 | 1 | 1 | 38 | |||||
| Sunmax Collagen Implant | 1 | 1 | |||||||||
| Unknown | 1 | 1 | |||||||||
| Total count | 5 | 36 | 125 | 1 | 9 | 4 | 23 | 8 | 1 | 1 | 213 |
The products injected were Restylane Lyft/Perlane, Restylane, Restylane Vital (Galderma Pharma SA, Uppsala, Sweden); Juvederm Voluma, Juvederm Volift, Juvederm Ultra Plus (Allergan PLC, Dublin, Ireland); Belotero Balance (Merz Pharma GmbH & Co. KGaA, Frankfurt am Main, Germany); Hyadermis Smile (SciVision Biotech Inc., Kaohsiung, Taiwan); Elravie Deep line (Humedix, Anyang-si, Republic of Korea); Princess Volume (Croma-Pharma GmbH, Leobendorf, Austria); Sculptra (Sinclair Pharma Ltd., London, United Kingdom); Sunmax Collagen Implant (Sunmax Biotechnoloy CO., Ltd., Tainan, Taiwan); and Artecoll (Canderm Pharma Inc., St. Laurent, Canada) (Tables 2 and 4).
Criteria for Positive Blood Aspiration
Each included case was considered a “positive blood aspiration case” if blood was visible in the needle hub during needle insertion or during aspiration in the initiation phase of the filler injection. Aspiration was consistently performed during each injection process (independent of the amount injected) and was characterized by the generation of negative pressure in the needle by retracting the plunger of the soft tissue filler syringe. The pullback volume until a positive case was noted was 0.05 to 0.5 cc. The generated pressure was not measured objectively because the cases occurred during daily clinical consults of consecutive patients.
If the aspiration procedure was positive (independent of the amount of blood visible in the needle hub), the procedure was immediately stopped, the needle removed, and digital pressure applied. After a minimum time of 5 minutes, a different location in the same facial region was targeted with a different injection angle utilizing a different soft tissue filler syringe. In no case was the injection procedure continued after blood appeared in the needle hub.
Statistical Analyses
Descriptive and comparative analyses were performed using SPSS Statistics 23 (IBM, Armonk, NY). Statistical significance was defined as P < 0.05.
RESULTS
General Description
Patient demographics noted were: 213 participants aged an overall mean of 43.9 ± 7.2 years (range, 24-64 years). Of those, 208 were female (mean age, 43.8 ± 7.2 years; range, 24-64 years) and 5 male (mean age, 46.8 ± 7.8 years; range, 37-57 years).
No adverse vascular adverse events and especially no injection related visual compromise occurred during the evaluation period in patients with a positive aspiration procedure.
Estimate of Minimum and Maximum Incidence Rates
Only positive blood aspiration cases were retrospectively analyzed; no negative injection cases were utilized for comparisons. During the 48-month study period, an approximate amount of 11,500 cc of filler material was utilized. The extrapolation for the computation of the incidence rate was performed based on the amount of filler material injected rather than from the number of patients treated. Various amounts of filler material, that is, various numbers of syringes and injection passes per patient, could be performed, thus influencing the number of performed aspiration procedures per patient.
The smallest amount of product injected during 1 pass (1 pass refers to 1 injection and 1 aspiration procedure per specific location) was 0.02 cc (commonly used for lips, periorbital areas, nasolabial folds), whereas the largest amount of product applied during 1 pass was 0.5 cc (commonly used for temples or chin). An approximate range of incidence for having a positive blood aspiration event may be estimated utilizing this information to derive the minimum and maximum values. If all of the filler material (11,500 cc) had been injected in boluses of 0.02 cc per pass during the observational period, there would have been 575,000 injection passes, resulting in a minimum incidence rate of 213/575,000 (0.037%), or 1 of 2700 injection passes. If all the filler material had been delivered in boluses of 0.5 cc per pass, there would have been 23,000 injection passes, resulting in a maximum incidence rate of 213/23,000 (0.926%), or 1 of 108 passes.
Injection Location
The most frequent location for positive aspiration was the pyriform fossa with a total of n = 56 (26.3%), followed by the injection into the deep midfacial fat compartments (n = 54; 25.4%), anterior superior temple (n = 42; 19.7%), and chin (n = 15; 7.0%). Detailed information about all facial regions injected can be found in Table 1 and Figure 1.
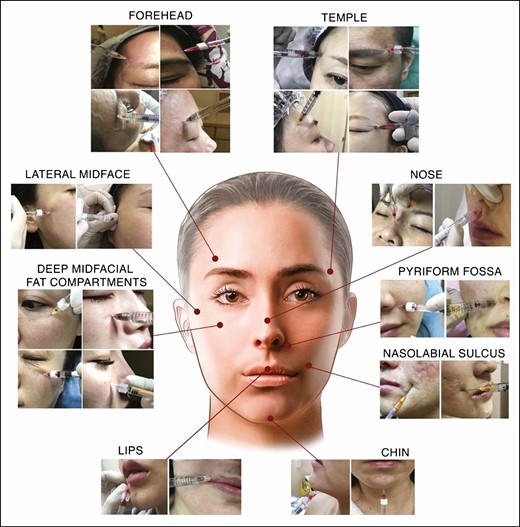
Collage of 24 positive blood aspiration cases arranged by region observed in the retrospectively investigated 213 clinical cases. The mean age of the displayed patients is 40.38 ± 7.9 years and the gender distribution is 22 females and 2 males.
Injection Depth
The most frequent plane where aspiration was positive for blood was the supra-periosteal plane, that is, the injection was performed with the needle tip being in constant contact with the bone during the product application with n = 195 (91.5%), followed by the subdermal plane with n = 17 (8.0%) and the submucosal plane in 1 case (0.5%) (Table 1). Subdermal/submucosal plane refers to a very superficial plane right deep to the dermis or the mucosa of the dry red lip.
In the supraperiosteal plane of the pyriform fossa and of the deep midfacial fat compartments, n = 54 positive case were recorded each, representing 25.4% of all cases. Positive cases were recorded in the subdermal plane of the superficial cheek fat compartments (n = 11; 5.2%), the nasolabial sulcus (n = 2; 1.0%), the pyriform fossa (n = 2; 1.0%), and the lips (n = 2; 1.0%) (Table 3).
Needles Utilized
The most frequently utilized needle size was a 27 G (n = 125; 58.7%), followed by 25 G (n = 44; 20.7%) and 29 G (n = 36; 16.9%). Detailed information about needle sizes and length utilized are given in Table 3.
Five positive cases (2.3%) were recorded utilizing a 30 G needle, and 1 case (0.5%) was recorded utilizing a 23G needle targeting the supraperiosteal plane of the anterior superior temple (Table 3).
Injection Technique
The injection angle of the performed needle injections ranged between 90°C (perpendicular to the bone surface) performed in 162 cases (76.1%), 45°C (oblique dermal access) in 38 cases (17.8%), and 10°C (almost parallel to the skin surface) in 13 cases (6.1%). The recorded positive cases injected at a 10°C angle were n = 11 in the superficial medial cheek fat compartment (5.2%) and 1 case each in the nasolabial sulcus and the lips (0.5%).
Needle Priming
In 175 of the positive cases (82.2%), the needle was primed with product (independent of the product utilized), whereas the needle was empty during the aspiration procedure in 38 of the positive cases (17.8%). No statistically significant difference in the distribution of the depth of injection vs the priming of the needle was detected (P = 0.890).
Time Until Blood Was Visible
In n = 210 (98.6%) cases, blood was visible in the needle hub within less than 2 seconds after the initiation of the aspiration procedure, whereas in n = 3 cases (1.4%) no aspiration was needed as the blood rushed into the hub during needle advancement. In the cases where no aspiration was needed, injections were performed in the supraperiosteal plane of the pyriform fossa, the subdermal plane of the pyriform fossa, and the subdermal plane of the nasolabial sulcus.
DISCUSSION
This retrospective clinical study analyzed n = 213 cases of soft tissue filler injections positive for blood aspiration performed in Asian patients by the same doctor during a period of 48 months. The results revealed that the most frequent location for positive aspiration was the pyriform fossa (n = 56; 26.3%), followed by the deep midfacial fat compartments (n = 54; 25.4%), anterior superior temple (n = 42; 19.7%), and chin (n = 15; 7.0%). The most frequent plane where aspiration was positive for blood was the supra-periosteal plane (n = 195; 91.5%), followed by the subdermal plane (n = 17; 8.0%) and the submucosal plane in 1 case (0.5%). Positive cases were recorded for various needle sizes: 27 G (n = 125; 58.7%), followed by 25 G (n = 44; 20.7%) and 29 G (n = 36; 16.9%). More positive cases were identified when the needle was primed compared with an unprimed needle (175 vs 38 cases), which was independent of the product present in the needle. In the great majority of the cases (n = 210; 98.6%), blood was visible in the needle hub within less than 2 seconds after the initiation of the aspiration procedure, whereas in n = 3 cases (1.4%) no aspiration was needed as the blood rushed into the hub during needle advancement. The latter 3 cases were observed in the pyriform fossa (2×) and in the nasolabial sulcus (1×). Utilizing the 213 positive cases evaluated in this study, we may estimate the incidence rate to be in the range of 0.04% to 0.9% per pass (1 pass refers to 1 injection and 1 aspiration procedure per specific location).
The major strength of the study is that this is the first in vivo case series, to our knowledge, analyzing cases positive for pre-injection blood aspiration. Previous studies analyzed influencing factors like pullback time,12 priming of the needle,11 or the pullback volume13 dependent on the type of product utilized.11-13 Despite their interesting diversity, all the referenced investigations employed an in vitro model for their analyses, which lacks real-life conditions. These in vitro models are absent of arterial blood pressure, which can influence the retrograde blood flow during the aspiration procedure. Additionally, these models lack appropriate tissue temperature, which can influence the physiochemical and rheological properties of the soft tissue filler and therefore increase the shear forces of the product inside the lumen of the needle at room temperature. Both factors can result in false lower aspiration rates compared with a clinical real-life scenario. Another strength of the present study is the large sample size, which was retrospectively evaluated during a 48-month period. This sample represents the largest case series of positive blood aspirations published to date. Another strength of this study is that all analyzed cases were injected and reevaluated by the same investigator. This assures consistency during the injection process, the aspiration procedure, and the retrospective case analyses and their interpretation. However, this could also be regarded as a limitation of the study because no verification of the data by a second expert injector occurred.
One weakness of the study is that only positive cases for blood aspiration were analyzed. No detailed information on negative aspiration cases, the number of total injection passes, or the precise amount of filler material injected during the 48-month observational period was collected. This unfortunately excludes the identification of influencing factors by which the specificity of aspiration procedures during soft tissue filler injections could be increased. This also limits the statistical analyses to descriptive analyses only. The reported incidence rates are based on the estimation of the average utilization of filler material over several months and then extrapolation to 48 months. Weekly/monthly fluctuations in filler material utilization might influence the numbers employed for extrapolation and might thus influence the incidence rates presented. The incidence rates presented could be additionally biased by the preference of the injector, for example, selection of injection plane, needle type utilized, injection angle, product type, or by regional/country-specific influencing factors, for example, products being approved in some countries only. It could thus be possible that the “real” frequency of positive cases significantly differs compared with other injectors, with a non-Asian study population, or with other centers. Future studies will need to validate the numbers presented herein. To account for the limitations inherited in this descriptive analysis (ie, selection bias), no clear recommendations can be given. The study was designed and is presented as a retrospective descriptive analysis of in vivo positive aspiration cases. However, to date this is the largest case series summarizing cases positive for blood aspiration. It is also the only systematically analyzed series obtained in a clinical scenario as opposed to an experimental setting.
The results of this retrospective case series reveal that in all 213 cases (100%), blood was visible in the needle hub within less than 2 seconds after the initiation of the aspiration procedure in contrast to other reports.11 This was independent of the type of product employed, the injected plane, the size of the needle utilized, or if the needle was primed. In 3 cases (1.4%), blood was visible even without aspiration. These results are contradictory to currently performed standards where a minimum time of 4 seconds is recommended to aspirate before injecting the product. One explanation why in 100% of the cases the aspiration time was less than 2 seconds could be the combination between the injected product and the utilized needle.14 This could be due to the preselection of the product/injector combination, which is supported by the results of previous experimental investigations (Table 5, correspondence with at least 50% of the published literature): Juvederm Voluma injected with a 27G 0.5-inch needle, Juvederm Volift injected with a 30G 0.5-inch needle, Juvederm Ultra Plus injected with a 27G 0.5-inch needle, Restylane Lyft injected with a 29G 0.5-inch needle, Restylane Vital injected with a 30G 0.5-inch needle. Respecting product/needle combinations could result in a reduced time until blood is visible in the needle hub because a variety of products are known to have inadequate aspiration times even ex vivo. A reduced time until blood is visible will increase the clinical applicability because aspiration times longer than 2 to 4 seconds would lead to increased discomfort of the patient and of the treating physician. Additionally, longer aspiration times would increase false negative results due to potential needle tip movement or reposition with subsequently decreased accuracy during injection procedures.
Review of Previous Studies Analyzing Product Type Injected, Needle Size/Length Utilized, Time Measured Until Blood Visible in Needle Hub, and Pull-Back Volume
| Study . | Present study . | . | . | Torbeck et al, 2019 . | . | . | Van Loghem et al, 2018 . | . | . | Casabona G, 2015 . | . | . | Carey et al, 2015 . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | In vivo retrospective, case series | In vitro using EDTA-coated vacutainers | In vitro using EDTA anticoagulated blood bag pressurized to 150 mmHg | In vitro ink solution (saline + water-based red ink) in small cup | In vitro slow pull test, using test tube heparinized blood with rubber stopper | ||||||||||
| Procedures performed | N = 213 clinical aspiration | N = 20 experimental aspiration | N = 340 experimental aspiration | N = 30 experimental aspiration | N = 153 experimental aspiration | ||||||||||
| Gauge/ length | Secs | mL | Gauge/ length | Secs | ML | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | |
| Restylane Lyft (Perlane) | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 29, ½” | 4; 2 | 0.2; 0.5 | 27, ½”; 29, ½” | >10; 1-10 | 0.5 | 29, ½”; 29 | Neg; 8 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 27, ½” | 4; 7 | 0.2; 0.5 | 27, ½”; 29, ½” | ~1 | 0.5 | 29, ½” | 3 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane Vital | 30, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 30, ½” | 2 | N/A | 29, ½”; 30, ½” | <5 | 0.1; 0.05 |
| Juvederm Voluma | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 2; 2 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | 27, ½” | 2 | N/A | 27, ½” | <5 | 0.3 |
| Juvederm Volift | 30, ½” | <2 | 0.05-0.5 | 30, ½” | 2; 2 | 0.2; 0.5 | 30, ½” | ~1 | 0.5 | 30, ½” | 1 | N/A | 30, ½” | <5 | 0.2 |
| Juvederm Ultra Plus | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 11; 8 | 0.2; 0.5 | 27, ½” | 1-10 | 0.5 | 27, ½”; 25 | Neg; 3 | N/A | 27, ½” | <5 | 0.2 |
| Belotero Balance | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 30; 23 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Hyadermis Smile | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Elravie Deep Line | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Princess Volume | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sculptra | 25, ½”, 5/8” 1”,1.5” 24, 1”; 23,1” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 27 | 3 | N/A | N/A | N/A | N/A |
| Sunmax Collagen Implant | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Artecoll | 25, ½”; 26, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Study . | Present study . | . | . | Torbeck et al, 2019 . | . | . | Van Loghem et al, 2018 . | . | . | Casabona G, 2015 . | . | . | Carey et al, 2015 . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | In vivo retrospective, case series | In vitro using EDTA-coated vacutainers | In vitro using EDTA anticoagulated blood bag pressurized to 150 mmHg | In vitro ink solution (saline + water-based red ink) in small cup | In vitro slow pull test, using test tube heparinized blood with rubber stopper | ||||||||||
| Procedures performed | N = 213 clinical aspiration | N = 20 experimental aspiration | N = 340 experimental aspiration | N = 30 experimental aspiration | N = 153 experimental aspiration | ||||||||||
| Gauge/ length | Secs | mL | Gauge/ length | Secs | ML | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | |
| Restylane Lyft (Perlane) | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 29, ½” | 4; 2 | 0.2; 0.5 | 27, ½”; 29, ½” | >10; 1-10 | 0.5 | 29, ½”; 29 | Neg; 8 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 27, ½” | 4; 7 | 0.2; 0.5 | 27, ½”; 29, ½” | ~1 | 0.5 | 29, ½” | 3 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane Vital | 30, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 30, ½” | 2 | N/A | 29, ½”; 30, ½” | <5 | 0.1; 0.05 |
| Juvederm Voluma | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 2; 2 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | 27, ½” | 2 | N/A | 27, ½” | <5 | 0.3 |
| Juvederm Volift | 30, ½” | <2 | 0.05-0.5 | 30, ½” | 2; 2 | 0.2; 0.5 | 30, ½” | ~1 | 0.5 | 30, ½” | 1 | N/A | 30, ½” | <5 | 0.2 |
| Juvederm Ultra Plus | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 11; 8 | 0.2; 0.5 | 27, ½” | 1-10 | 0.5 | 27, ½”; 25 | Neg; 3 | N/A | 27, ½” | <5 | 0.2 |
| Belotero Balance | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 30; 23 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Hyadermis Smile | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Elravie Deep Line | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Princess Volume | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sculptra | 25, ½”, 5/8” 1”,1.5” 24, 1”; 23,1” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 27 | 3 | N/A | N/A | N/A | N/A |
| Sunmax Collagen Implant | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Artecoll | 25, ½”; 26, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
EDTA, ethylenediaminetetraacetic acid; mL, pullback volume; secs, aspiration time until blood was visible in the needle hub.
Review of Previous Studies Analyzing Product Type Injected, Needle Size/Length Utilized, Time Measured Until Blood Visible in Needle Hub, and Pull-Back Volume
| Study . | Present study . | . | . | Torbeck et al, 2019 . | . | . | Van Loghem et al, 2018 . | . | . | Casabona G, 2015 . | . | . | Carey et al, 2015 . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | In vivo retrospective, case series | In vitro using EDTA-coated vacutainers | In vitro using EDTA anticoagulated blood bag pressurized to 150 mmHg | In vitro ink solution (saline + water-based red ink) in small cup | In vitro slow pull test, using test tube heparinized blood with rubber stopper | ||||||||||
| Procedures performed | N = 213 clinical aspiration | N = 20 experimental aspiration | N = 340 experimental aspiration | N = 30 experimental aspiration | N = 153 experimental aspiration | ||||||||||
| Gauge/ length | Secs | mL | Gauge/ length | Secs | ML | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | |
| Restylane Lyft (Perlane) | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 29, ½” | 4; 2 | 0.2; 0.5 | 27, ½”; 29, ½” | >10; 1-10 | 0.5 | 29, ½”; 29 | Neg; 8 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 27, ½” | 4; 7 | 0.2; 0.5 | 27, ½”; 29, ½” | ~1 | 0.5 | 29, ½” | 3 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane Vital | 30, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 30, ½” | 2 | N/A | 29, ½”; 30, ½” | <5 | 0.1; 0.05 |
| Juvederm Voluma | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 2; 2 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | 27, ½” | 2 | N/A | 27, ½” | <5 | 0.3 |
| Juvederm Volift | 30, ½” | <2 | 0.05-0.5 | 30, ½” | 2; 2 | 0.2; 0.5 | 30, ½” | ~1 | 0.5 | 30, ½” | 1 | N/A | 30, ½” | <5 | 0.2 |
| Juvederm Ultra Plus | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 11; 8 | 0.2; 0.5 | 27, ½” | 1-10 | 0.5 | 27, ½”; 25 | Neg; 3 | N/A | 27, ½” | <5 | 0.2 |
| Belotero Balance | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 30; 23 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Hyadermis Smile | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Elravie Deep Line | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Princess Volume | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sculptra | 25, ½”, 5/8” 1”,1.5” 24, 1”; 23,1” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 27 | 3 | N/A | N/A | N/A | N/A |
| Sunmax Collagen Implant | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Artecoll | 25, ½”; 26, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Study . | Present study . | . | . | Torbeck et al, 2019 . | . | . | Van Loghem et al, 2018 . | . | . | Casabona G, 2015 . | . | . | Carey et al, 2015 . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | In vivo retrospective, case series | In vitro using EDTA-coated vacutainers | In vitro using EDTA anticoagulated blood bag pressurized to 150 mmHg | In vitro ink solution (saline + water-based red ink) in small cup | In vitro slow pull test, using test tube heparinized blood with rubber stopper | ||||||||||
| Procedures performed | N = 213 clinical aspiration | N = 20 experimental aspiration | N = 340 experimental aspiration | N = 30 experimental aspiration | N = 153 experimental aspiration | ||||||||||
| Gauge/ length | Secs | mL | Gauge/ length | Secs | ML | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | Gauge/ length | Secs | mL | |
| Restylane Lyft (Perlane) | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 29, ½” | 4; 2 | 0.2; 0.5 | 27, ½”; 29, ½” | >10; 1-10 | 0.5 | 29, ½”; 29 | Neg; 8 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane | 27, ½”; 29, ½” | <2 | 0.05-0.5 | 27, ½” | 4; 7 | 0.2; 0.5 | 27, ½”; 29, ½” | ~1 | 0.5 | 29, ½” | 3 | N/A | 29, ½”; 30, ½” | <5 | 0.2; 0.3 |
| Restylane Vital | 30, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 30, ½” | 2 | N/A | 29, ½”; 30, ½” | <5 | 0.1; 0.05 |
| Juvederm Voluma | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 2; 2 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | 27, ½” | 2 | N/A | 27, ½” | <5 | 0.3 |
| Juvederm Volift | 30, ½” | <2 | 0.05-0.5 | 30, ½” | 2; 2 | 0.2; 0.5 | 30, ½” | ~1 | 0.5 | 30, ½” | 1 | N/A | 30, ½” | <5 | 0.2 |
| Juvederm Ultra Plus | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 11; 8 | 0.2; 0.5 | 27, ½” | 1-10 | 0.5 | 27, ½”; 25 | Neg; 3 | N/A | 27, ½” | <5 | 0.2 |
| Belotero Balance | 27, ½” | <2 | 0.05-0.5 | 27, ½” | 30; 23 | 0.2; 0.5 | 27, ½” | ~1 | 0.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Hyadermis Smile | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Elravie Deep Line | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Princess Volume | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sculptra | 25, ½”, 5/8” 1”,1.5” 24, 1”; 23,1” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | 27 | 3 | N/A | N/A | N/A | N/A |
| Sunmax Collagen Implant | 27, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Artecoll | 25, ½”; 26, ½” | <2 | 0.05-0.5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
EDTA, ethylenediaminetetraacetic acid; mL, pullback volume; secs, aspiration time until blood was visible in the needle hub.
The differences between the presented results and previous investigations (Table 5) could be caused by the different study setup because the aspiration procedures reported in this retrospective analysis relied exclusively on clinical cases, whereas previous studies obtained their results from an experimental study design. It is hoped that the results of the present investigation show that aspiration can be performed with an acceptable aspiration time, that is, less than 2 seconds, if a suitable product/needle combination is chosen. A larger needle diameter should be chosen if a more viscous product is injected. A large pullback volume, that is, >0.2 cc, could help to prevent false negative errors. However, a thorough understanding of facial anatomy should be mandatory for each practitioner. It is important to understand the layered arrangement of facial structures and to be aware of facial danger zones and especially of the variations in facial arterial vasculature.
CONCLUSIONS
The analysis of this retrospective case series of 213 positive blood aspiration procedures revealed that almost all positive aspirations were evident within 2 seconds (1.4% of the cases, blood rushed into the needle hub under its own pressure). The pyriform fossa was the most frequent location (n = 56; 26.3%), the most frequent depth was the supra-periosteal plane (n = 195; 91.5%), and the most frequent needle utilized was a 27 G needle (n = 125; 58.7%). The estimated incidence rate for a positive aspiration event ranged from 0.04% to 0.9% per pass. However, the results are biased towards a preselected product/needle combination; this is in line with previous experimental studies. Accurate relative risk assessment or causal relationships cannot be concluded from the data presented.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Dr Tseng is a physician in private practice in Taipei, Taiwan.
Mr Bommareddy is a medical student, Division of Anatomy, Department of Medical Education, Albany Medical College, Albany, NY, USA.
Dr Frank is a resident, Department for Hand, Plastic, and Aesthetic Surgery, Ludwig – Maximilians University, Munich, Germany.
Dr DeLorenzi is a plastic surgeon in private practice in Toronto, Canada.
Dr Green is a dermatologist in private practice, Coral Gables, FL, USA.
Dr Sadick is a physician, Department of Dermatology, Weill Cornell Medical College, New York, NY, USA.
Dr Fitzgerald is a physician, Division of Dermatology, University of California Los Angeles (UCLA), Los Angeles, CA, USA.
Dr Onishi is a physician in private practice in Manila, Philippines.
Dr Swift is a physician in private practice, Montreal, Canada.
Dr Cotofana is an associate professor, Department of Clinical Anatomy, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.



