-
PDF
- Split View
-
Views
-
Cite
Cite
Mildred Lopez Pineiro, Jeremy B Green, Joely Kaufman, Patricia L Blackwelder, David L Freytag, Konstantin Frank, Michael Alfertshofer, Sebastian Cotofana, Deformation of Needle Tips During Facial Soft Tissue Filler Injections: An Electron-Microscopic Study, Aesthetic Surgery Journal, Volume 41, Issue 12, December 2021, Pages NP2011–NP2019, https://doi.org/10.1093/asj/sjab211
Close - Share Icon Share
Abstract
Injectable soft tissue fillers are used on a global scale for a variety of aesthetic indications. Despite their widespread use, there is a dearth of information regarding needle deformation following injection procedures. Repeated injections with the same needle could lead to progressive needle tip deformation, potentially resulting in decreased precision and increased patient discomfort.
The objective of this study was to quantify the magnitude of needle tip deformation utilizing scanning electron microscopy (SEM) image analysis.
An observational study was performed evaluating 4 differently sized needles following soft tissue filler injections for 5 different aesthetic indications (zygomatic arch, infraorbital, midcheek, nasolabial sulcus, and perioral) in patients aged 36 to 64 years. Following treatment, each needle was visualized and imaged through SEM, and the percentage of deformation in relation to the total amount of needle tip surface was calculated.
The factor most influencing needle tip damage was revealed to be the number of injection passes, ie, dermal transitions. Per injection procedure, an increase in needle tip damage of 4.7% occurred. Touching the bone deformed the needle tip by 9.6% and an increase in needle size resulted in 0.13% more damage.
To the authors’ knowledge, this is the first SEM investigation to provide objective evidence for the deformation of needle tips after repeated facial soft tissue filler injections. These data may help improve patient safety and comfort during these minimally invasive procedures.
Soft tissue filler injections of the face are frequently performed for various aesthetic indications.1 The techniques employed seek to provide optimal outcomes while prioritizing patient safety and comfort.2 Injection safety has gained greater relevance due to the increase in severe adverse events related to the inadvertent application of filler material into the facial arterial vasculature.3,4
An intimate knowledge of facial anatomy may allow injectors to mitigate the risk of severe adverse events.5 Recent studies have revealed that in addition to this knowledge, the injection angle, the injector type (needle vs cannula) and size, the layer of product application, and the viscoelastic properties of the injected material can also influence the outcome and incidence of adverse events.6-11
Previous studies have provided evidence that cannulas are less likely to penetrate facial arterial vasculature than needles when a cannula size of 25G or larger is used,8 and that cannula injections can provide greater precision when precision is defined as the injected product remaining in the same fascial layer as the location of the tip of the cannula/needle.7 This finding is contrary to the general consensus, as published in an online survey of 58 experts (77% aesthetic physicians, 15% dermatologists, and 2% plastic surgeons) who opined that a needle can place filler more precisely in the supraperiosteal plane than a cannula.6
Although much is known regarding the tissue effects of injected fillers, there is a dearth of evidence regarding what effects the introduction into tissue has on the needle. In the authors’ experience, after repeated injections, the needle tents the skin and more force is required to achieve cutaneous penetration, increasing discomfort for the patient. Manufacturers anticipate that clinicians will need to switch needles at least once to inject the full volume of the syringe (1.0-1.5 mL), as they provide 2 needles with each US FDA-approved injectable filler (hyaluronic acid or calcium hydroxyapatite) container. The accompanying needle size is chosen to facilitate ease of use depending on the viscoelastic properties of the filler material. It could be assumed that the tip of the needle might lose its acuity and its structural integrity during multiple injection passes and that this could result in a reduced injection precision and a potentially increased patient discomfort when the skin is penetrated while advancing into deep layers. These delicate potential changes in needle deformation are difficult to assess unless electron-microscopy (EM) imaging is performed.
Thus, the objective of the present study was to investigate the magnitude of needle tip deformation following sequential injection passes during soft tissue filler injection of the face. It is hoped that the results of this study will enhance the understanding of the mechanics behind needle injections to ultimately increase precision during facial injections and to reduce patient discomfort.
METHODS
Study Sample
Injection procedures were conducted in consecutive patients treated at Skin Associates of South Florida and Skin Research Institute, Coral Gables, FL, USA for aesthetic indications during July to September 2020. The needles utilized during the injection procedures were collected and further investigated by EM imaging.
Needles Used During Injections
A total of 4 differently sized needles were used and further investigated with EM imaging: 27G × 1/2 inch (0.4 × 13 mm) (TSK Laboratory, Tochigi, Japan), 29G × 1/2 inch (0.33 × 12 mm) (K-Pack II Terumo Europe, Leuven, Belgium), 30G × 1/2 inch (0.3 × 12 mm) (K-Pack II), 33G × 1/2 inch (0.24 × 13mm) (TSK Laboratory). All needles were collected after the various injection procedures. For each needle type, a new not previously utilized needle was additionally sent for EM imaging; this needle served as a reference.
Injection Procedures
Informed consent for the aesthetic treatment was obtained by each patient as per standard practice. Soft tissue fillers were used in 5 different aesthetic indications. Areas to be treated were prepped with alcohol followed by chlorhexidine, and patients were treated according to the standard of care described below. All injection procedures were performed with primed needles. Following the injection procedures, all areas were iced for 5 to 10 minutes. No complications were reported, and the procedures were well tolerated by all patients.
Zygomatic Arch (Bone Contact)
A hyaluronic acid (HA) filler (Juvéderm Ultra Plus XC, Allergan, Irvine, CA) was injected along the zygomatic arch with the aforementioned 27G needle packaged with the syringe. The needle was introduced perpendicular to the skin surface and advanced until contact with the zygomatic bone was established. After a standardized aspiration procedure was performed, 0.1 mL of HA was injected while bony contact was maintained. After 3 such injections were performed, the needle was removed from the syringe and collected. Another needle was used performing the same injection technique and after 5 injection passes, each in contact with bone, the needle was removed and collected for EM analysis.
Tear Trough (Bone Contact)
An HA filler (Restylane-L, Galderma, Uppsala, Sweden) was used to treat infraorbital hollows with the aforementioned 29G needle packaged with the syringe. The needle was inserted perpendicular to the surface of the skin in the tear trough region until bony contact was established. After an aspiration procedure was performed, 0.05-mL aliquots of HA filler were injected supraperiosteally and in constant contact with the bone. The needle was removed and collected for EM analysis after 1, 2, 3, 4, and 5 supraperiosteal injection passes, each in contact with bone during the injection.
Middle Face (Subdermal)
Another HA filler (Restylane Silk, Galderma, Uppsala, Sweden) was injected as mesotherapy in the cheeks with the 30G needle packaged with the syringe. The needle was inserted almost parallel to the skin surface with the bevel up and aliquots of approximately 0.025 mL of HA filler were injected into the immediate subdermal plane by a serial puncture technique. The needle was removed and collected for EM analysis after 5 and 10 subdermal injections.
Nasolabial Sulcus (Intradermal)
An HA filler (Belotero Balance, Merz Aesthetics, Frankfurt, Germany) was used to treat the etched lines of the nasolabial folds with the aforementioned 30G needle packaged with the syringe. The needle was oriented perpendicular to the nasolabial fold and inserted with the bevel up almost parallel to the skin surface. Tiny aliquots of HA were injected intradermally by a serial puncture technique. The needle was removed and collected for EM analysis after 2, 3, and 4 cutaneous insertions.
Perioral (Intradermal)
Lastly, an HA filler (Belotero Balance) was used to treat the etched perioral lines of the superior cutaneous lip with the aforementioned 33G needle, which was not packaged with the syringe but was acquired separately. The needle was oriented perpendicular to the etched rhytides and inserted with the bevel up almost parallel to the skin surface. Tiny aliquots of HA were injected intradermally by a serial puncture technique. The needle was removed and collected for EM analysis after 3 and 4 cutaneous insertions.
EM Imaging
Each collected needle (following the facial injections) was mounted on a scanning EM vice mount (Electron Microscopy Sciences, Hatfield, PA) and imaged in a Philips XL-30 scanning electron microscope (Thermo Fisher Scientific, Hillsboro, OR) at magnifications ranging from less than ×100 to ×2000. The microscope was operated at 20 kV, spot size 3, and was maintained at a working distance of approximately 20 mm. Images were then digitally captured as TIFF files. A total of 166 EM needle tip images were generated.
EM Image Analysis
The generated EM images were imported into the picture analysis software ImageJ (NIH, Bethesda, MD) for further analysis. Based on the varying image angles, a circle was superimposed on the external outline and on the internal outline of each needle. The internal and external diameters of each needle were manually determined for each EM image based on the reference standard length included in each EM image; this was done to account for differences in image angle and image focal length. The diameters were used to calculate the effective needle surface adjusted for each EM image (Figure 1). The amount of deformation as a percentage of the total amount of needle surface was calculated to estimate the extent of deformation that occurred after each treatment (Figure 2). Deformation of the needle surface was determined visually as degrees of a circle with the respective needle diameter as radius and was classified if the needle surface was irregular or different in color compared to the remainder of the needle (Figure 3).
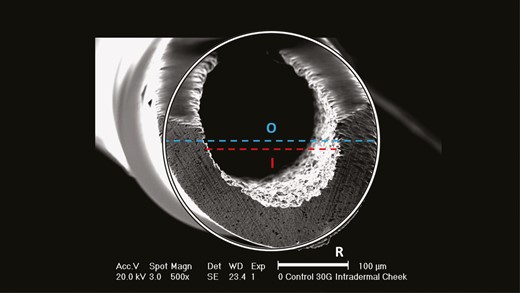
Needle surface area calculation based on the inner (I) and outer (O) diameters.
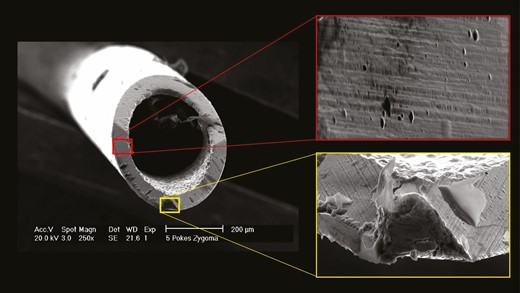
Comparison of damaged areas (yellow) and undamaged areas (red) on the surface of a 27G needle.
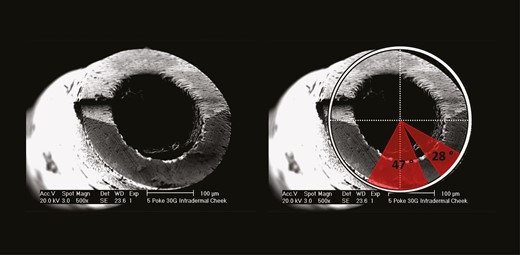
Analysis pattern of damaged areas on the needle surface. The red borders indicate damaged areas on the surface of a 30G needle after 5 subdermal injections into the cheek. The size of damaged areas is presented in degrees.
Statistical Analysis
The percentage of needle deformation (deformed area of needle surface divided by the intact area of needle surface) adjusted for each needle size was used as the variable of interest. Influencing factors were needle size (in gauge), number of injection procedures performed per needle, and plane of injection, ie, if the bone was touched (yes or no). Statistical testing was performed with SPSS Statistics 23 (IBM, Armonk, NY) and results were considered statistically significant at a probability level of ≤0.05 to guide conclusions.
RESULTS
General Observations
The mean age of the treated patients (n = 1 males, n = 4 females) was 51 years (range, 36-64 years) and the mean body mass index (BMI) was 21.02 kg/m2 (range, 18.0-24.4 kg/m2). All patients were of Fitzpatrick skin type II to IV. Immediately following the injection procedures, visual inspection was performed on all needles by the injectors. No needle tip deformation was appreciable on gross examination.
Regional Analyses
Needle Surface Area
The calculated and adjusted mean [standard deviation] needle surface was 0.0561 [0.0] mm2 for the 27G needle, 0.0548 [0.0] mm2 for the 29G needle, 0.047 [0.0] mm2 for the 30G needle, and 0.0324 [0.0] mm2 for the 33G needle. This indicated a statistically significantly decrease in effective needle surface with increasing gauge size with rp = –0.926 with P < 0.001.
Zygomatic Arch (Bone Contact)
Conducting deep injections at the zygomatic arch with a 27G needle and bone contact during the injection procedure resulted in a percentage area of damage of 38.50% when performing 3 injections and in an area of damage of 51.52% when performing 5 injections (Figure 4).
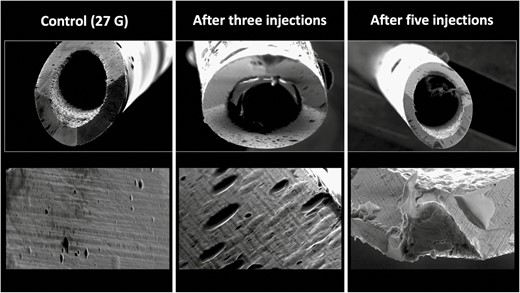
Deformational changes of a 27G needle after 3 and 5 injections into the zygomatic area with bony contact. The control image was taken prior to any injection. Note the smooth surface of the control needle on the left in comparison to the rougher texture and deformation of the needle tips after use.
Infraorbital Area (Bone Contact)
Injecting the tear trough with a 29G needle in contact with the bone resulted in a percentage area of damage of 15.23% after 1 injection, 30.19% after 2 injections, 33.24% after 3 injections, 38.50% after 4 injections, and 55.68% after 5 injections (Figure 5).
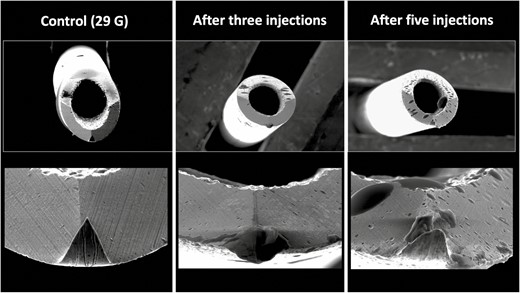
Deformational changes of a 29G needle after 3 and 5 supraperiosteal injections into the tear trough region. The control image was taken prior to any injection. Note the smooth surface of the control needle on the left in comparison to the rougher texture and deformation of the needle tips after use.
Middle Face (Subdermal)
Immediate subdermal injections of the cheek with a 30G needle mimicking mesotherapy resulted in a percentage needle tip damage of 21.88% after 5 injections and 24.65% after 10 injections (Figure 6).
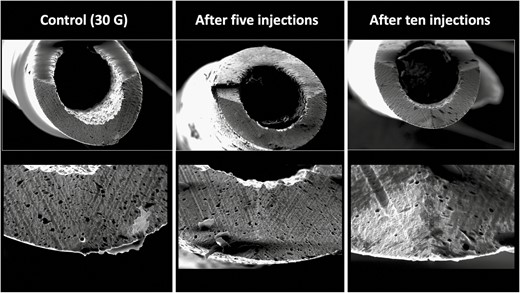
Deformational changes of a 30G needle after 5 and 10 subdermal injections into the cheek. The control image was taken prior to any injection. Note the smooth surface of the control needle on the left in comparison to the rougher texture and deformation of the needle tips after use.
Nasolabial Sulcus (Intradermal)
Intradermal injections of the nasolabial fold with a 30G needle resulted in a percentage needle tip damage of 25.21% after 1 injection (retrograde HA injection into the contralateral nasolabial fold with the single injection point), 28.53% after 2 injections, 29.09% after 3 injections, and 46.81% after 5 injections (Figure 7).
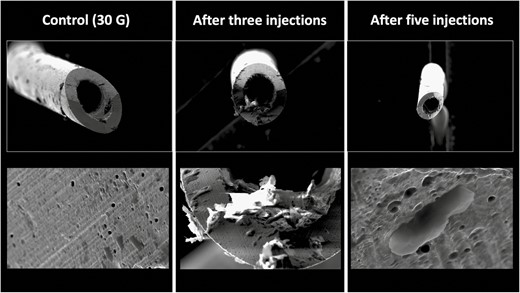
Deformational changes of a 30G needle after 3 and 5 intradermal injections into the nasolabial fold. The control image was taken prior to any injection. Note the smooth surface of the control needle on the left in comparison to the rougher texture and deformation of the needle tips after use.
Perioral (Intradermal)
Intradermal injections of perioral rhytides of the upper cutaneous lip with a 33G needle resulted in a percentage area of needle tip damage of 17.45% after 3 injections and in 36.56% after 4 injections (Figure 8).
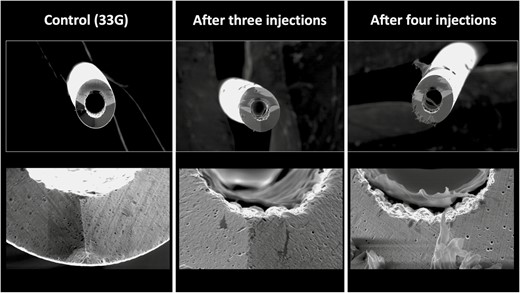
Deformational changes of a 33G needle after 3 and 4 intradermal injections into the cutaneous upper lip. The control image was taken prior to any injection. Note the smooth surface of the control needle on the left in comparison to the rougher texture and deformation of the needle tips after use.
Multivariate Analyses
Adjusting the percentage damage of the needle tip for the number of injection procedures performed, needle size, and injection depth revealed that the number of injection passes was the most important influencing factor. Per each performed injection, ie, dermal transition, the tip of needle deformed by 4.7% with P = 0.002. Touching the bone deformed the needle tip by 9.6% (P = 0.309) and the decrease in needle size allowed for 0.13% (P = 0.961) more damage. However, both parameters (injection depth and needle size) failed to reach a statistically significant level when trying to relate to the extent of damaged needle tip area.
DISCUSSION
EM imaging has been employed to assess the deformation effects on needle tips used for a variety of transcutaneous procedures, including spinal anesthesia, acupuncture, and dental nerve blocks.12-14 For the first time in aesthetics, this study aimed to investigate the deformation occurring at the tip of a needle following multiple soft tissue filler injection procedures utilizing EM imaging. The imaging-based results revealed that the needle tip undergoes deformation during multiple injection passes which can be related statistically significantly to the number of injection passes performed (P = 0.002). Although no postprocedure gross changes to the needle tip were appreciated by the injectors, EM imaging revealed structural changes of the needle tip. The magnitude of deformation (as a percentage) increased by 4.7% with every injection procedure performed and when bone contact was established the percentage area of deformation increased by 9.6% compared with subdermal or to intradermal injections. Incorporating the needle size into a multivariate model showed that the needle tip deformation increased by 0.17% with every decrease in gauge size.
The results presented herein provide objective evidence for what one would presume: with more mechanical stress to the needle tip its material integrity diminishes. The magnitude of mechanical stress is proportional to the number of times the needle tip penetrates the skin, and with deeper injections, the subcutaneous fat and the fasciae and/or muscles located in deeper planes. Based on the results of the multivariate models, the number of injection procedures performed seems to be strongest factor (the only one to reach statistical significance) influencing the percentage area of deformation. The magnitude in percentage deformation, however, is greater when the needle tip was advanced until bony contact was established. This confirms what is intuitive: that contact with the hard bone surface has the greatest mechanical impact on the needle tip. However, we did not measure the pressure during the needle insertion to standardize for bone contact pressure and it would be plausible to assume that with greater bone contact pressure the area of damage increases; this should be regarded as a limitation of this study.
However, the current study is a reflection of daily clinical practice and its inherent variability might thus be more representative of performed injection procedures (and bone contact pressure) vs standardized laboratory settings. FDA-approved HA fillers are packaged with needles sized according to the rheology of the product, ranging from 27G for more robust products intended for deeper plane injections, to 32G for products intended for superficial injections. For this investigation, the most commonly used needles accompanying the syringes were chosen: 27G, 29G, and 30G. The 33G needle studied is sold separately and is favoured by the authors for precision and patient comfort when injecting low-viscosity HA products intradermally. The greatest impact on the needle tip with bony contact is consistent with the experience of the authors: needles repeatedly used for injections on bone seem to dull more quickly than repeated in injections performed in more superficial planes. All of the injection procedures were well tolerated by the patients treated and each of the injectors performing the injections in this study have more than 10 years of injectable experience. The fact that bone contact yes/no did not reach statistical significance could be related the small sample size of 16 (37.2%) injection passes out of 43 total performed in this study. Needle size did likewise fail to reach statistical significance in our multivariate model but it seems plausible that larger needles have a greater needle surface. This greater needle surface provides greater mechanical stability which was observed as a percentage decrease of needle tip damage by 0.13% per increase in needle gauge size. Here again, the results failed to reach statistical significance which could most probably be attributed to the small number of different needle strata investigated.
The small number of investigated needles is due to the funding nature of this study; no industry-related support was obtained. All imaging procedures in the EM facility, all injectable filler materials, all needles utilized, and all personal costs were self-funded by the authors. Future studies with greater research budgets could expand upon the findings presented herein and investigate a larger number of needles following injection procedures.
Another limitation of this study is that patient comfort was not assessed. Anecdotally, patient discomfort increases with repeated soft tissue filler injections with the same needle; however, pain scores were not captured during the routine injection procedures. Future investigations could attempt to correlate needle tip deformation with repeated injections and its impact on patient comfort. An additional limitation of the study is that the injection procedures were not all performed on the same patient, thus differences in tissue thickness/anatomy were not controlled for. However, this could be considered a strength of this study because it represents an objective EM-based analysis of the needle surface effects of soft tissue filler injections in live patients from a high-volume aesthetic practice.
The results of this study reveal that with repeated usage of the same needle during soft tissue filler injections the tip of the needle is increasingly damaged; this is reflected by the percentage area of deformation per injection pass. It can be hypothesized (despite the fact that it was not objectively tested in the study) that a deformed needle tip is less sharp and could potentially result in less accurate product placement. A structurally damaged needle tip (up to 56% following 5 repeated supraperiosteal tear trough injections) could additionally cause greater tissue damage due to the nonhomogeneous needle tip and the less sharp edges. In areas of delicate structural arrangement such as the tear trough, tissue rupture during needle advancement instead of accurate tissue transection could affect the function of the orbicularis oculi muscle, which could potentially result in impaired lymphatic drainage with resulting tissue edema and product visibility.15 The latter is caused by weakening of muscle fibers which can facilitate superficial product migration, thus underscoring the importance of utilizing a sharp, homogeneously surfaced needle tip. Whether the deformation of the needle tip is influenced by the hardness of the metal used during needle manufacturing, or if other metallurgical factors might have a different outcome on needle tip deformation, is subject to further investigations.
In the nasolabial fold, less precise needle advancement could pose a greater risk for intra-arterial product placement as more force might be required to move a deformed needle tip within the facial soft tissues; this increase in force could potentially facilitate the intra-arterial product placement into the angular artery as the needle could be forced into the artery abruptly.16
Based on the findings of this study combined with clinical experience, the authors recommend changing needles after 3 injection procedures where bone is contacted. When bone is not contacted there is less needle deformation, and therefore injectors may consider changing needles after 5 subcutaneous injections. With an intradermal serial puncture technique in which small needles are used to inject tiny aliquots of product, there are multiple injections, and thus it is not practical to change needles with such frequency. In this case the authors recommend changing needles after approximately 10 injections. Future studies correlating EM needle deformation with patient comfort may provide more valuable objective evidence for how often needles should be changed while injecting a syringe of filler.
CONCLUSIONS
To the authors’ knowledge, this is the first EM investigation to provide objective evidence for the deformation of needle tips after repeated aesthetic filler injections in different tissue planes. The findings confirm that with repeated injections there is increased needle tip deformation, and this damage is greatest when injections are performed in contact with bone. It is hoped that injectors will better appreciate the impact of repeated soft tissue filler injections on the needle and consider the potential clinical implications of repeated needle use including precision of product placement, patient comfort, and most importantly, patient safety.
Acknowledgments
Drs Pineiro and Green made an equal contribution to this work as co-first authors.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article. The study (imaging, materials used) was entirely self-funded by the authors.
References



