-
PDF
- Split View
-
Views
-
Cite
Cite
Su-Ben Tsao, Lih-Ren Jong, Yue-Chiu Su, Yur-Ren Kuo, Progress in Microdermal Grafting for Color Regeneration of White Scars, Aesthetic Surgery Journal, Volume 41, Issue 11, November 2021, Pages NP1758–NP1768, https://doi.org/10.1093/asj/sjab301
Close - Share Icon Share
Abstract
Microdermal grafting with knife-cut, partially de-epithelialized skin can regenerate color in white (hypopigmented) scars. However, the scalp has more melanocytes, and dermabrasion can preserve more melanocytes than knife cutting during partial de-epithelialization.
The aim of this study was to evaluate the color regeneration results and complications of various microdermal grafting procedures for white scar color regeneration.
Two refinements to an existing microdermal grafting technique for treating white scars were described: dermabrasion, rather than knife cutting, was used to partially de-epithelialize skin, and melanocyte donor sites were harvested from the scalp, rather than from skin. A review was performed of 65 cases in which various combinations of these refinements were used to treat scars on the face and forearms.
Sixty-five patients (36 forearms; 29 faces) were treated, 40 receiving 1 session, 23 receiving 2 sessions, and 2 receiving 3 sessions of treatment. The follow-up was 6.5 months (range, 4-16 months). The use of both technique refinements produced approximately 15% better color generation than the original procedure after 1 session of treatment and approximately 20% better than the original procedure after 2 sessions. Histologic immunostaining showed that the dermabrasion method preserved more melanocytes around the epidermal-dermal region, and that the scalp has richer melanocytes than skin. The complication rate was reduced.
The use of the scalp as the donor site and partial de-epithelialization by dermabrasion can be safely incorporated into a previously developed microdermal grafting procedure for better color regeneration of white scars.

A mature hypopigmented scar, referred to herein as a white scar, on the face or other body parts can be cosmetically, psychologically, or socially disturbing.1 White scars from self-inflicted wounds on the forearm are easily recognized and carry some social stigma.2 Previously, we reported performing microdermal grafting to regenerate color in white scars on the faces or forearms of 38 patients.3 At that time, to perform this procedure, we harvested non–hair-bearing skin from the retroauricular area near the occipital hairline or from the groin area of the patients, defatted the skin with scissors, cut it into 1- to 2-mm-long strips, partially de-epithelialized the strips with a knife cut, then cut the strips into 1- to 2-mm approximately square microdermal grafts, and transplanted these grafts into puncture holes created with an 18G needle in the white scars. In that study, we reported a 49% objective color improvement in 79% of the cases receiving 1 session of treatment. In those who received 3 sessions, the objective color improvement reached 90%.
However, because hair is known to be a richer reservoir of melanocytes,4 and follicular grafting has been used to treat vitiligo,4-6 we began to speculate whether we could improve color regeneration in white scars if we used hair-bearing scalp instead of non–hair-bearing skin as the donor site for microdermal grafts. In addition, we reasoned that we could reduce melanocyte loss in our grafts if we used dermabrasion instead of knife cutting to partially de-epithelialize the donor scalp tissue. Our clinics have evolved our procedures over time. In this article, we first explain 2 technical refinements of our method for performing microdermal grafting to regenerate pigmentation in white scars. For the purposes of this study, we refer to these 2 technique refinements as “amended procedures,” in contrast to our original procedures, which refer to our original microdermal grafting to regenerate pigmentation in white scars, which we reported previously.3 Following the explanation of the amended procedures, we report the scar color improvement results of a retrospective review of 65 patients with face or forearm white scars, divided into those receiving the original procedures (knife cutting and retroauricular skin), those receiving 1 amended procedure (dermabrasion instead of knife cutting), those receiving the other amended procedure (hair-bearing scalp instead of skin), and those receiving both amended procedures (dermabrasion and scalp instead of knife cutting and skin) over an 18-month period. The benefits and complications of the original and amended procedures are discussed. For the purposes of this report, we also sent recent specimens of skin and hair-bearing scalp to the pathology department of a major medical center for histologic immunostaining examination to compare the availability of melanocytes undergoing partial de-epithelialization by knife cutting and by dermabrasion, and compare the amounts of melanocytes in skin and scalp; this enabled us to compare the preservation of melanocytes by these 2 techniques.
METHODS
We retrospectively reviewed 65 cases (53 females and 12 males) with a mean age of 34 years (range, 22-55 years) who received microdermal grafting procedures to regenerate skin color in their white scars between June 2019 and December 2020, including all patients treated for white scars and excluding all those who had been followed-up for less than 4 months and those who had been lost to follow-up. Sixty-one percent (40/65) of these patients received 1 session of treatment, 37% (23/65) received 2 sessions of treatment, and 2% (3/65) received 3 sessions of treatment. Table 1 describes the 4 treatment groups. The first group was the Original Group, which consisted of 11 patients receiving our original microdermal grafting procedures, which involved the use of non–hair-bearing skin donated from the retroauricular area and de-epithelialization by surgical knife cutting. The second group was the Dermabrasion Group, consisting of 13 patients receiving the original grafting procedures except for 1 variation, which was the use of dermabrasion instead of knife cutting for de-epithelialization. The third group was the Scalp Group, consisting of 19 patients receiving the original procedure except for 1 variation, which was the use of retroauricular hair-bearing scalp instead of retroauricular skin as the donor site. The fourth group was the Dermabrasion + Scalp Group, consisting of 22 patients receiving the grafting procedures except for 2 patients who received dermabrasion instead of knife cutting and hair-bearing scalp instead of skin. All of the cases were followed up for 4 to 16 months (mean, 6.5 months). Photographs were obtained before the initial treatment and at the last follow-up. The degree of color improvement in the scars was evaluated by 4 nonphysicians at our clinics who were asked to compare pretreatment photographs to photographs taken at the final follow-up. These 4 nonphysicians assessed the scars in terms of a simple objective pigmentation recovery scale, which ranged from 0 (no recovery) to 100 (complete recovery) and was roughly based on the “Pigmentation” subscale of the Vancouver Scar Scale—a scale frequently used for scar assessment in clinical studies which has been found to be reliable.7 In our first published white scar article 2 years ago, we reported that the results of this objective scale closely paralleled those of the patients’ self-assessment results.3 Thus, although no formal validation study has been performed specifically on our scale, we believe it to offer a valid measurement. In addition, to verify that the hair-bearing scalp was a richer source of melanocytes than skin for this report, we sent specimens (1 scalp and 1 skin) collected from patients for histologic examination. We also sent partially de-epithelialized scalp specimens, 1 by knife cutting and 1 by dermabrasion, to the pathology department to compare the preservation of melanocytes achieved by these 2 methods.
| . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|
| Case number | 11 | 13 | 19 | 22 | 65 |
| Female/male ratio | 9:2 | 11:2 | 16:3 | 17:5 | 53:12 |
| Age (years) | 30.6 (23-42) | 32.5 (24-47) | 35.9 (22-51) | 35.9 (25-55) | 34.0 (22-55) |
| Scar location | |||||
| Face | 5 | 6 | 10 | 8 | 29 (45%) |
| Forearm | 6 | 7 | 9 | 14 | 36 (55%) |
| Scar length (cm) | 10.4 (2-25) | 22.1 (4.5-70) | 8.0 (1-18) | 15.4 (3-34) | 14.3 (1-70) |
| Grafts per case | 91 (11-256) | 166 (47-577) | 69 (7-200) | 122 (23-300) | 114 (7-577) |
| Follow-up (months) | 7.1 (4-14) | 5.4 (4.5-7) | 6.2 (6-16) | 6.7 (5-8) | 6.5 (4-16) |
| . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|
| Case number | 11 | 13 | 19 | 22 | 65 |
| Female/male ratio | 9:2 | 11:2 | 16:3 | 17:5 | 53:12 |
| Age (years) | 30.6 (23-42) | 32.5 (24-47) | 35.9 (22-51) | 35.9 (25-55) | 34.0 (22-55) |
| Scar location | |||||
| Face | 5 | 6 | 10 | 8 | 29 (45%) |
| Forearm | 6 | 7 | 9 | 14 | 36 (55%) |
| Scar length (cm) | 10.4 (2-25) | 22.1 (4.5-70) | 8.0 (1-18) | 15.4 (3-34) | 14.3 (1-70) |
| Grafts per case | 91 (11-256) | 166 (47-577) | 69 (7-200) | 122 (23-300) | 114 (7-577) |
| Follow-up (months) | 7.1 (4-14) | 5.4 (4.5-7) | 6.2 (6-16) | 6.7 (5-8) | 6.5 (4-16) |
Values are mean (range) or n (%).
| . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|
| Case number | 11 | 13 | 19 | 22 | 65 |
| Female/male ratio | 9:2 | 11:2 | 16:3 | 17:5 | 53:12 |
| Age (years) | 30.6 (23-42) | 32.5 (24-47) | 35.9 (22-51) | 35.9 (25-55) | 34.0 (22-55) |
| Scar location | |||||
| Face | 5 | 6 | 10 | 8 | 29 (45%) |
| Forearm | 6 | 7 | 9 | 14 | 36 (55%) |
| Scar length (cm) | 10.4 (2-25) | 22.1 (4.5-70) | 8.0 (1-18) | 15.4 (3-34) | 14.3 (1-70) |
| Grafts per case | 91 (11-256) | 166 (47-577) | 69 (7-200) | 122 (23-300) | 114 (7-577) |
| Follow-up (months) | 7.1 (4-14) | 5.4 (4.5-7) | 6.2 (6-16) | 6.7 (5-8) | 6.5 (4-16) |
| . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|
| Case number | 11 | 13 | 19 | 22 | 65 |
| Female/male ratio | 9:2 | 11:2 | 16:3 | 17:5 | 53:12 |
| Age (years) | 30.6 (23-42) | 32.5 (24-47) | 35.9 (22-51) | 35.9 (25-55) | 34.0 (22-55) |
| Scar location | |||||
| Face | 5 | 6 | 10 | 8 | 29 (45%) |
| Forearm | 6 | 7 | 9 | 14 | 36 (55%) |
| Scar length (cm) | 10.4 (2-25) | 22.1 (4.5-70) | 8.0 (1-18) | 15.4 (3-34) | 14.3 (1-70) |
| Grafts per case | 91 (11-256) | 166 (47-577) | 69 (7-200) | 122 (23-300) | 114 (7-577) |
| Follow-up (months) | 7.1 (4-14) | 5.4 (4.5-7) | 6.2 (6-16) | 6.7 (5-8) | 6.5 (4-16) |
Values are mean (range) or n (%).
This study was not sent for IRB approval because it was a retrospective study, performed in private practice clinics with only a few full-time physicians and nurses. However, the study and procedures were performed in accordance with the principles outlined by the Declaration of Helsinki, informed written consent was obtained from each participant, and permission was granted for each photograph chosen for publication.
Surgical Technique
All of the operative procedures were performed under local anesthesia (xylocaine injection) and loupe magnification. In the Original Group, the previously developed original microdermal grafting procedures were used.3 Briefly, non–hair-bearing skin from the retroauricular area near the occipital hairline or from the groin area was harvested, defatted with scissors, cut into 1- to 2-mm-long strips with a surgical knife (No. 11), and partially de-epithelialized by cutting the upper part of the epidermis out under loupe magnification (Figure 1). Then, the strips were cut into 1- to 2-mm approximately square microdermal grafts for later implantation into puncture holes created with an 18G needle in the white scars. In the Dermabrasion Group, we first dermabraded the skin in the same area with a surgical burr until we observed slight bleeding in some spots, partially de-epithelializing the upper part of the epidermis but preserving the basal layer of the epidermis containing melanocytes (Video 1, available online at www.aestheticsurgeryjournal.com). During this procedure, the dermabraded area was continuously irrigated with normal saline for cooling to prevent thermal injury to the basal layer melanocytes. The skin was then excised along the inner margin of the dermabrasion zone. In the Scalp Group, we harvested the retroauricular scalp behind the occipital hairline instead of the skin. After shaving the hair, an elliptical piece of scalp was excised. After the wound was closed, the harvested scalp was defatted, and its hair follicles were removed with scissors. The harvested skin was then cut into 1- to 2-mm wide long strips, partially de-epithelialized with a No. 11 knife, and cut into 1- to 2-mm approximately square microdermal grafts, each with the hair shafts removed by forceps, and then these grafts were implanted into the puncture holes with microforceps. In the Dermabrasion + Scalp Group, the same area of the scalp was first dermabraded until some slight bleeding spots were noted (Video 2, available online at www.aestheticsurgeryjournal.com). The upper part of the epidermis was removed by dermabrasion to preserve more melanocytes. The scalp was excised and treated as described above except that dermabrasion, rather than knife cutting, was used for the de-epithelialization process. In all 65 patients, microforceps were used to implant the microdermal grafts into puncture holes in the white scars. The puncture holes were created by inserting an 18G needle into the deep dermal layer at an oblique angle of approximately 30° spaced at 1- to 2-mm intervals. Based on our years of experience, an oblique angle of approximately 30° is optimal because this seems to provide more contact with the surrounding dermis for better survival of the grafted pieces than perpendicular insertion. This oblique angle also prevents needle penetration into the subcutaneous layer. Microdermal grafts were implanted without the need for suturing. The treated white scar area was finally covered with antibiotic ointment and oil gauze, and then compression dressing was applied. On the second day, the oil gauze was replaced with extra-thin DuoDerm dressing, which was changed once per day for the subsequent 6 days.
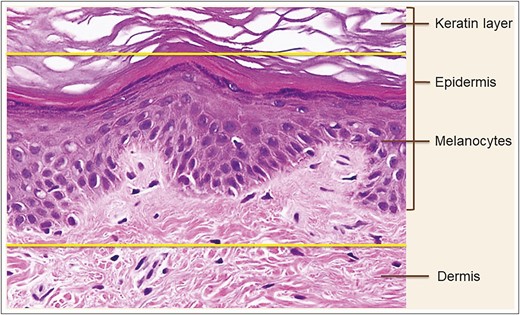
Hematoxylin-eosin staining of skin biopsy prior to microdermal grafting. Partial de-epithelialization: the upper part of epidermis above the upper yellow line is removed. The zone between the upper and lower yellow lines represents the tissue to be harvested for microdermal grafting. Published with permission from Oxford University Press.3
RESULTS
As shown in Figures 2 and 3, histologic immunostaining of recent non–hair-bearing skin and hair-bearing scalp specimens confirmed that the scalp was a richer source of melanocytes than the skin. Figures 4 and 5 show the results of histologic examination of partially de-epithelialized scalp specimens by knife cutting and dermabrasion, revealing greater preservation of melanocytes by dermabrasion. Table 1 shows the characteristics of our patients. There were 11 patients in the Original Group, 13 patients in the Dermabrasion Group, 19 in the Scalp Group, and 22 in the Dermabrasion + Scalp Group. White scars were treated on 29 faces (45%) and 36 forearms (55%).
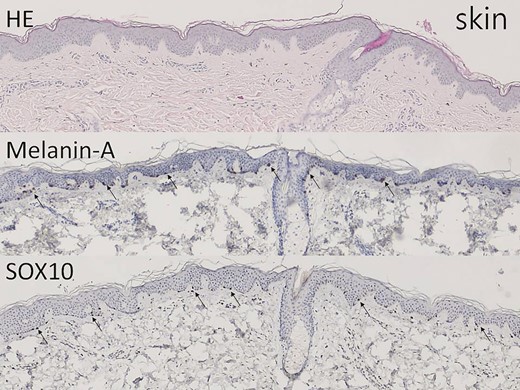
The non–hair-bearing skin tissue shows epidermis and upper dermis on hematoxylin-eosin–stained section (×40). Melanin-A and SOX10 immunostains highlight the melanocytes in the basal layer (arrow, ×40).
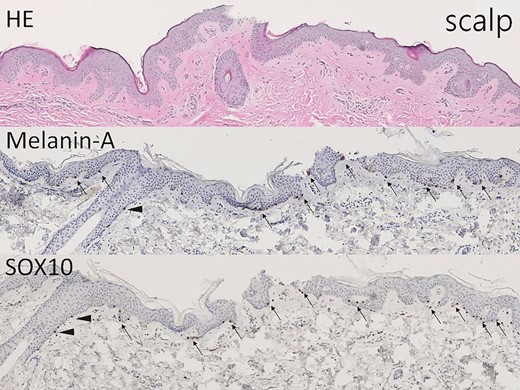
The hair-bearing scalp tissue shows epidermis and upper dermis on hematoxylin-eosin–stained section (×40). Melanin-A and SOX10 immunostains highlight the melanocytes in the basal layer (arrow, ×40) and at the outer root sheath of hair (arrowhead, ×40).
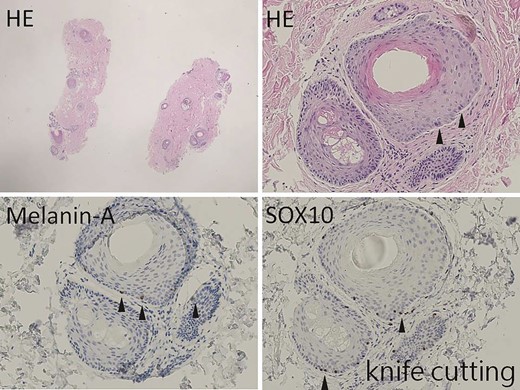
The hair-bearing scalp tissue after knife cutting shows scanty epidermis (×10). However, scattered melanocytes are found at the outer root sheath of hair on hematoxylin-eosin–stained section (arrowhead, ×100). Melanin-A and SOX10 immunostains highlight the melanocytes at the outer root sheath of hair (arrowhead, ×100).
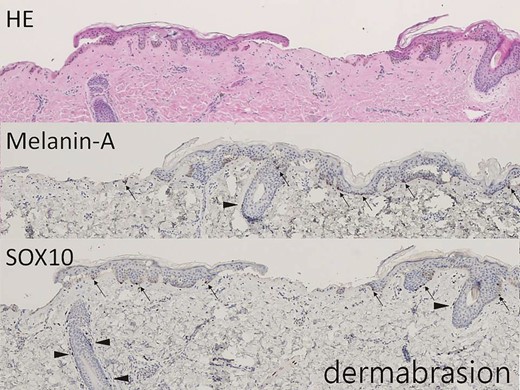
The hair-bearing scalp tissue after dermabrasion shows intermittent lower epidermis on hematoxylin-eosin–stained section (×40). Melanin-A and SOX10 immunostains highlight the melanocytes in the basal layer (arrow, ×40) and at the outer root sheath of hair (arrowhead, ×40).
The outcomes in Table 2 show white color improvement results per treatment session and complications in the 4 treatment groups. The objective statistical average color regeneration improvement rates after 1 session of treatment were 49% for the Original Group, 53% for the Dermabrasion Group, 59% for the Scalp Group, and 64% for the Dermabrasion + Scalp Group. After 2 treatment sessions, these rates were 68%, 74%, 80%, and 84%, respectively. In general, the color regeneration improvement rate for the Dermabrasion + Scalp Group was approximately 15% better than that for the Original Group, and it was approximately 20% better for those receiving 2 treatment sessions than for those receiving 1 treatment session only.
| . | . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|---|
| Objective mean scar improvement average rate after treatment | First session (40 cases) | 49% (47%-51%) | 53% (50%-56%) | 59% (58%-60%) | 64% (61%-67%) | |
| Second session (23 cases) | 68% (66%-70%) | 74% (70%-78%) | 80% (79%-81%) | 84% (82%-86%) | ||
| Cyst incidencea | 11/641 (1.72%) | 3/698 (0.43%) | 25/1666 (1.50%) | 3/1221 (0.24%) | 42/4226 (0.98%) | |
| PIH incidence | Face | 0/5 (0%) | 0/6 (0%) | 0/10 (0%) | 0/8 (0%) | 0/29 (0%) |
| Forearm | 2/6 (33%) | 2/7 (29%) | 2/9 (22%) | 3/14 (21%) | 9/36 (25%) |
| . | . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|---|
| Objective mean scar improvement average rate after treatment | First session (40 cases) | 49% (47%-51%) | 53% (50%-56%) | 59% (58%-60%) | 64% (61%-67%) | |
| Second session (23 cases) | 68% (66%-70%) | 74% (70%-78%) | 80% (79%-81%) | 84% (82%-86%) | ||
| Cyst incidencea | 11/641 (1.72%) | 3/698 (0.43%) | 25/1666 (1.50%) | 3/1221 (0.24%) | 42/4226 (0.98%) | |
| PIH incidence | Face | 0/5 (0%) | 0/6 (0%) | 0/10 (0%) | 0/8 (0%) | 0/29 (0%) |
| Forearm | 2/6 (33%) | 2/7 (29%) | 2/9 (22%) | 3/14 (21%) | 9/36 (25%) |
Values are mean (range) or n (%). PIH, postinflammatory hyperpigmentation.
aNumber of cysts divided by total number of microdermal grafts.
| . | . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|---|
| Objective mean scar improvement average rate after treatment | First session (40 cases) | 49% (47%-51%) | 53% (50%-56%) | 59% (58%-60%) | 64% (61%-67%) | |
| Second session (23 cases) | 68% (66%-70%) | 74% (70%-78%) | 80% (79%-81%) | 84% (82%-86%) | ||
| Cyst incidencea | 11/641 (1.72%) | 3/698 (0.43%) | 25/1666 (1.50%) | 3/1221 (0.24%) | 42/4226 (0.98%) | |
| PIH incidence | Face | 0/5 (0%) | 0/6 (0%) | 0/10 (0%) | 0/8 (0%) | 0/29 (0%) |
| Forearm | 2/6 (33%) | 2/7 (29%) | 2/9 (22%) | 3/14 (21%) | 9/36 (25%) |
| . | . | Original Group . | Dermabrasion Group . | Scalp Group . | Dermabrasion + Scalp Group . | Total . |
|---|---|---|---|---|---|---|
| Objective mean scar improvement average rate after treatment | First session (40 cases) | 49% (47%-51%) | 53% (50%-56%) | 59% (58%-60%) | 64% (61%-67%) | |
| Second session (23 cases) | 68% (66%-70%) | 74% (70%-78%) | 80% (79%-81%) | 84% (82%-86%) | ||
| Cyst incidencea | 11/641 (1.72%) | 3/698 (0.43%) | 25/1666 (1.50%) | 3/1221 (0.24%) | 42/4226 (0.98%) | |
| PIH incidence | Face | 0/5 (0%) | 0/6 (0%) | 0/10 (0%) | 0/8 (0%) | 0/29 (0%) |
| Forearm | 2/6 (33%) | 2/7 (29%) | 2/9 (22%) | 3/14 (21%) | 9/36 (25%) |
Values are mean (range) or n (%). PIH, postinflammatory hyperpigmentation.
aNumber of cysts divided by total number of microdermal grafts.
Figures 6 and 7 show the color regeneration of forehead and upper-lip white scars treated with microdermal grafts harvested from retroauricular skin and scalp separately, both partially de-epithelialized by knife cutting. Four nonsurgeon judges evaluated the color regeneration results from photographs obtained 3 months after the first treatment session. Microdermal grafts made from the scalp produced a better color regeneration improvement rate than grafts made from the skin (60% and 50%, respectively).

The scalp donor site of a 35-year-old female patient with a white upper-lip scar: (A) preoperative and (B) 3 months after the first treatment session. The scars were treated with microdermal grafts harvested from retroauricular scalp and partial de-epithelialization by knife cutting. The color regeneration improvement rate was assessed to be about 60%.
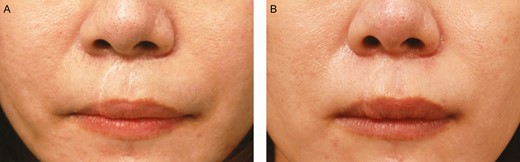
The skin donor site of a 20-year-old female patient with a white forehead scar: (A) preoperative and (B) 3 months after the first treatment session. The scars were treated with microdermal grafts harvested from retroauricular skin and partial de-epithelialization by knife cutting. The color regeneration improvement rate was assessed to be about 50%.
Figures 8 and 9 show the color regeneration of forearm white scars treated by microdermal grafts harvested from the retroauricular scalp, and partial de-epithelialization by knife cutting and dermabrasion separately. Four nonsurgeon judges evaluated the color regeneration results 6 months after the first treatment session. Microdermal grafts made by partial de-epithelialization with dermabrasion produced a better color regeneration improvement rate than partial de-epithelialization by knife cutting (65% and 60%, respectively).
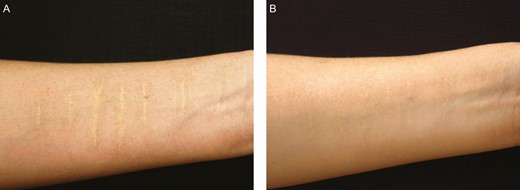
A partial de-epithelialization by knife cutting for a 41-year-old female patient with a white forearm scar: (A) preoperative and (B) 6 months after the first treatment session. The scar was treated with microdermal graft harvested from retroauricular scalp and partial de-epithelialization by knife cutting. The color regeneration improvement rate was assessed to be about 60%.
A partial de-epithelialization by dermabrasion for a 34-year-old female patient with a white forearm scar: (A) preoperative and (B) 6 months after the first treatment session. The scar was treated with microdermal graft harvested from retroauricular scalp and partial de-epithelialization by dermabrasion. The color regeneration improvement rate was assessed to be about 65%.
Figure 10 shows forehead scars treated with grafts from scalp donors partially de-epithelialized by dermabrasion. Color regeneration improvement rates 3 months after the first treatment session and 3 months after the second treatment session were assessed to be approximately 65% and 85%, respectively.
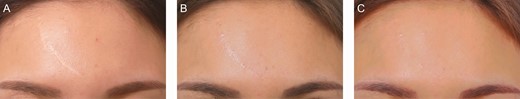
A scalp donor de-epithelized by dermabrasion for a 32-year-old female patient with a white forehead scar: (A) preoperative; (B) 3 months after the first treatment session, when the color regeneration improvement rate was assessed to be about 65%; and (C) 3 months after the second treatment session, when the color regeneration improvement rate was assessed to be about 85%.
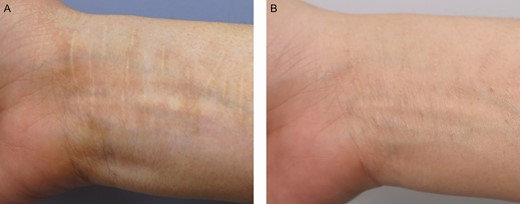
Figure 11 shows forearm scars treated with grafts from scalp donors de-epithelialized by dermabrasion. Color regeneration improvement rates 6 months after the first treatment session and 6 months after the second treatment session were assessed to be approximately 60% and 80%, respectively. The surgical scar at the scalp donor site was barely visible 6 months postoperatively (Figure 12).
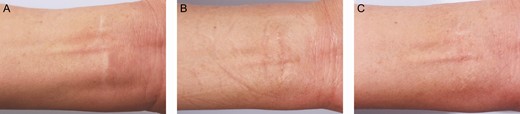
A scalp donor de-epithelized by dermabrasion for a 45-year-old female with a forearm white scar: (A) preoperative; (B) 6 months after the first treatment session, when the color regeneration improvement rate was assessed to be about 60%; and (C) 6 months after the second treatment session, when the color regeneration improvement rate was assessed to be about 80%.
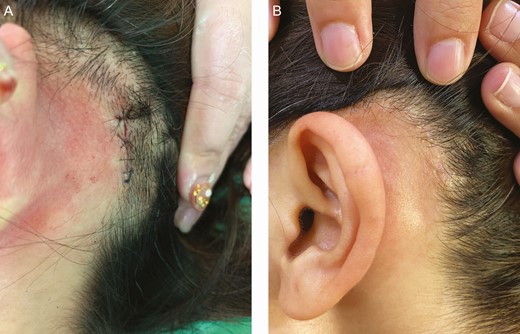
The surgical scar at the donor site of a 40-year-old female patient immediately after scalp donor site harvesting (A) and 6 months later (B). The donor site scar is barely visible.
Complications and Management
None of the cases had postsurgical hair growth on their treated white scar area. There were 2 complications: epidermal inclusion cysts and postinflammatory hyperpigmentation (PIH). For all patients, the complication rate for epidermal inclusion cysts was 0.98%. For the Dermabrasion + Scalp Group, the rate was 0.24%. Those treated with dermabrasion had a lower incidence of cyst formation than those treated with knife cutting. Epidermal inclusion cysts were unroofed and removed, and new microdermal grafts regrafted simultaneously. Color regeneration after regrafting in these patients was almost the same as in patients who had no cyst formation (Figure 13). PIH usually develops 1 month after the operation. It progresses early but regresses over the following year, usually between 6 and 12 months. For patients whose white scars were located on their faces, there was no PIH, assessed in this study as absence of hyperpigmentation. In fact, we did not attempt to quantify hyperpigmentation; we only noted its presence/absence. However, every treatment group receiving treatment for forearm white scars had some incidence of PIH (average, 25%; range, 21%-33%), and the lowest incidence was found in the Dermabrasion + Scalp Group. In all treatment groups, PIH developed over the surrounding normal skin around the punctured white scar, usually appearing approximately 1 month after the operation and disappearing spontaneously approximately 1 year later. From our perspective, PIH must be treated because patients are not happy to see the whiteness of their scars replaced with dark spots, which they consider a new problem. However, it is difficult to distinguish between the color regenerated by our procedure and hyperpigmentation due to PIH. Therefore, the sooner PIH is resolved, the sooner we can assess the success of the procedure. In this study, PIH was treated aggressively with topical whitening agents starting approximately 1 month after the operation when PIH appeared. During the early PIH period, azelaic acid ointment was applied nightly for 1 month. If PIH worsened, then the ointment was replaced with hydroquinone ointment nightly, applied for 3 to 6 months. PIH was usually resolved after applying this treatment for 4 to 6 months (Figure 14).
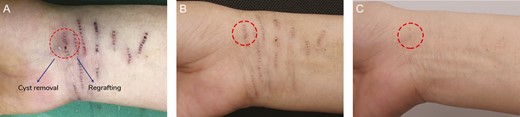
Cyst removal results for a 34-year-old female patient: (A) immediately after removal; (B) 7 days after removal; and (C) 1 year after removal. The second microdermal grafting was performed 6 months after the first treatment session. The epidermal inclusion cyst was removed and regrafted. After 1 year, color regeneration was almost the same as that in sites where cysts had not formed.
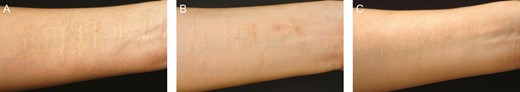
A 41-year-old female patient presenting with white forearm scars: (A) before; (B) 1 month after microdermal grafting treatment, postinflammatory hyperpigmentation appeared; and (C) 5 months after the first treatment session with azelaic acid and hydroquinone, postinflammatory hyperpigmentation was effectively resolved.
Discussion
Burm et al described their use of a nonfractional CO2 laser to create holes when treating facial hypopigmented (white) scars.8 They reported excellent improvement when applying this technique to superficial or thin scars, but minimal or partial improvement when applying it to deep or thick hypopigmented scars. They believed that it would be possible for superficial or thin scars to have healthier deep dermal layers containing dermal or follicular melanocytes, so when laser holes were created in the deep dermis, these melanocytes were activated and produced melanin pigment. When deep or thick white scars are to be treated, it is necessary to take melanocytes from the normal skin of other areas and transplant them into scar sites. This goal can be achieved by suction blister grafting, split-thickness skin grafting, minigrafting (punch grafting), follicular grafting, cultured melanocyte transplantation, and noncultured melanocyte transplantation.4 Blister induction takes approximately 2 hours, and de-epithelialization of the linear white scars by delicately placing suction blister grafts on them, is difficult to perform. Split-thickness skin grafting brings risks of stuck-on side effects and persistent hyperpigmentation, as well as residual scars at the donor site. Many plastic surgery clinics are not equipped to provide cultured or noncultured melanocyte transplantation. Transplanting melanocytes in this way manner can be expensive.
Punch grafting has been used as a relatively simple, safe, and inexpensive way to treat vitiligo. Punch holes are made on both the donor and recipient sites. Then, punch grafts are placed in the recipient holes. This method has been found to be a successful way of treating vitiligo, although one potential side effect of punch grafting is a cobblestone appearance,4 which is usually considered aesthetically unacceptable. The follicular grafting technique is based on an understanding that hair follicles are an important reservoir of melanocytes.4 Therefore, we used the retroauricular scalp as the donor site for the microdermal grafts. The scalp graft was defatted and partially de-epithelialized. The follicles were completely excised to prevent hair growth from the treated white scar area, and the hairs were removed by forceps. The scalp grafts were then cut into microdermal grafts and implanted into puncture holes created with an 18G needle into the white scars. The scalp donor site scar was well hidden and there was no hair growth at the recipient site. Lay judges rated color regeneration better in grafts made from the scalp than those made from the skin, as confirmed by histologic examination of these two sites (Figures 2, 3). The lay judges also rated color regeneration better with dermabrasion, which was also confirmed by histologic examination (Figures 4, 5). An added advantage of dermabrasion was that it greatly reduced the partial de-epithelialization operation time compared with knife cutting.
Dermal grafts have been used to correct cutaneous depressions and found to have a long-lasting effect. To decrease the incidence of epidermal inclusion cysts, Swinehart selected the skin on the crease behind the ear.9 The skin in this area is devoid of skin appendages. He dermabraded the skin down to the level of the deep dermis to remove dermal appendages. In our patients, because the melanocytes located in the basal layer of the epidermis and in the dermis appendage should not be removed, we dermabraded the skin just down to the level of the deep epidermis to preserve as many melanocytes as we could. In contrast to Swinehart, the incidence of epidermal inclusion cysts in our series was very low (0.98%), suggesting that our use of dermabrasion instead of knife cutting for partial de-epithelialization of the scalp did not increase cyst formation. If cysts happened to form, they were effectively treated by simultaneous unroofing, removal, and regrafting. The final color regeneration result in the cyst area was almost the same as that in areas where no cysts had formed.
PIH was suggested by Burm et al to be one mechanism inducing color regeneration. They used a laser hole technique to treat superficial or thin hypopigmented scars, and hypothesized that color regeneration could largely be attributed to persistent PIH coming from the activation of melanocytes in healthy deep dermis, although they were not working with deep or thick scars. In our case series, PIH was found not only in superficial and thin scars but also in the deep or thick scars of our patients, and we did not find PIH in our facial cases although their color regeneration results were quite good. Another finding was that color regeneration in forearm cases persisted even when PIH finally disappeared. These findings suggest that Burm et al’s hypothesis regarding PIH arising from healthy deep dermis could be problematic. We agree that the source should be healthy skin but consider that color regeneration possibly originated from melanocytes in the implanted microdermal grafts, not from PIH, and PIH may arise from puncture hole stimulation of the normal skin surrounding the punctured white scar and then gradually migrate into the puncture holes thereafter. From our case follow-up observations, PIH usually disappeared spontaneously 1 year after surgery and was not permanent. PIH develops more frequently in individuals with Fitzpatrick skin types IV to VI, including Asians, because of increased melanocyte reactivity within the skin.10 The incidence of PIH is higher in the forearm than in the face.11 However, PIH was effectively treated in our patients with the use of whitening topical agents, including azelaic acid and hydroquinone, applied for 4 to 6 months.
Microdermal grafts made from scalp produced better color regeneration than grafts made from skin because the hair possesses richer reservoirs of melanocytes. The microdermal grafts made by partial de-epithelialization with dermabrasion produced better color regeneration than those made by knife cutting, indicating that dermabrasion can reduce melanocyte loss during the partial de-epithelialization procedure. The color regeneration improvement results on the face in our study were overall better than those on the forearm. In addition, the time that it took to achieve color regeneration goals for the face was also shorter (3 months) than it was for the forearm (6 months), possibly because blood circulation is better in the face than in the forearm. This difference would also likely explain why the incidence of PIH complications on the face was different from that on the forearm, from 0% to 25% (Table 2).
This study was limited by the small number of cases reviewed and its short follow-up period. It was also slightly limited by the inconsistency of the lighting when photographing the target sites of the patients, a limitation that we could not overcome in retrospect.
Conclusions
This study concluded that the scalp was a better, richer melanocyte donor site for microdermal grafts than the skin, and that the use of dermabrasion for partial de-epithelialization preserved more melanocytes than knife cutting, as confirmed by histologic immunostaining examination. Amended microdermal grafting procedures produced better white scar color regeneration results and lower complication rates, including a much lower incidence of epidermal inclusion cysts (0.24%). There was no incidence of PIH on the face. Although the incidence of PIH on the forearm was rather high (25%), this condition could be effectively treated by topical application of azelaic acid and hydroquinone.
Presented at: the International Annual Congress of the Taiwan Society of Aesthetic Plastic Surgery, November 2020, Taipei, Taiwan.
Acknowledgments
The authors would like to thank Clair Lee for administrative assistance, James Steed for comments regarding English usage, and Drs Yue-Chiu Su and Yur-Ren Kuo for their help with histologic immunostaining examination and explanation.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
References
Author notes
Dr Tsao is a plastic surgeon in private practice in Kaohsiung, Taiwan.
Dr Jong is a plastic surgeon in private practice in Taipei, Taiwan.



