-
PDF
- Split View
-
Views
-
Cite
Cite
Luiz Charles-de-Sá, Natale Ferreira Gontijo-de-Amorim, Sydney Coleman, Gino Rigotti, Regen Fat Code: A Standardized Protocol for Facial Volumetry and Rejuvenation, Aesthetic Surgery Journal, Volume 41, Issue 11, November 2021, Pages NP1394–NP1404, https://doi.org/10.1093/asj/sjab016
Close - Share Icon Share
Abstract
Facial aging is a degenerative process that impairs contour and angle prominence. Rejuvenation is based on tissue replacement, volumization of the atrophic areas, and improving flaccidity and cutaneous photoaging.
The aim of this study was to apply structural fat grafting to manage volumetric deficits of the face, following a new systematic protocol called “Regen Fat Code” (RF Code) that was created to standardize structural lipotransfer methods.
This is a prospective clinical trial involving 80 healthy candidates for facial rejuvenation who were split into 2 groups. Group A underwent only structural lipotransfer; Group B underwent replacement of deep facial structures by face-lifting plus structural lipotransfer. Structural lipotransfer followed the protocol “RF Code” and 3 clinical tools were adopted for pre- and postoperative facial volumetric analysis.
Total volume (mL) of lipotransfer in Groups A and B ranged between 1 and 20 mL (mean [standard deviation], 12 [5] mL), distributed to the different areas as follows: nasolabial fold, 3.32 [0.92] mL; superior lip, 2.0 [0.62] mL; inferior lip, 2.76 [0.71] mL; malar, 8.51 [5.25] mL; inferior eyelid, 1.2 [0.54] mL; and chin, 7.18 [1.99] mL. Areas with less mobility showed a lower absorption index than dynamic areas.
The development of the RF Code protocol demonstrated the potential of grouping many parameters based on the lipotransfer method used to volumize and regenerate atrophic areas of the face. The protocol is easy to apply, and allows different volumizing and regenerative effects to be proposed, according to the demands of each surgical area.

Facial aging is a degenerative process that affects both the skin and the deep structures of the face.1,2 The aging process is the result of major intrinsic factors associated with extrinsic factors, such as exposure to solar ultraviolet radiation3,4 resulting in volume loss of the facial soft tissues, which impairs contour and angle prominence. Facial fat, such as malar fat pads, is distributed into superficial and deep compartments,5-7 which undergo redistribution, displacement, and/or atrophy, leading to more noticeable nasolabial and jowl folds. This study addresses the behavior of various fat compartments during the aging process. Facial rejuvenation is based on tissue replacement, volumization of the atrophic areas, and also on improving flaccidity and cutaneous photoaging.8,9 The volumizing approach for facial compartments, mainly through lipotransfer, is a technique that has been increasingly used in recent years, either in isolation or combined with face-lifting.10-19
One of the most important contributions to lipotransfer was developed by Coleman,20 who standardized a new protocol to collect, process, transfer, and infiltrate adipose tissue, known as structural fat grafting. Surgeons around the world have achieved remarkable results with the Coleman technique. However, the rejuvenating and regenerative effects obtained with lipotransfer are not completely understood.21-24
In order to improve our lipotransfer techniques it is necessary to consider the anatomic distribution of facial fat, the depth of the fat grafted, cannula diameter, particle size, and the effect of surrounding tissues.24,25 One parameter that may predict the ability of grafted adipose tissue to survive after a transfer is the notion of fat particles. Fat particles are defined as intact globules of adipocytes and interconnecting mesenchymal structures. Particle units are formed after the intact adipose tissue is aspirated, and cannula holes often create a range of fat particle sizes regardless of the cannula diameter.26
The autologous stromal vascular fraction (SVF) from fat can be added to the transferred fat to improve volume retention and neovascularization, a technique named cell-assisted lipotransfer by Yoshimura and co-workers.27,28 In a previous report of our clinical comparative study, we demonstrated improvements in fat retention after facial volumizing with mechanically SVF-enriched lipotransfer and better facial skin quality with the addition of adipose-derived stem cells (ADSCs).9,29-31
There is currently no consensus in the literature regarding standards of fat injection or technique protocols for facial lipotransfer, and guidelines should take into consideration the area, level, volume, tissue effect, cannula, and particle size.32-36 The aim of this study was to apply a standard protocol of structural fat grafting to manage volumetric deficits of the face, considering the demand area, plane, particle size, volume grafted, cannula, and tissue effect, creating a systematic protocol we called the “Regen Fat Code” (RF Code).
METHODS
This is a clinical prospective consecutive series study, conducted between January 2014 and June 2018, involving 80 healthy subjects, 64 females and 16 males, aged 25 to 65 years old, all of whom were candidates for facial rejuvenation. All individuals eligible for facial lipotransfer were screened by selective criteria. At the end of the selection process, all the individuals were consecutively split into 2 groups based on an individual’s need for face-lifting.
Group A underwent only primary structural fat grafting of the face. Group B underwent replacement of deep facial structures (by face-lifting) together with structural fat grafting. All patients underwent structural fat grafting to manage the volumetric deficit of the face following the RF Code. The surgical procedures were done at the authors’ private clinic (L.C.S., N.F.G.A., G.R.) and the Pedro Ernesto University Hospital—UERJ. Patients were treated according to the ethical principles of the Declaration of Helsinki 2000, and the present study was approved by the Brazilian Medical Investigation Ethical Board (protocol number 3.408.473).
All patients received extensive information on the protocols and outcomes; they were informed clearly of the benefits, risks, operative complications, and postoperative care, and they signed informed consent to participate in the study. Subjects who were smokers, had hematologic or hemodynamic disorders, autoimmune diseases, connective tissue diseases, diabetes type I or II, other metabolic diseases, were chronic users of corticosteroids, or had undergone recent facial volumization or injection of permanent fillers were excluded. The direct endpoint of the study was to assess the benefits provided by facial structural lipotransfer following the RF Code protocol.
Fat grafting placement depths were selected according to the RF Code guide to address local demand and achieve an adequate particle size to improve the facial volume and regenerative aspects, while avoiding complications.
The preoperative workup included laboratory studies, blood tests, cardiac examinations, photography and 3-dimensional (3D) image evaluations (Vectra H1 software; Canfield Scientific, Parsippany, NJ). Patient screening involved evaluating the volumetric and facial asymmetries, areas of displacement and atrophy, as well as photoaged skin.
The majority of patients were submitted to surgery under sedation and locoregional anesthesia, in a hospital environment. Patients of Group A, who received less than 40 mL of fat graft, were operated in an ambulatory environment.
RF Code
The RF Code was developed to adequately treat the need for replacement and to refill the specific fatty compartments that underwent major changes during the aging process. In both Groups A and B, the areas of volumetric loss were individual and specifically volumized within a system of codes, adopting a specific strategy. The RF Code system uses letters (anatomic unit), colors (plane application), number (cannula diameter), upper- and lowercase letters (particle size/volumizing-regenerative effect), and symbols (risk areas) (Table 1, Figure 1). In the cases associated with face-lifting (Group B), an adequate tissue repositioning of the deep structures, according to Pitanguy and Backer principles,12,37-39 was performed before structural lipotransfer, in the same surgical time.
| Letters | Facial anatomic unit |
| Number | Cannula diameter |
| Uppercase letter (macro size >1.5mm) Lowercase letter (micro size <1.5 mm) | Particle size (associated with SVF)a |
| Color | Application plane |
| Letters | Facial anatomic unit |
| Number | Cannula diameter |
| Uppercase letter (macro size >1.5mm) Lowercase letter (micro size <1.5 mm) | Particle size (associated with SVF)a |
| Color | Application plane |
SVF, stromal vascular fraction. aIn cases in which the regenerative approach is required, microfat is supplemented with SVF.
| Letters | Facial anatomic unit |
| Number | Cannula diameter |
| Uppercase letter (macro size >1.5mm) Lowercase letter (micro size <1.5 mm) | Particle size (associated with SVF)a |
| Color | Application plane |
| Letters | Facial anatomic unit |
| Number | Cannula diameter |
| Uppercase letter (macro size >1.5mm) Lowercase letter (micro size <1.5 mm) | Particle size (associated with SVF)a |
| Color | Application plane |
SVF, stromal vascular fraction. aIn cases in which the regenerative approach is required, microfat is supplemented with SVF.
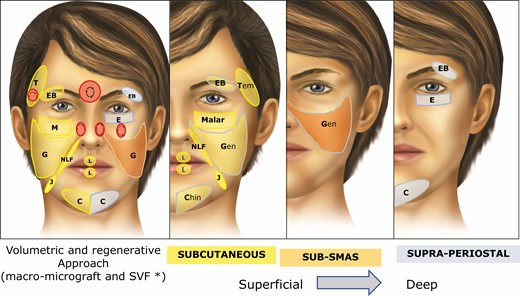
(A) The “Regen Fat Code” facial system protocol was adopted as a guide to facial lipotransfer, considering the volumetric and regenerative approaches (area, plane, cannula, particle size, and SVF enrichment). For example, “C” indicates chin; grey and yellow indicate supraperiosteal and subcutaneous planes, respectively; uppercase letters indicate macrograft (volumetric approach) and lowercase letters indicate micrograft. In cases where the regenerative approach is demanded, the microfat is supplemented with SVF. Danger areas (red circle) must be handled carefully to avoid vascular phenomena, with use of the of 2% lidocaine with epinephrine to promote local vasoconstriction, as well as a blunt cannula to reach the safety plane in retrograde injection mode. The lipotransfer is addressed from the superficial to the deepest plane in the face. (B) Subcutaneous approach. (C) Sub-superficial muscular aponeurotic system approach. (D) Supraperiosteal approach. All the anatomic areas shown in the figure may receive micrograft. In this case, the letter would be in lowercase, as t (temporal), eb (eyebrow), e (eyelid), m (malar), g (genian region), nlf (nasolabial fold), j (jowl), l (lip), c (chin). In the case that lipotransfer is associated with SFV-enriched fat, it is placed in the subdermal layer. An example: m-1-yellow* (m, malar; lowercase letters, micrograft + SFV-enriched fat; 1, 1 mm cannula; yellow, subcutaneous/subdermal). SVF, stromal vascular fraction; SMAS, superficial muscular aponeurotic system.
Clinical Evaluation
We have adopted 3 clinical tools for facial volumetric analysis preoperatively and postoperatively at 2-, 6- and 12-month follow-ups: direct observation, and 2D and 3D photography. The last of these tools is based on the use of Vectra H1 3D software for high-resolution image analysis. For 3D image acquisition, patients were instructed to sit upright while the operator held the camera to capture 3 facial images (lateral and frontal views). Once these images have been acquired, the software uses them to create 3D images for volumetric analysis. The preoperative and postoperative 3D images were submitted to the software for image analysis and measurements of absorption rates of lipotransfer areas.
Fat Harvesting
The fat was collected from the lower abdomen, anterior flanks, or inner thighs areas by the super-wet technique. A modified Klein solution containing normal saline with carbocaine 20 mg/mL, ropivacaine 7.5 mg/mL, and epinephrine 1:500,000 with NaHCO3 was injected through a 22-gauge spinal needle before harvesting. This solution was infiltrated into the area of liposuction at a ratio of 1 mL of solution per 1 mL of harvested tissue wet solution. Macro- and micrografts were harvested with 3-mm (hole size, 1.5 mm) and 2-mm (hole size, 0.8 mm) diameter, 20-cm blunt cannulas with 12 to 16 holes (custom made), respectively. Between 20 and 60 mL of adipose tissue was aspirated to manage the volumizing demand. The inserted cannula is attached to a 10 mL Luer-Lok syringe by a Luer-Lock connector. The plunger is pulled back gently by manual manipulation to minimize negative pressure.
Fat Processing
To prepare the collected fat for structural grafting, sedimentation followed by decantation is used. After sedimenting for 15 minutes, the collected fat separates into layers. In order to eliminate the lower aqueous and sometimes bloody layer, we proceed with decantation. The piston was never removed from the harvesting syringe during decantation to avoid tissue exposure to air (closed method).
After discarding the lower aqueous/bloody layer, the upper layer, consisting of a firmer yellow tissue with less blood, was transferred to a 1-mL insulin syringe through the adapter. This upper layer represents the more purified tissue in the sample. By filling the syringe with fat to a maximum 0.5 mL each time, we are able to control the injection pressure.
On the other hand, to prepare the mechanical SVF-enriched microfat grafting, the processing followed the same method published in previous studies.9,29 Half of the lipoaspirate was processed by washing and gravity separation. The other half of lipoaspirate was processed by centrifugation to enable the isolation of the SVF. Specifically, 10-mL syringes containing the lipoaspirates were capped and placed in a centrifuge (IEC Medilite Microcentrifuge, Thermoelectron Corporation, Byron Medical, Waltham, MA) set to 3000 rpm (1286 g) for 3 minutes. After centrifugation, 4 layers were observed. The lowest layer comprised a pellet that corresponded to the SVF. This pellet was composed of regenerative cells, growth factors, and cytokines, which were collected and mixed in a 10-mL syringe containing conventionally washed fat lipoaspirate to prepare the mechanical SVF-enriched micrograft sample.
Facial Structural Fat Grafting With the RF Code
Preoperative markings were made on the face, identifying areas of volumetric deficit. Supraorbital, infraorbital, and mental nerve blocks were achieved with 1 to 2 mL of 2% lidocaine with epinephrine when surgery is under sedation. In the cases of aged face, a solution of saline, 0.5% lidocaine, 1:200,000 epinephrine was injected subcutaneously fanwise around the areas to be treated with lipofilling, because in these cases, a midface lift was executed to replace the deep structures and malar fat pad12,29 before the lipotransfer. In undermined areas in the midface, where lipofilling seemed necessary, lipotransfer was done in the sub-superficial muscular aponeurotic system (SMAS) layer, at the same surgical time. The volume of injected fat in each area was recorded in milliliters and the structural fat grafting procedure, as codified by Coleman, was adopted.10,20 In cases presenting low skin quality (acne sequel, severe photoaging skin, multiple fine wrinkles), the microfat grafting was enriched with SVF, obtained by mechanical dissociation of the fat, to increase the skin regenerative effect. This SVF-enriched fat sample was placed into the subdermal layer with a blunt-tipped cannula, 5 to 10 cm long, 1 to 2 mm in diameter, with a 1-mm distal hole, connected to a 1- to 3-mL syringe, following the protocol adopted in previously published clinical trials.9,29
In order to carry out the macrofat grafting implantation, small scalp incisions were made with an 18-gauge needle at the level of the temporal hairline, at the lateral base of the alar cartilage, at the nasolabial fold (NLF), and in other areas according to demand. Finally, the fat graft was injected into the target sites. The macrofat graft was injected with a 3-mm blunt-tipped cannula, with a 1.5-mm distal hole (RF Cannulas, São Paulo, Brazil), connected to a 1- to 3-mL syringe of lipoaspirated sample; injection was performed by a multichannel technique retrogradely in each movement. To create a suitable approach for the whole facial aesthetic unit, the lipotransfer was addressed in 2 or 3 direction vectors to give a homogeneous distribution in the tissue. The amount of fat to be implanted was assessed based on the degree of volume deficit, avoiding overcorrection. Fat was deposited in the subcutaneous level to achieve volume augmentation in most cases; otherwise, fat was deposited in a sub-SMAS layer and supraperiosteal plane, providing a more volumizing effect. The cannula access points were sutured with 6.0 nylon sutures. All of these fat grafting transfers were carried out following the protocol of the RF Code (Figures 2–4). The danger areas (red circle) were handled carefully to avoid vascular phenomena, with use of 2% lidocaine with epinephrine to promote local vasoconstriction, and we used a blunt cannula to reach the safety plane when working in retrograde injection mode in the superficial plane.
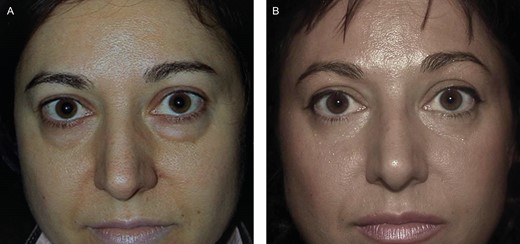
(A) Preoperative photograph of a Group A female patient, 43 years old, who underwent lipotransfer in the tear trough in the subperiosteal layer. (B) Postoperative photograph (1 year), showing great improvement in the tear trough and the disappearance of the swollen aspect of the lower eyelids.
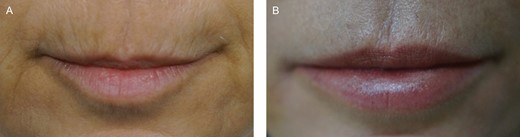
(A) Preoperative photograph of a Group B female patient, 45 years old, who underwent macrograft and SVF-enriched micrograft in the perioral area and lips. (B) Postoperative photograph (1 year); the increase in skin quality is noticeable.
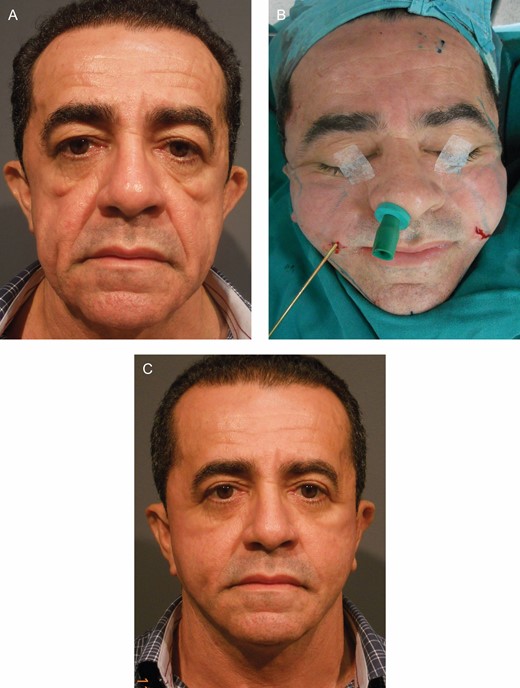
A male Group B patient, 51 years old, who undewent macro lipotransfer combined with face-lifting by the Regen Fat Codes protocol. (A) Preoperative, (B) during surgery, and (C) 1 year postoperative.
![(A) Total volume (mL) of fat grafted in transferred in Groups A and B per area. The overall volume transferred ranged between 1 and 20 mL (mean [standard deviation], 12 [5] mL). The mean volumes transferred to the different areas were: nasolabial fold (NLF), 3.32 [0.92] mL; superior lip (Sup.Lip), 2.0 [0.62] mL; inferior lip (Inf.Lip), 2.76 [0.71] mL; malar, 8.51 [5.25] mL; inferior eyelid (Inf.Eyelid), 1.2 [0.54] mL; and chin, 7.18 [1.99] mL. (B) The mean volumes of fat grafted according to area and group: NLF-A and NLF-B, 3.32 [0.92] mL and 2.94 [0.69] mL; Sup.Lip-A and Sup.Lip-B, 2.0 [0.64] mL and 2.2 [0.64] mL; Inf.Lip-A and Inf.Lip-B, 2.76 [0.71] mL and 3.05 [0.63] mL; malar-A and malar-B, 8.51 [5.25] mL and 7.25 [4.16] mL; Inf.Eyelid-A and Inf.Eyelid-B, 1.23 [0.54] mL and 1.05 [0.37] mL; and chin-A and chin-B, 7.25 [2.69] mL and 7.48 [2.53] mL).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/asj/41/11/10.1093_asj_sjab016/4/m_sjab016_fig5.jpeg?Expires=1748026833&Signature=1ywyRZ2Cd8lMZdBZwXHAgjTPw3pR9g6pqDzmnuz247pKT7zEnk-Ez9I3gdyyavbBtOy3w3rurd~ywd1DE1GeCIYNtOgzKr3Q9pGT7MoUXCEMs8hLf8Me4EjAMWmO~w6FUAPYg1h32oeFicgfXY6kfNRBkrfZQ-z-kz7YNPM~tLHMBUTttRSJC1VUpTzEuzY0kXwtNWvUy2wQhYj6eBD-YOcrOYSO3aO3c0ifbs7poadV63LLyxLvlskYR79RTQXfB2qA7AfuPN3te5gelFnkgyojqO7a8W2E5VIgh7bLEryEQKji3gE-F3MWgHlMZ2uBMlSZd1ugWE~yazjVMSJsUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(A) Total volume (mL) of fat grafted in transferred in Groups A and B per area. The overall volume transferred ranged between 1 and 20 mL (mean [standard deviation], 12 [5] mL). The mean volumes transferred to the different areas were: nasolabial fold (NLF), 3.32 [0.92] mL; superior lip (Sup.Lip), 2.0 [0.62] mL; inferior lip (Inf.Lip), 2.76 [0.71] mL; malar, 8.51 [5.25] mL; inferior eyelid (Inf.Eyelid), 1.2 [0.54] mL; and chin, 7.18 [1.99] mL. (B) The mean volumes of fat grafted according to area and group: NLF-A and NLF-B, 3.32 [0.92] mL and 2.94 [0.69] mL; Sup.Lip-A and Sup.Lip-B, 2.0 [0.64] mL and 2.2 [0.64] mL; Inf.Lip-A and Inf.Lip-B, 2.76 [0.71] mL and 3.05 [0.63] mL; malar-A and malar-B, 8.51 [5.25] mL and 7.25 [4.16] mL; Inf.Eyelid-A and Inf.Eyelid-B, 1.23 [0.54] mL and 1.05 [0.37] mL; and chin-A and chin-B, 7.25 [2.69] mL and 7.48 [2.53] mL).
Statistical Analysis
Paired t test and nonparametric tests were performed when necessary to evaluate the differences between the groups. Graft Pad Prism 5 software was used to analyze the data. The results were expressed as mean [standard deviation]. Values of P < 0.05 were considered statistically significant.
RESULTS
This prospective study comprises 80 patients who were submitted to lipotransfer following the guiding protocol of the RF Code and were split into 2 groups (A and B) according to the surgical approach.
Overall, there were 64 females and 16 males (31 women and 9 men in Group A; 33 women and 7 men in Group B). They ranged in age from 25 to 65 years old (group A mean, 33 years; group B mean, 55 years). Their BMIs ranged from 22.6 to 27.2 kg/m2 (mean, 26.2 [2.8] kg/m2). In Group A, the BMIs ranged from 22.6 to 26.9 kg/m2 (mean, 25.9 [2.9] kg/m2), and in Group B from 24.1 to 27.2 kg/m2 (mean, 26.62 [2.6] kg/m2).
Between 1 and 20 mL (mean, 12 [5] mL) fat was grafted by the structural fat graft technique. The final clinical results of all the patients were assessed by observation, examination with palpation, and pre- and postoperative 2D photographs and 3D imaging analysis.
The 3D image evaluation was carried out for all the patients at each pre- and postoperative time. For quantitative facial volumetric analysis to determine the absorption index, only Group A (facial lipotransfer isolate) was enrolled, due to the association with the deep structural replacement by face-lifting in Group B.
Local transitory effects were occasionally observed immediately in all patients after the fat transfer, such as edema (100%), erythema (60%), and bruising or induration in small areas (35%). They did not require any pharmaceutical intervention and returned to the normal skin condition within 7 to 10 days. We did not observe any subsequent skin reactions, such as persisting inflammation, necrosis, lumps, and irregularities, or any other adverse vascular events, such as embolism and vasculitis. The clinical analysis found, through subjective observation, an improvement in the quality of the skin, mainly in areas submitted to SFV-enriched micrograft technique (Supplemental Figure 1A-D).
The RF Code was adopted to standardize the structural lipotransfer method (Figures 2–6).
![(A) Total absorption index (%) per area in group A (after 2 months): NLF, 24.46% [4.06%]; Sup.Lip, 33.28% [5.23%]); Inf.Lip, 30.62% [5.85%]); malar, 29.21% [5.06%]; and chin, 24.51% [5.09%]. (B) Mean volume loss (%) per area and time (2, 6, and 12 months): NLF, 24.59% [4.9%], 30.21% [4.39%], and 32.03% [5.27%]; Sup.Lip, 33.35% [5.06%], 36.72% [4.95%], and 40.10% [5.26%]; Inf.Lip, 31.05% [5.06%], 34.79% [4.95%], and 37.54% [5.13%]; malar, 29.33% [4.88%], 33.44% [4.96], and 37.21% [4.81%]; and chin, 24.69% [4.86%], 29.72% [4.79%], and 32.79% [4.83%]. See Figure 5 for abbreviations.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/asj/41/11/10.1093_asj_sjab016/4/m_sjab016_fig6.jpeg?Expires=1748026833&Signature=bru~JsRcIZpbGE4AVkdJT9grd98XKmt5UiRE3emSvAYOvp8cNsKb6KBBHlJnN2UsY4hkXb9Ms93yQwqTd2DohXtYnNV-~3bv0iODX65HXreAYYG-0ohSAuF~Mls0p6qdr8~tMZy8RVprN3iTKqK-FTJJfUNCCqEVOZCHUdD9U0m80V9QRWPmM~3ZPq9C3LaQ1xXEbACTZGKU-JikNYDQ7R-53kLAtEvXQ4sPjT5gNkYr1VvUdvAdtr14znBrzRlLazBZvWzkJkpftaMObE1lu8LhNRhjA9jtVIuDLyZPg5YgSeWosEst~Zi4PoAzVPiIS6bEMPFIEz7ojVetGxUmXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(A) Total absorption index (%) per area in group A (after 2 months): NLF, 24.46% [4.06%]; Sup.Lip, 33.28% [5.23%]); Inf.Lip, 30.62% [5.85%]); malar, 29.21% [5.06%]; and chin, 24.51% [5.09%]. (B) Mean volume loss (%) per area and time (2, 6, and 12 months): NLF, 24.59% [4.9%], 30.21% [4.39%], and 32.03% [5.27%]; Sup.Lip, 33.35% [5.06%], 36.72% [4.95%], and 40.10% [5.26%]; Inf.Lip, 31.05% [5.06%], 34.79% [4.95%], and 37.54% [5.13%]; malar, 29.33% [4.88%], 33.44% [4.96], and 37.21% [4.81%]; and chin, 24.69% [4.86%], 29.72% [4.79%], and 32.79% [4.83%]. See Figure 5 for abbreviations.
Both groups and the areas grafted were analyzed:
Group A: 40 patients were submitted to structural facial lipotransfer only. The majority of this group were female (77.5%). The mean volumes of fat graft varied according to the area being treated (Figure 6).
Group B: 40 patients were submitted to structural facial lipotransfer combined with face-lifting. The majority were female (82.5%). The amount of fat transferred per area of tissue replacement directly affected by facelifting (malar, NLF, inferior eyelid) was lower than the amount of fat transferred per area not directly affect by facelifting, such as chin. On the other hand, the areas that did not have a direct effect on tissue replacement were treated with more volume by lipotransfer (lips and chin) (Figure 3).
The following subgroups were created based on the number of associated treated areas:
Subgroup 1 (15%): 1 treated area—NLF (47%); lip (32%); malar (18%); and others (3 %).
Subgroup 2 (55%): 2 associated treated areas—NLF + lip (55%); NLF + malar (32%); and others (23%).
Subgroup 3 (30%): 3 associated treated areas—NLF + malar + lip (70%); NLF + malar + palpebral inferior (20%); and others (10%). In the inferior eyelid, the fat graft transferred to the tear trough area ranged from 0.5 to 1 mL.
The retention index was measured in Group A through a comparative 3D image analysis between the preoperative period, 6 months and 1 year after surgery. The lower-mobility areas submitted to lipotransfer, such as the NLF and chin, showed a lower absorption index (NLF, 24.46%; chin, 24.51%) than dynamic areas such as malar (29.21%) and lips (30%-33%). The degree of absorption was minimal at 2 months postoperatively by clinical observation (Figure 6).
The final clinical results of both Groups A and B were assessed by observation, examination with palpation, as well as pre- and postoperative 2D photographs at least during the postoperative period of 1 year (Figures 2-6). Follow-up of all patients was performed 1 year postoperatively to collect the data and final results.
DISCUSSION
The use of adipose tissue has been shown to be attractive because it is readily available, inexpensive, host compatible, and can be harvested repeatedly as needed without any concern over allergic or foreign-body reactions.30-33 Adipose tissue is a promising source of adipocytes and adult stem cells, and offers a range of useful characteristics and metabolic properties, such as volumizing and angiogenic potential, antioxidative effects, immunotolerance, and paracrine and inflammatory modulations. It has been demonstrated to affect grafted fat and its surrounding tissues.9
Facial aging can be attributed to volume deflation and tissue laxity and displacement.33 Age-related loss of facial fat rarely exists as an isolated event. Successful surgical rejuvenation aims to address tissue laxity in conjunction with strategic volume restoration.34 Patients presenting significant facial sagging and skin redundancy who undergo isolated fat injections can experience an overfilled face, which gives an unnatural and unpleasant appearance (“pillow face”). Although aggressive filling of the sagging face can produce improved contour and face projection, it seems to be more logical and appropriate to perform fat injections in association with face-lifting to achieve successful results.
There is currently no standard protocol to guide facial lipotransfer that considers the area, layer, volume, cannula, tissular effect, and particle size.36-39 In practice, fat transplantation often has unpredictable long-term results because of its absorption and volume loss, and several different lipofilling techniques have been developed in recent years.40-48 In this study, however, we present a guiding protocol, the RF Code, to support suitable fat transfer in primary facial lipotransfer, both alone and combined with face-lifting. Our method uses decanted lipoaspirate for volumization and SVF-enriched fat in those areas where the skin improvement was sought.
The RF Code has been adopted as a practical visual method of autologous fat transfer that provides easy, safe, and reproducible guidance for simplifying the surgery of facial lipotransfer. This method increased the efficacy of autologous lipo injections, resulting in higher volumizing rates and volume maintenance of transplanted fat and decreased adverse effects of lipo injection.19-21
According to the RF Code, structural fat grafting for the management of volumetric deficit of the face demonstrated that multiple planes can be used in the same area to improve the final volumizing effect, disregarding the absorption index, because there will be a summation of the final retention volume in each layer of the same treated area, without producing irregularity caused by a larger lobular size in the superficial plane of the fat graft.46 Considering the particle size, Kato et al47 emphasized that smaller fat particles may better maintain volume by diffusion. On the other hand, Del Vecchio and Rohrich48 claimed that smaller particles may lack stromal components for structural support of adipocytes and proliferating stem cells.
The RF Codes protocol has adopted the macrograft for deeper planes and micrografts in superficial plane applications (subcutaneous/subdermal). The mechanically SVF-enriched fat was associated with the regenerative proposes when necessary, creating a more skin regenerative effect, and avoiding lumps and irregularities. It is interesting to note that the effects of this regenerative protocol are visible in the epidermis also if the injection takes place in the subcutaneous region; it is evident that this is attributable to a sequence of events and represents an important role in regenerative medicine.9,30,31 In part, these roles are attributed to the action of several growth factors released by ADSCs, and one hypothesis is that the cutaneous modification is linked to an angiogenic action operating at the junction between the dermis and the subcutaneous tissue. In the aging skin, a functional disconnection between the microcirculation of the dermis and that of the subcutaneous tissue exists, and therefore the new formation of microvessels could play a role in the modification observed after treatment with SVF-enriched fat or ADSCs.9,30,31
Most of the facial units were analyzed pre- and postoperatively in both groups. We used Vectra H1 3D software to analyze and measure all the transferred areas in Group A pre- and postoperatively to compare the absorption index per area. Only the primary lipotransfer-treated area in patients from Group A was analyzed by the 3D software; patients of Group B underwent both midface lifting and lipotransfer, and the image-analysis system has difficulty discriminating between elevation and filling of the deep structures. MRI could be an excellent method because of its high sensitivity for soft tissue, but the need for frequent follow-up for volume analysis means that repeated MRI exams are not cost effective.49 Other imaging systems are unsuitable because they do not permit reliable measurements in the range of 1 mm. Furthermore, these alternative images would be operator dependent and costly.33
When analyzing the areas grafted per group, it was observed that Group B was less grafted, mainly in the areas mobilized during face-lifting, such as the midface (Figure 5). Most patients in Group A needed more volumization, despite being younger and having less volumetric loss. In Group B, represented by older patients with major volumetric demands, the tissue replacement during face-lifting decreases the need for volumization with lipotransfer in the surgically lifted areas (malar, NLF, inferior eyelid). On the other hand, the areas that did not have a direct effect on tissue replacement were treated with more volume by lipotransfer (lips and chin) (Figure 3). The strategy adopted in Group B was structural lipotransfer after the replacement of the deep tissue structures, within the same surgical time, due to a new volumetric configuration of the face that is established by face-lifting. This new scenario allows for a more accurate evaluation of a more appropriate volumization approach.19,29,32-35 Other surgeons prefer lipotransfer before replacement of the deep structures, claiming more precise results, lower edema, and higher efficiency.19,43,44 Perhaps a lack of graft absorption could lead to some overprojection in certain areas submitted to lipotransfer before the replacement of deep structures. As example, the NLF is attenuated by the repositioning of the malar fat pad during face-lifting, and requires less volumization.38,50 Our results showed less volume demand in some areas, such as malar, NLF and inferior eyelid in Group B, due to the volume replacement by face-lifting. However, the areas that do not suffer direct and/or indirect action with face-lifting (lips, chin, temporal) do not undergo volumetric variation, and are thus suitable for prior filling (Figure 3).19 Therefore, we recognize that the best moment for fat grafting during face-lifting is after the repositioning of the deep structures, at the end of the surgery.45-48 It was also observed that, in general, the most dynamic areas (lips and malar) presented a higher rate of absorption. The grafted volume stabilized at 2 months,51-59 although it continues to undergo minor modifications up to 12 months (Figure 6). It should also be taken into account that fat retention is less favorable in older patients.56-59
The limitations and weaknesses of the study were the lack of a comparative image analysis such as MRI scans, no quantitative clinical skin improvement analysis, a longer postoperative time to follow-up, and no comparative analysis of fat compartments of the face.
CONCLUSIONS
The development of the present guide protocol demonstrated the potential of grouping many parameters, based on the fat transfer method to volumize and regenerate the atrophic areas. This new protocol enrolled parameters that facilitate selection of the best option for placement of fat tissue in each facial segment, with all patients showing improvement after a single lipotransfer surgery.
Acknowledgments
The authors would like to thank Colton J. Tucker (BSc at the University of Central Florida, Orlando, FL) for his contributions in reviewing the manuscript and Canfield Scientific (Parsippany, NJ) for the 3D image support.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES



