-
PDF
- Split View
-
Views
-
Cite
Cite
Francis D Graziano, Peter W Henderson, Jordan Jacobs, C Andrew Salzberg, Hani Sbitany, How to Optimize Prepectoral Breast Reconstruction, Aesthetic Surgery Journal, Volume 40, Issue Supplement_2, December 2020, Pages S22–S28, https://doi.org/10.1093/asj/sjaa214
Close - Share Icon Share
Abstract
Prepectoral breast reconstruction has become a popular method of postmastectomy breast reconstruction due to its numerous benefits in properly selected patients. Prepectoral reconstruction, as compared with retropectoral position, offers the advantage of leaving the pectoralis muscle undisturbed and in its original anatomic position, resulting in significantly decreased acute and chronic pain, improved upper extremity strength and range of motion, and avoidance of animation deformity. The use of acellular dermal matrices (ADMs) allows for precise control of the breast pocket, resulting in aesthetic outcomes and high patient satisfaction. ADMs have the added benefit of reducing capsular contracture, especially in the setting of postmastectomy radiation therapy. Although prepectoral breast reconstruction is effective, the breast implant is placed closer to the skin flap with less vascularized soft tissue coverage. Therefore, optimizing outcomes in prepectoral breast reconstruction requires careful patient selection, intraoperative mastectomy flap evaluation, and perioperative surgical algorithms specific to prepectoral reconstruction.
Prepectoral breast reconstruction has emerged as a popular technique for postmastectomy breast reconstruction due to the numerous benefits it offers patients. This technique involves the placement of a breast implant prosthesis completely above the pectoralis major and serratus muscles, thereby leaving the chest wall muscles in their original anatomic locations.1 Reported benefits of this technique include decreased acute and chronic postoperative pain, complete avoidance of animation deformities, high patient satisfaction rates, and precise aesthetic outcomes.2-4 Furthermore, prepectoral reconstruction avoids enveloping the implant in pectoralis muscle, which may become fibrotic and tight following irradiation, resulting in impaired upper extremity strength and range of motion, as well as implant malposition, which can be seen in some cases of submuscular reconstruction.
Although prepectoral breast reconstruction is effective, the breast implant is placed closer to the skin flap with less vascularized soft tissue coverage, and therefore it is critical for the surgeon to assess preoperative and perioperative factors to ensure safe outcomes. With properly selected patients and optimal surgical technique, prepectoral breast reconstruction can be performed with minimal morbidity and high patient satisfaction. In this article, the authors discuss preoperative, intraoperative, and postoperative considerations to optimize outcomes in prepectoral breast reconstruction.
PREOPERATIVE CONSIDERATIONS
Patient Selection
As with any operation, patient selection is key to successful outcomes. Relative contraindications to prepectoral breast reconstruction include obesity (body mass index over 35 kg/m2), active smokers, and patients with poorly controlled diabetes mellitus. These patient comorbidities may result in compromised microvascular perfusion, therefore increasing the risk of mastectomy skin flap necrosis and implant exposure or extrusion. Other comorbidities to consider are long-term steroid use, immunosuppression, peripheral vascular disease, and connective tissue disorders (Ehler-Danlos, etc).
Another important factor to consider is the radiation status of the patient at the time of the mastectomy. Prepectoral reconstruction should be avoided in patients treated with radiation prior to mastectomy. Patients with prior radiation therapy are predisposed to increased wound-healing complications, including wound dehiscence and implant exposure, and therefore are less optimal candidates for any implant-based reconstruction, including prepectoral placement.5 Therefore, it is our opinion that these patients benefit more from autologous reconstruction. In contrast, patients receiving postmastectomy radiation therapy (PMRT) can be considered for prepectoral reconstruction. Although the risk of complications after prepectoral reconstruction with PMRT is increased, it is comparable to that in complete submuscular breast reconstruction patients receiving PMRT.6 Thus, prepectoral reconstruction should be considered in this subset of patients.
Although prepectoral breast reconstruction is predominantly used as a means of immediate breast reconstruction, patients undergoing delayed breast reconstruction may also be optimal candidates for prepectoral reconstruction. In patients with delayed reconstruction, the mastectomy flaps have undergone vascular “delay,” thereby theoretically increasing the likelihood of reconstructive success. Another subset of patients who benefit from delayed prepectoral reconstruction are patients who have completed submuscular implant reconstruction, but now report significant muscle pain and tightness or animation deformity.7 These patients can be converted from the subpectoral to prepectoral plane with placement of ADM. These patients typically can have full resolution of the pain and immediate resolution of the animation deformity.
Tumor-Specific Considerations
In addition to patient selection, oncologic considerations must be analyzed preoperatively. Preoperative tumor location should be assessed before mastectomy and reconstruction. Tumors that invade the chest wall or are within 0.5 cm of the pectoralis muscle should be considered contraindications to prepectoral reconstruction.8 Given the location of the tumor, these patients have a higher risk of chest wall tumor recurrence.9 These patients could potentially benefit from submuscular implant reconstruction, thereby placing the pectoralis muscle anterior and closer to the skin. This would aid in self-palpation and monitoring for recurrence. The most common presentation of breast cancer is the presence of a palpable mass, whether noted by self-exam or during a clinical breast examination.10 However, if a recurrence were to occur on the deep chest wall margin with prepectoral breast reconstruction, it would be difficult to detect due to the overlying prosthesis and may delay detection. Other oncologic considerations and relative contraindications for prepectoral breast reconstruction include patients with Stage IV breast cancer, aggressive axillary adenopathy, and inflammatory breast cancer.11 In these patients, the nature of the pathology often requires aggressive adjuvant therapy that may make them suboptimal candidates for prepectoral reconstruction or potentially any form of breast reconstruction.
Furthermore, although not a contraindication to prepectoral breast reconstruction, superficial tumors that require additional or atypical skin resection to obtain negative margins should be considered preoperatively. These skin excisions may extend outside of standard incisions which can lead to distortion of the mastectomy skin flap envelope and implant position. These considerations should be discussed with the patient preoperatively to manage expectations.
INTRAOPERATIVE CONSIDERATIONS
Intraoperative Mastectomy Flap Assessment
Mastectomy skin flap evaluation is an important component in the decision-making process for prosthetic breast reconstruction. This is especially true in the context of prepectoral breast reconstruction, given that the mastectomy flap is the only vascularized soft tissue covering the prosthesis. Mastectomy flap evaluation starts with intraoperative clinical assessment of perfusion and viability. Key aspects of evaluation include flap warmth, color, capillary refill, and visible bleeding at incision edges. Additionally, the underside of the mastectomy flap should be inspected for preservation of subcutaneous fat. Preservation of subcutaneous tissue on the entire underside of the mastectomy flap indicates that the subdermal dermal plexus has been preserved, and the skin is more likely to be perfused (Figure 2).
Although tissue thickness is often associated with improved perfusion, this is not always the case. Patients with thinner body habitus have less subcutaneous fat, and thus will have thinner flaps.12 In addition to clinical assessment of mastectomy flaps, intraoperative use of perfusion devices, such as indocyanine green (ICG) laser angiography can be a useful adjunct to assess tissue perfusion.13 ICG laser angiography can provide real-time visual feedback to indicate areas with compromised perfusion and allow for immediate excision, thus preventing areas of potential future necrosis.14 ICG laser angiography is used roughly 75% of the time in the senior author’s practice. Moreover, ICG laser angiography can help guide the amount of intraoperative tissue expander filling that can be safely performed by indicating when there is excessive tension and decreased perfusion to the mastectomy flap. In the authors’ experience, areas with clinical concern for compromised perfusion are highly correlated with compromised areas identified by ICG laser angiography.
Exposure of dermis on the underside of the skin flap indicates likely damage to the subdermal vascular plexus and loss of skin perfusion in large areas; in these cases, consideration should be given to delaying the reconstruction. In such cases, the authors’ preference is to close the incision primarily and delay the prepectoral reconstruction for at least 3 weeks to allow for the mastectomy flap to revascularize. Adjunct fat grafting prior to delayed tissue expander placement has also been shown to be a viable option to augment subcutaneous tissue and the overall mastectomy flap.15 However, another option in this scenario is to proceed with immediate complete submuscular coverage of the prosthesis if deemed safe.
In patients with thin, but uniform, preservation of subcutaneous fat, the skin flap should be evaluated both clinically and with perfusion devices such as ICG laser angiography. Patients with thin but viable flaps are typically better candidates for 2-stage reconstruction. Smooth-walled tissue expanders should be filled with minimal or no volume to prevent tension and impairment of venous return, at the time of mastectomy. In the authors’ experience, lower tissue expander fill rates in thin mastectomy flaps have resulted in reduced rates of mastectomy skin necrosis. The vast majority of the senior author’s implant-based reconstructions are performed with 2-stage tissue expander reconstruction, with minimal direct-to-implant cases. After at least 3 weeks, when the incision has healed, serial expansions can typically begin.
Optimal Acellular Dermal Matrix Use
Historically, attempts at subcutaneous breast reconstruction after mastectomy without the use of ADMs have led to high rates of capsular contracture and implant exposure, requiring conversion to complete submuscular reconstruction.16 However, the advent of ADMs has made prepectoral breast reconstruction a safe option with the potential for reliable aesthetic outcomes (Figure 1). Prior studies have shown that use of ADMs with prepectoral breast reconstruction has similar safety outcomes to complete submuscular breast reconstruction.17 Moreover, the use of ADMs with implant reconstruction has been shown to decrease capsular contracture rates and to be protective in reducing complications with PMRT.18 Lastly, the use of ADMs allows the pectoralis muscle to be left in its anatomic position and eliminates the risk of animation deformities seen with submuscular reconstruction.
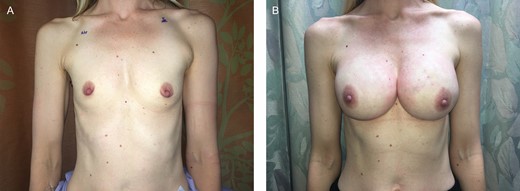
(A) A 42-year-old female presented with right breast cancer and a desire for bilateral nipple-sparing mastectomies. She desired a very large size postoperatively, and thus underwent bilateral prepectoral tissue expander placement. At the second stage, the expanders were exchanged for round cohesive silicone gel implants. (B) At 2 years postoperatively, she maintains a stable, soft breast reconstruction on each side.
Precise placement of the ADM allows for control of the boundaries of the reconstructed breast, and therefore is key to identify the anatomic borders along the chest wall. The breast surgeon’s primary goal is to improve oncologic safety, and this sometimes includes tissue removal beyond the native breast borders. Manual palpation and visual inspection are important in confirming the original inframammary fold (IMF) location along the lateral, medial, and caudal borders of the breast, and carefully marking these following completion of mastectomy. Then, the ADM can then be used to re-establish and/or reinforce these borders in the reconstructed breast. The authors recommend a double-suture technique, with a cuff of ADM, at the IMF to bolster the strength and implant support at the lower pole. In this technique, the inferior 2 cm of the ADM is placed posterior to the lower edge of the prosthesis and sutured in place to the chest wall. The most inferior portion of the ADM sling (at the IMF portion that transitions from the posterior to the anterior surface of the implant) is then sutured to the chest wall as a 2-layer cuff (Figure 3). This technique allows for long-lasting support at the IMF and reduces the incidence of implant descent over the long term.18 Additionally, for patients with thin inferior mastectomy flaps, the authors elevate a serratus anterior fascia advancement flap in the areas of the fourth to seventh ribs to provide vascularized soft tissue coverage and support to the inferior pole in addition to the ADM.
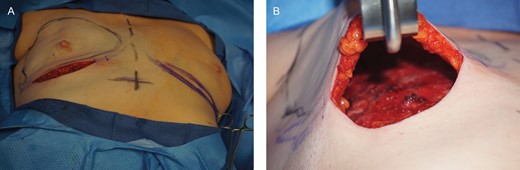
(A) A 48-year-old female presented with left breast cancer, and underwent a 2-stage expander-based prepectoral breast reconstruction on the left breast, and a right breast augmentation for symmetry. (B) After mastectomy was completed, mastectomy flaps were assessed and deemed to be adequate.
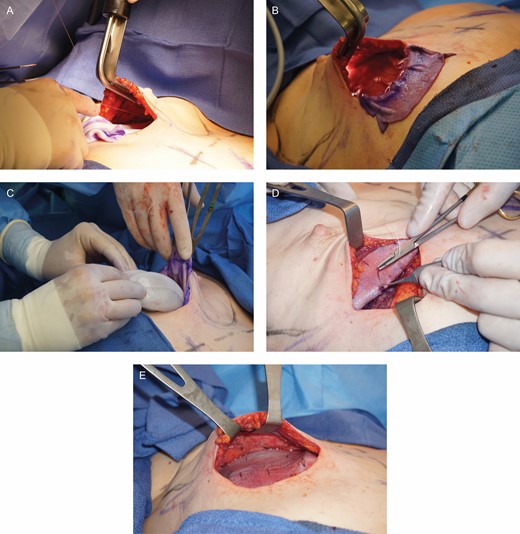
(A-E) ADM was used to hold the tissue expander in the prepectoral pocket.
Larger amounts of ADM are used with prepectoral breast reconstruction than with submuscular ADM reconstruction. Studies have confirmed the higher incidence of seromas with the use of ADMs.19,20 Therefore, consideration should be given to proper drain management with prepectoral breast reconstruction to reduce the risk of seroma formation. The authors recommend installing 2 drains in larger breasts to prevent the formation of seroma in the potential space between the mastectomy flap and the ADM during the period of ADM integration. The use of premeshed or intraoperative meshing of the ADM may also improve the egression of fluid and prevent the accumulation of seromas, as well as increase the rate of incorporation.21 Lastly, the authors recommend leaving the drains in place for 2 to 3 weeks or until there is less than 30 mL of drain output for 2 consecutive days to ensure the integration of the ADM and mastectomy flap.
Aesthetic Considerations
Although being able to control the precise breast pocket with ADM has led to reproducible results and high patient satisfaction, one of the more frequent aesthetic issues with prepectoral breast reconstruction is rippling. Specific techniques can be used to minimize this issue. First, during expander filling, it is ideal to underfill the expander by roughly 100 to 200 mL relative to the anticipated final implant size. This allows the final implant to fit into a tighter pocket and reduces the potential for implant mobility.
Second, implant selection is key to minimizing rippling. Typically, silicone implants result in less rippling than saline implants.22 If silicone implants are being used, it is preferable to use a highly cohesive gel or implants filled to maximum capacity (maximum gel-to-shell fill ratio). This approach utilizes implants that have minimal or no rippling on their surface before implantation, and therefore are less likely to show rippling after implantation. Likewise, overfilling saline implants can also ameliorate visible rippling but increase implant firmness. The authors’ preference is to place smooth, round, cohesive silicone implants. In addition to reducing rippling, these implants have a less acute edge shape, which results in a more natural appearance in prepectoral reconstruction.
Lastly, another strategy that can be used to minimize rippling is autologous fat transfer to the mastectomy skin flap, especially in the upper pole.18 Providing upper pole fullness is critical in prepectoral reconstruction because the patient has only the thickness of the mastectomy flap and ADM to provide volume in this area. In the authors’ experience, autologous fat transfer is used frequently at the time of expander-to-implant exchange (Figure 4). In addition to minimizing rippling, autologous fat transfer can minimize the potential “shelf appearance” at the superior portion of the implant.
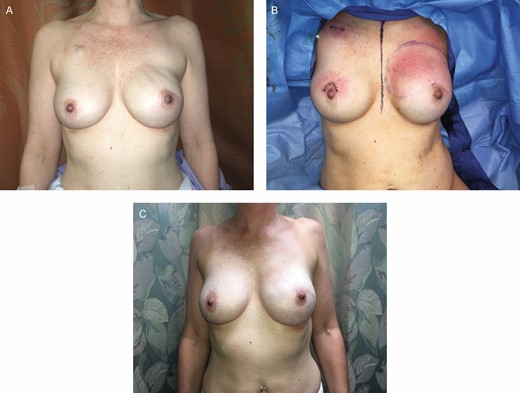
(A) After completion of reconstruction, the patient had notable rippling and upper pole implant visibility on the left reconstruction breast. (B) She underwent a third-stage procedure, where fat grafting alone was performed to the left breast reconstruction in the upper pole skin flaps (90 mL). (C) Another 9 months following this revision with left breast fat grafting, there is complete correction of the left breast rippling. This highlights the importance of autologous fat grafting in prepectoral patients, which is routinely done by the authors at the second-stage expander-to-implant exchange operation.
POSTOPERATIVE CONSIDERATIONS
Infection
Postsurgical site infection should be given special attention in the prepectoral reconstruction patient. Without muscle coverage, breakdown of the skin is likely to lead to ADM and implant exposure. Therefore, a patient with a suspected infection should have oral antibiotics administered and be followed closely as an outpatient. Any patient showing signs of an incipent implant exposure requires immediate surgical debridement, excision of threatened skin, implant removal, and intravenous antibiotics. A prior study by the senior author found that in 51 patients (84 breasts), there was a 5% rate of cellulitis and a 2.4% rate of infections requiring a washout during a mean follow-up period of 11.1 ± 5.8 months.7 Furthermore, in the setting of postmastectomy radiation therapy, no difference in explantation rates between prepectoral and submuscular reconstruction patients was found (15.4% vs 19.3%; P = 0.695).6 Postoperative surgical site infection in the context of prior implant placement requires immediate operative management due to the risk of biofilm formation. Biofilms are difficult to eradicate, even with prolonged intravenous antibiotics. Prior studies have shown a roughly 15% infection rate after breast reconstruction with direct-to-implant and 2-stage implant reconstruction. However, no significant differences have been found between the prepectoral and submuscular cohorts.23
CONCLUSIONS
Achieving optimal prepectoral breast reconstruction results depends on numerous strategies in the preoperative, intraoperative, and postoperative settings. Patient selection is key to minimizing potential risks and complications. It is also important to understand that certain oncologic factors will play a significant role in whether to pursue a prepectoral reconstruction. Intraoperatively, assessment of mastectomy flap perfusion by both clinical examination and with perfusion devices, such as ICG laser angiography, is critical for reconstructive success. Implant selection and fat grafting can play an important role in minimizing rippling.
Prepectoral reconstruction offers numerous benefits, including elimination of animation deformity, decreased pain, precise control of breast pocket with ADM, and aesthetic outcomes with high patient satisfaction. Plastic surgeons should critically evaluate patient selection and perioperative surgical techniques to obtain optimal prepectoral breast reconstruction results.
Disclosures
Dr Sbitany is a consultant for Allergan, Inc. He received no compensation or support for this study. The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This supplement is sponsored by Allergan Aesthetics, an Abbvie Company (Irvine, CA), Mentor Worldwide, LLC (Irvine, CA), and 3M+KCI (St. Paul, MN).
REFERENCES



