-
PDF
- Split View
-
Views
-
Cite
Cite
Patricia G Gensel, Comparative anatomy, fossils, genomes and development coming together: a Commentary on ‘A protoxylem pathway to evolution of the pith?’, Annals of Botany, Volume 130, Issue 6, 1 December 2022, Pages i–ii, https://doi.org/10.1093/aob/mcac124
Close - Share Icon Share
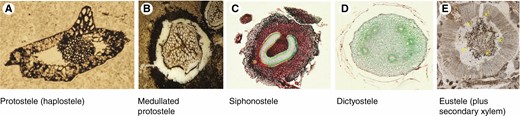
Very often one can identify a plant as belonging to a certain major group (fern, lycopod, seed plant) by making a cross-section of a stem and noting the organization of its vascular bundle(s) – or stele – a concept that has existed for over a century. The term stele refers to xylem, phloem and its associated tissues (formulated by Van Tiegham and Douliot, 1886; and expanded upon by subsequent botanists), all presumably derived from the embryonic procambial tissue (at least) and bounded to the outside by the inner layer of the cortex, the endodermis or a parenchymatous sheath (Tomescu, 2021). Stelar types vary from a solid central core of xylem (haplo- or protostele) to those with a central region of parenchyma or sclerenchyma (a pith), or both (siphonosteles, solenosteles, dictyosteles, eusteles; Fig. 1). Stelar organization and variation have been extremely important in the study of fossil and extant vascular plants, particularly seedless vascular plants given that the greatest variation occurs in them.
(A) Psilophyton coniculum (Early Devonian); (B) Ankyropteris glabra (Carboniferous) – brown areas are regions where parenchyma was present; (C) Adiantum sp. (extant); (D) Polypodium sp. (extant); (E) Lyginopteris oldhamia (Carboniferous) – primary xylem is indicated by a yellow x1. Most Devonian vascular plants exhibit different types of protosteles or eusteles (with a pith region); Devonian and Carboniferous plants exhibit some parenchyma intermixed with xylem as in B, supporting some of the ideas regarding the origin of pith as proposed by Tomescu and McQueen (2022). Anatomy similar to that in C may initially have supported the ‘extrastelar origin’ hypothesis of Jeffrey (1898).
From early studies on, botanists have noted ontogenetic changes in stelar morphology, as well as changes associated with change in size (Bower, 1930), the relative location of xylem vs. phloem, and the presence, absence, size and composition of the pith. As the steles of the earliest vascular plants featured a central core of xylem lacking pith (a haplostele), the question of how the pith originated is a fundamental one that is the subject of two competing hypotheses: E. C. Jeffrey advocated an extrastelar origin of the pith, namely an ‘invasion’ of the central tracheary region by cortical cells (Jeffrey, 1898) while other botanists supported an ‘intrastelar origin’ (Van Tiegham and Douliot, 1886; Boodle, 1901, 1903), suggesting the pith originated by developmental change in centrally located cells of the xylem. Considerable support for the intrastelar origin of pith ensued, although the process(es) causing such a ‘developmental change’ remained vague. Tomescu and McQueen (in this issue of Annals of Botany), building from a novel anatomical xylem pattern of a permineralized Early Devonian plant, Leptocentroxyla tetrarcha, provide a hypothesis about the origin of the pith that incorporates discoveries of developmental biologists on the genetic and regulatory control of cell patterning and differentiation of vascular tissues.
Leptocentroxyla tetrarcha is used by Tomescu and McQueen (2022) as a starting point for considering the processes of pith evolution. The metaxylem of both main axis and lateral traces consists of two zones of nearly similar-sized cells (the central cells are slightly smaller) that differ in wall thickness and pitting patterns, the outer showing P-type pitting and the inner showing scalariform pitting. It is not unusual to find more than one type of secondary wall thickening in metaxylem but in this case the difference in wall thickness is rather unusual, and according to Tomescu and McQueen, so too is the difference in pitting. The scalariform pitting of these thinner walled cells is interpreted as incompletely developed P-type pitting as occurs in the outer metaxylem. A few short projections from the scalariform bars are used to support the incompletely developed P-type pitting hypothesis, in contrast to a degradation hypothesis. They hypothesize that the central metaxylem in both types of axes is homologous to protoxylem. Given data from developmental genetic studies about controls on the generation of pitting pattern and cell wall thickness during differentiation, the factors invoked by Tomescu and McQueen are that a pith may arise as a result of (1) a delay in differentiation of cells destined to become protoxylem until after stem elongation (thus forming metaxylem), and (2) a shortening of the differentiation phase – which they consider a form of paedomorphosis (if shortened enough, forming pith). This ‘delayed and shortened protoxylem differentiation’ hypothesis for the origin of pith cells agrees in a general way with the intrastelar origin of pith, but is novel in suggesting specific genetic and developmental bases for it. Implicit in this hypothesis is that the embryo and developing sporeling/seedling exhibit a centrally located procambium, not ground meristem tissue, and that such a pith is derived from procambium rather than ground meristem.
Considering the ‘delay in differentiation’ step, one might immediately ask: why protoxylem? Might these cells already be committed to a certain developmental pathway? Possibly the alteration of the developmental trajectory occurs at the stage of stem cells (e.g. preprocambial or procambial cells), first by a delay in differentiation so they elongate and enlarge similar to other cell types, and then are patterned following stem elongation in a genetic/regulatory environment causing cell wall patterning typical of metaxylem, but evolutionarily they experience increasingly shorter phases of differentiation or, carrying this further, would remain as parenchyma, derived from procambium. This places the genetically based regulatory change at an earlier stage than the protoxylem – perhaps being more of a ‘procambium to pith’ or ‘delayed and shortened differentiation of procambium’ hypothesis. One can also deduce that if this were the sequence of events, it confirms early interpretations (Brebner, 1902) that pith, in some plants at least, is derived from procambial cells via specific gene-controlled developmental alterations. Allusions to this possibility were made by Evert and Eichhorn (2013, p. 569), who cite as an example the roots of the monocot Zea (corn). Whether all piths are derived in this manner requires further investigation.
Thus, while the hypothesis of Tomescu and McQueen suggests specific developmental processes for the origin of the pith, many questions remain. First, does the pith in all plants arise in a similar way, particularly those with eusteles? Second, as the authors recognize, the expression and activity of auxin and other growth regulators, the interactions of the several types of transcription factors (Class III HD-Zips, NACs, VNDs, Wrky, miRNAs and others) that determine cell fate as to proto- vs. metaxylem and that control establishment of a tracheid developmental pathway is mostly known from highly derived angiosperms (Ramachandran et al., 2017). Some of these gene classes and their products are present in seedless vascular plants and bryophytes (Floyd and Bowman, 2007), but their function is mostly unknown. What kind(s) of changes in function occur from one plant group to another? This hypothesis, along with other papers by Tomescu and colleagues (e.g. Tomescu, 2021; Woudenberg et al., 2022), demonstrates the very real need for genome sequencing of additional seedless vascular plants and for further comparative studies of their developmental pathways. It may be worth noting that the stelar concept, as an organizing principle for systematic and evolutionary relationships, arose out of a need to make sense of the enormous amount of comparative anatomical knowledge of all groups of plants acquired in the late 1800s to early 1900s (Beck, 1982). As such, the stelar concept is really a family of models relating stem structure first to evolutionary history, and now to developmental genetics. In this sense, it seems likely to remain an essential tool for linking genome- and physiology-based information to comparative anatomical structures in fossil and living plants.



