-
PDF
- Split View
-
Views
-
Cite
Cite
Kate E Wulf, James B Reid, Eloise Foo, Auxin transport and stem vascular reconnection – has our thinking become canalized?, Annals of Botany, Volume 123, Issue 3, 15 February 2019, Pages 429–439, https://doi.org/10.1093/aob/mcy180
Close - Share Icon Share
Abstract
The presence of a polar auxin transport stream has long been correlated with the differentiation and patterning of vascular cells across vascular plants. As our understanding of auxin transport and vascular development has grown, so too has evidence for the correlation between these processes. However, a clear understanding of the cellular and molecular mechanisms driving this correlation has not been elucidated.
This article examines the hypothesis that canalization via polar auxin transport regulates vascular reconnection and patterning in the stem after wounding or grafting. We examine the evidence for the causal nature of the relationship and the suggested role that other hormones may play. Data are presented indicating that in grafted plants the degree of auxin transport may not always correlate with vascular reconnection. Furthermore, data on grafting success using plants with a range of hormone-related mutations indicate that these hormones may not be critical for vascular reconnection.
In the past, excellent work examining elements of auxin synthesis, transport and response in relation to vascular development has been carried out. However, new experimental approaches are required to test more directly the hypothesis that auxin transport regulates stem vascular reconnection after wounding or grafting. This could include studies on the timing of the re-establishment of auxin transport and vascular reconnection after grafting and the influence of auxin transport mutants and inhibitors on these processes using live imaging.
INTRODUCTION
The evolution of vascular tissues has enabled plants to adapt and thrive in terrestrial environments for >420 million years (Silvestro et al., 2015). These tissues provide mechanical support as well as the ability to transport water, photoassimilates, nutrients and signalling molecules. This has allowed plants to overcome the physical and environmental constraints of the land, enabling them to grow in stature and diversity and to inhabit the majority of the Earth’s terrestrial surface. Due to the importance of the vasculature, plants have evolved mechanisms for its repair, enabling the vasculature to recover from physical damage by the environment or pests and diseases, as well as to connect the plant to symbionts (Melnyk, 2017).
The phytohormone auxin has long been implicated in both the development and maintenance of vascular tissue. While Charles Darwin and his son Francis first proposed the presence of a downward travelling plant messenger (Darwin, 1880), it was not until 1928 that the Dutch botanist Frits Went first described its role in plant growth and named the class of plant hormones auxins (Went, 1928). Went, and later William Jacobs (1952), suggested that the presence and concentration of auxin aided in vascularization, and subsequently Tsvi Sachs conducted a series of simple yet elegant vascularization experiments in Pisum sativum that indicated a specific role for auxin (Sachs, 1969). These experiments led to the development of what has become known as the ‘canalization theory’ (Sachs, 1981).
In one key experiment, Sachs removed the apex from pea seedlings and, in the remaining epicotyl tissue, separated a small section of epicotyl that had no existing vascular tissue (Fig. 1A). He observed that application of auxin to the separated tissue initiated xylem regeneration and reconnection to the established vasculature. However, this did not occur if auxin was also applied to the existing vascular bundle, unless more auxin was applied to the separated tissue than to the existing vascular bundle (Sachs, 1969; Fig. 1A). This has been explained by the observation that the flow of auxin through cells of high auxin content (source) towards cells of low auxin content (sink) polarizes and upregulates auxin transport (Fig. 1B). This positive feedback process leads to the channelling or canalization of auxin through files of cells, some of which have been proposed then to differentiate to form vascular strands (Sachs, 1981; Bennett et al., 2014). High auxin transport in existing strands has been proposed to suppress connection to new vasculature (Fig. 1A) due to hypercanalization of the existing tissue (Sachs, 1981; Bennett et al., 2014).
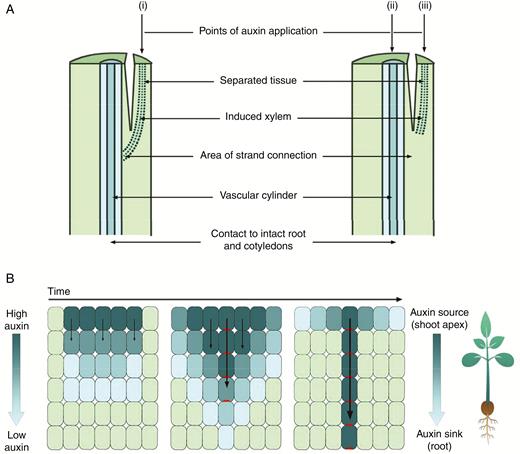
Auxin canalization model of vascular differentiation. (A) Sachs’ experiments on pea. (i) Auxin was applied to a separated section of the stem, and strands of xylem were differentiated, connecting the source of auxin with the existing vascular cylinder. (ii) Auxin applied to the existing vascular cylinder and the (iii) separated tissue prevented differentiation of xylem in separated tissue. NB: when the amount of auxin applied to the separated stem tissue was increased, but the amount of auxin applied to the existing vascular cylinder was kept constant, differentiation in the separated tissue did occur. (Based on image and information from Sachs, 1969.) (B) The canalization theory-based model of auxin flow. Auxin moves from cells of high to low concentration, and the resulting auxin influx polarizes PIN1 efflux carrier proteins to the plasma membrane opposite the influx of auxin. This positive feedback loop results in the gradual narrowing of cells with polarized PIN1 and canalization of auxin transport. Dark blue cells contain high concentrations of auxin, light green cells contain low concentrations of auxin, and polarized PIN1 proteins are shown in red.
Sachs’ postulations have had a powerful influence on the field to this day. Subsequent studies have added significant detail to the model, in particular aided by a deeper understanding of the auxin transport machinery, computer modelling and studies with mutants displaying altered auxin transport, synthesis or perception (e.g. Scarpella et al., 2006; Melnyk et al., 2015). In this article, we examine the role of auxin transport in the control of stem vascular development during recovery from wounding and grafting, as this is a powerful model system to understand the processes that drive vascular reconnection. In addition, we outline an experimental system of grafts between species that indicates that auxin transport may not always be correlated with stem vascular reconnection.
STEM VASCULAR DEVELOPMENT
Vascular tissues in mature angiosperms consist of highly specialized cells, comprised of three main tissue types: xylem, phloem and associated cambial tissue. Within the xylem a variety of cell types exist, from distinctive tracheids and vessel elements to xylem fibres and parenchyma. Xylem elements and tracheids are formed from highly lignified cells, which undergo apoptosis to form water-impermeable tunnels capable of transporting water and dissolved nutrients upwards from the roots to the shoots in the transpiration stream (Broderson, 2016). Phloem in most angiosperms is primarily made up of three cell types: sieve elements, companion cells and phloem parenchyma. Similar to xylem elements, sieve elements are elongated and tubular, allowing movement of essential constituents through the interior of the cell from source to sink (Heo et al., 2014). In contrast to xylem, phloem cells are living and have thinner cell walls, are separated by sieve plates and are capable of bi-directional transport.
Following primary growth, procambial cells from the vascular cambium, made up of cambial cells, drive secondary growth (Schuetz et al., 2012). In many herbaceous dicots, xylem and phloem are limited to vascular bundles, with the cambium between the xylem and phloem known as the fascicular cambium. However, cambium may be present not only in vascular bundles, but also between vascular bundles in the cylindrical interfascicular cambium (Sehr et al., 2010; De Rybel et al., 2016). The cambium facilitates secondary growth leading to an increase in the diameter of the stem and secondary vascular tissue (Cho et al., 2017).
AUXIN TRANSPORT – A COMPLEX, MULTI-TRANSPORT REGIME
Auxin is thought to move through plant stems in two main ways. First, exogenous auxin is capable of unregulated bulk movement through mature phloem cells (Petrasek and Friml, 2009). The second route, often referred to as the polar auxin transport (PAT) stream, is regulated and occurs via influx and efflux transport proteins in the plasma membrane. Auxin is transported primarily in a basipetal direction through (pro)cambial cells in established vasculature, although it can move short distances through other tissue types such as parenchyma (Goldsmith, 1977; Galweiler et al., 1998; Petrasek and Friml, 2009). This is consistent with recent data showing that auxin concentrations are highest in dividing cambium (Immanen et al., 2016).
The AUX1 and LAX protein families of auxin influx carriers are transmembrane proteins that actively transport auxin from the extracellular environment into the cell cytoplasm. Several studies using aux1/lax mutants have shown impacts on vascular development (Peret et al., 2012; Fabregas et al., 2015). It is believed that influx carriers play an integral role in maintaining auxin gradients and the maxima needed for canalization to occur throughout the plant (Friml et al., 2003; Reinhardt et al., 2003; Swarup and Peret, 2012).
Auxin transport is also regulated by efflux carriers, including the well characterized PIN protein family. pin1 mutants were first described in arabidopsis and failed to develop floral organs and produced pin-like inflorescences, leading to the name PIN FORMED (PIN) (Okada et al., 1991). The PIN family of proteins can differ in their expression, location and activity, which in turn influence auxin efflux (Krecek et al., 2009). PIN1 efflux carriers have been shown to accumulate on the basal plasma membrane, opposite to the influx of auxin (e.g. Gälweiler et al., 1998; Friml et al., 2003; Omelyanchuk et al., 2016). The manner in which PIN1 proteins become polarized is thought to be through sub-cellular trafficking and polarity maintenance in response to auxin in the cell cytoplasm, via intracellular auxin receptors (Muday and Murphy, 2002; Adamowski and Friml, 2015). This dynamic, actin-dependent process is thought to occur continually, with PIN1 cycling between endosomal compartments and the plasma membrane (Muday and Murphy, 2002). These intracellular processes appear to be influenced by a range of both endogenous and environmental signals, such as cytokinins, gibberellins, strigolactones, nutrient availability and gravitrophic response, as outlined in Adamowski and Friml (2015).
Whilst the majority of research has focused on PIN proteins, other known efflux proteins are also involved in auxin transport. These include the ABCB family that also shows polarity in developing vascular tissue and is essential for the stabilization and function of PIN1 (Blakeslee et al., 2007; Mravec et al., 2009; Titapiwatanakun et al., 2009; Armengot et al., 2016). Recent studies have also identified a variety of other potential efflux proteins, such as PIN-like proteins (PILS; Mohanta et al., 2015) and ABCG-type auxin transporters. The latter have been found to affect vascular bundle development, density and patterning in arabidopsis stems (Hir et al., 2013; Borghi et al., 2015).
Mathematical modelling has suggested that the PIN1-driven model of PAT is not sufficient to account for the total movement of auxin (Ongaro et al., 2008; Prusinkiewicz et al., 2009). A recent study by Bennett et al. (2016) confirmed that a single polar transport regime is insufficient to explain the dynamics of auxin movement through a stem. This widespread low conductance movement most probably involves less-polar contributions of PIN3, PIN4 and PIN7 (Bennett et al., 2016). It was implied that the PAT stream, once established, is the conclusion of canalization, and is not capable of responding to differing auxin fluxes, in contrast to the previously held assumptions outlined above. Therefore, while PIN1 has been implicated as the integral auxin efflux carrier and driver of auxin transport canalization, multiple efflux carriers probably work in concert, and multiple transport regimes exist (Bennett et al., 2016).
DOES AUXIN TRANSPORT CONTROL STEM VASCULAR DEVELOPMENT AND PATTERNING?
Examining the role of auxin transport in the vascular patterning of intact plants using mutants disrupted in auxin transport or hormone application studies is complicated by the fact that auxin influences many important developmental processes. This means that it is difficult to determine if the changes in vascular patterning observed are directly related to auxin transport or are due to secondary effects. This could include changes in plant development (e.g. cell size, division and organ shape) that then indirectly influence vascular development, or changes in the level of or response to other hormones influenced by auxin (e.g. gibberellin; Reid and Ross, 2011). Indeed, quite different outcomes for vascular development and patterning in the stem can be induced by mutations that disrupt auxin transport in intact plants. For example, altered xylem differentiation and a reduction in the number of vascular bundles occur in the inflorescence stem of the arabidopsis aux1 lax1 lax2 lax3 quadruple auxin influx mutant compared with comparable wild-type plants (Frabregus et al., 2015). In contrast, the auxin efflux mutant pin1, which has a reduction in auxin sources (i.e. young leaves) and auxin transport in stem segments, has a vascular phenotype similar to that of plants that overproduce auxin, with increased xylem production and vascular bundle development (Galweiler et al., 1998; Benjamins and Scheres, 2008). This is also seen in pin1 pin2 double mutants (Fabregas et al., 2015). In intact plants, auxin transport inhibitors such as 1-naphthylphthalamic acid (NPA) have also been shown to not only impair vascular continuity but also increase the amount of vascular tissue compared with plants grown on a non-NPA medium (Mattsson et al., 1999).
Perhaps some of the strongest evidence for auxin transport controlling post-embryonic stem vascular development has been obtained through studies using wounded or grafted plants. Plants have evolved wound response mechanisms that enable survival after herbivory, mechanical damage or pathogen attack (Asahina et al., 2011; Reid and Ross, 2011; Ikeuchi et al., 2013). The amazing ability of plants to restore vascular connections has also been harnessed in horticultural grafting practices to produce superior plants made up of selected root and shoot combinations (Mudge et al., 2009; Melnyk and Meyerowitz, 2015). Wounding and grafting studies sever existing vascular strands within the plant stem and allow the observation of the reconnection of the vasculature, either around the wound, through the wound site or across the graft junction (Sauer et al., 2006; Asahina et al., 2011; Sawchuck and Scarpella, 2013; Fig. 2). In incomplete severing of the stem, new vascular tissue will often form around the wound sites through pre-existing parenchyma tissue or through callus (Fig. 2A). In the case of complete severing of the vascular tissue, as occurs in grafting, new vascular tissue must form across a callus layer, a mass of totipotent parenchyma cells that aids in binding the plant sections together (Fig. 2A).
Sachs’ pioneering experiments have shown that in the presence of applied auxin, cells appear to be capable of xylem specification and differentiation (Sachs, 1969; Fig. 1). Auxin application to grafts may accelerate successful graft unions (Shimomura and Fujihara, 1977). Melnyk et al. (2015) have proposed that auxin and/or sucrose may drive reconnection and the wound response in the rootstock. The auxin canalization model of vascular development would also predict that disruption of PAT by chemical inhibitors would result in altered vascular patterning. While the PAT inhibitor NPA disrupts key PAT efflux proteins, grafts with NPA applied to the graft junction showed no disruption to phloem reconnection or reduced vascular strand reconnection across the graft junction (Melynk et al., 2015). Although NPA may not entirely block auxin transport (Bishopp et al., 2011), this result suggests that reducing PAT may not necessarily reduce vascular reconnection. However, use of another auxin transport inhibitor, 2,3,5-triiodobenzoic acid (TIBA), did suppress tissue reunion in incised inflorescence stems (Asahina et al., 2011) and reduced the width and length of vascular tissue formed at the graft junction in arabidopsis (Matsuoka et al., 2016).
CORRELATION OF PIN1 POLARIZATION AND/OR AUXIN TRANSPORT DURING STEM VASCULAR RECONNECTION
The link between PAT and vascular reconnection has been explored by examining PIN1 polarization and its role in canalization. The canalization theory proposes that following wounding of the stem, plants re-establish PAT and subsequent vascular reconnection through PIN1 polarization (Adamowski and Friml, 2015). It is proposed that existing vascular strands below a wound would act as an auxin sink. This sink would orient the auxin flow from above the wound site, gradually polarizing PIN proteins in an arc around the site of wounding as the flow of auxin becomes concentrated or canalized (Fig. 2). Positive feedback of polarized transport of auxin would lead to preferential transport along files of cells, some of which could then undergo differentiation to produce vascular cells (Sawchuk and Scarpella, 2013; Fig. 2B).
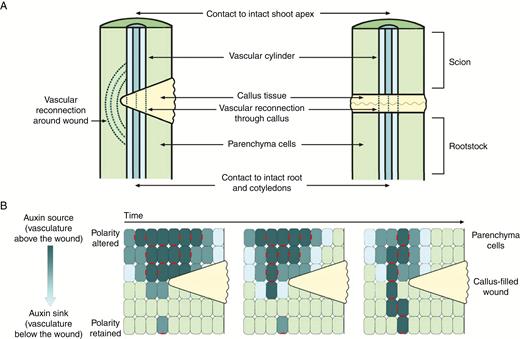
Vascular and polar auxin transport re-establishment after wounding or complete severing of the stem. (A) Vascular reconnection after wounding of the stem (left-hand side) or complete severing of the stem (right-hand side). (B) Polar auxin transport re-establishment over time after wounding, through polarization of PIN1 auxin transport proteins. Dark blue cells contain high concentrations of auxin, light green cells contain low concentrations of auxin, callus is shown in yellow, and polarized PIN1 proteins are shown in red.
PIN1 antibody studies have enabled patterns of PIN1 protein localization to be visualized and correlated to vascular reconnection in wounded and grafted plants (Sauer et al., 2006; Balla et al., 2011; Mazur et al., 2016; Weller et al., 2017). For example, antibody studies undertaken in pea epicotyl tissue show that the number of cells exhibiting PIN1 first increases in response to wounding and then decreases as PIN1 basal polarity is achieved and polarized cells extend towards cells that retain polarization below the wound site (Sauer et al., 2006; Fig. 2B). It has been hypothesized that, due to the involvement of PIN1 in auxin transport, as well as the positioning of polarized cells between existing vasculature, this process initiates vascular differentiation (e.g. Weller et al., 2017).
There are two important issues to consider in the interpretation of these PIN1 studies. The first is that it should be noted that these studies examine PIN1 and not auxin transport directly. Given this, and the potential for other proteins to be involved in active auxin transport as outlined above, studies that more directly examine the timing of re-establishment of auxin transport across or around the wound site and associated vascular reconnection would be useful. Indeed, elegant physiological systems such as those used by Sachs (1969) and Gersani (1985) could be harnessed to establish the exact timing of these events. Another point to consider is that the staining process for PIN1 renders the plant incapable of further growth. Therefore, it is impossible to determine whether cells in which PIN1 polarization occurs genuinely differentiate into vascular tissue, and if this is phloem and/or xylem. Therefore, currently, we cannot categorically determine whether auxin transport via PIN1 polarization drives vascular development, plays a non-initiating role in vascular patterning, or if these two processes simply co-occur.
Another approach has been to monitor auxin response in the days following wounding or grafting. Auxin response can be monitored by examining the expression of auxin-responsive genes or by transgenic plants containing auxin-responsive promoters fused to reporters, such as DR5. DR5::reporter constructs are particularly powerful, as they allow the spatial distribution of auxin responses to be visualized (Benkova et al., 2003). In arabidopsis, the beginnings of vascular reconnection are visible within 3 d after wounding, while DR5 staining above and below the graft junction was visible 2–3 d after wounding (Yin et al., 2012; Mazur et al., 2016). Several studies have examined the expression of genes above and below a wound site in the stem or a graft union in the days following the treatment and found a large number of genes that are up- and downregulated. This includes a range of hormone-responsive genes, including several auxin-responsive genes (Asahina et al., 2011; Yin et al., 2012; Asahina and Satoh, 2015; Melnyk et al., 2015; Qiu et al., 2016). Several of these auxin-responsive genes follow a pattern suggesting that changes in auxin response correlate with the timing and position of vascular reconnection. For example, Mazur et al. (2016) conclude that the increased auxin response above the wound site preceded the formation of PIN1 auxin transporter-marked channels, and the transient, gradual changes in PIN1 localization preceded the polarity of newly formed vascular tissue. It is important to note that the expression of DR5 and of other auxin-responsive genes does not indicate auxin concentration or transport, only auxin response, and therefore does not provide direct evidence that auxin transport itself is required for vascular reconnection.
USE OF GRAFTS TO EXPLORE THE ROLE OF PAT IN STEM VASCULAR RECONNECTION
Grafts carried out between different genotypes are also a useful tool to examine the role of PAT in stem vascular reconnection. The different genotypes used in grafting studies can differ by a single gene, such as a gene encoding a key step in hormone biosynthesis, signalling or transport, can be different plant varieties or even different species or genera of plants. Recent work in arabidopsis examined grafting success, including new vascular connections in grafts between wild-type plants and 30 mutants defective in various aspects of auxin biosynthesis, response and transport (Melnyk et al., 2015). Of these 30, only four graft combinations [wild-type scions expressing pSUC2::GFP (green fluorescent protein) grafted to rootstocks of alf4, axr1 iaa18 or to the triple mutant tir1 afb2 afb3] were found to have a reduced rate of phloem reconnection. These mutations have previously been implicated in phenotypic effects in arabidopsis root development and are involved in auxin perception and signalling (Leyser et al., 1993; Uehara et al., 2008; Chen et al., 2011; Chupeau et al., 2013; Takato et al., 2017). More detailed studies with alf4 self-grafts also showed a reduction in xylem transport capacity compared with self-grafted wild-type plants (Melnyk et al., 2015). It is interesting to note that axr1 or alf4 scions grafted onto wild-type rootstocks showed similar vascular reconnection rates to wild-type self-grafts. Together these results have been interpreted as evidence that auxin response genes below the graft junction are essential for vascular reconnection by both Melnyk et al. (2015) and others (Kuempers and Bishopp, 2015; Lup et al., 2016).
Melnyk et al. (2015) hypothesized that auxin transport across the graft junction was a critical step that occurs prior to vascular reconnection. However, it is important to note that auxin transport was not directly assessed in this study. The only mutants disrupted in auxin transport used in this study, namely the PIN efflux carrier mutants, pin3, pin4, pin7 and the triple mutant line, showed wild-type-like vascular reconnections after grafting. Furthermore, this study did not include any of the other well characterized arabidopsis mutants disrupted in auxin transport proteins, namely pin1, abcb, aux and lax (Mravec et al., 2008; Adamowski and Friml, 2015; Armegot et al., 2016). Other key mutants implicated in severe vascular patterning abnormalities were also neglected. For example, the quadruple kan mutant, which is thought to regulate expression of PIN genes and produces astonishing radial vascular bundles in intact plants, with xylem completely surrounding phloem tissue (Emery et al., 2003), was not included.
Another recent approach to examine the role of PAT in vascular reconnection after grafting has involved monitoring PAT in heterografts between different legume species. Heterografts between lupin/pea (scion/rootstock) and lupin/broadbean produce vigorous plants, similar to homografts between lupin/lupin, broadbean/broadbean and pea/pea (Fig. 3A; Foo et al., 2015). The transport of applied 3H-radiolabelled indole-3-acetic acid (IAA) from the apex across the graft junction into the root in homografts (lupin/lupin, pea/pea, broadbean/broadbean) occurred at approx. 1 cm h–1 (Fig. 3B; Foo et al., 2015), consistent with previous reports for the speed of auxin movement in the PAT stream (e.g. Goldsmith, 1977; Morris et al., 2005). However, transport of [3H]IAA from the apex across the graft junction into the root is significantly disrupted in the heterografts compared with self-grafts (Fig. 3B, D; Foo et al., 2015). Indeed, the majority of [3H]IAA accumulates above the graft junction in lupin/broadbean and lupin/pea grafts and very little reaches the rootstock, in stark contrast to self-grafts (Fig. 3D; Foo et al., 2015). However, when sections were taken through mature graft junctions significant amounts of vascular tissue were observed in both homo- and heterografts (Fig. 3C).
![Vascular development and transport of [3H]indole-3-acetic acid (IAA) in lupin and broadbean 18 d after grafting. (A) Photograph of a typical lupin/broadbean (scion/rootstock) wedge graft. (B) Percentage of radiolabel (% CPM, counts per minute) in 1 cm segments of plants 20 h after the application of 37 000 Bq 3H-labelled IAA in 1 μL of ethanol to the intact apex. (C) Longitudinal sections of the graft junctions stained with toluidine blue and safranin O. The graft junctions (yellow line) between the scions (S) and the rootstocks (R) are indicated. (D) Percentages of radiolabel recovered from the scion (excluding the apex and the section immediately above the graft junction), the scion immediately above the graft junction (S), the rootstock immediately below the graft junction (R) and the remainder of the rootstock are shown. Within-tissue values with different letters are significantly different (one way-ANOVA and Tukey’s HSD test, P < 0.05). For (B) and (D), values are the mean ± s.e. (n = 3). Grafts and transport experiments were carried out as described in Foo et al. (2015).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/aob/123/3/10.1093_aob_mcy180/1/m_mcy18003.jpeg?Expires=1750328293&Signature=iLmowCOIT9uq2djQfttFdatTPfNqjczuUHvT8D-dPDfuQcZSHJW6Ux645rWTWMI9y9Cuozn2qMNbjWm69aDC~VkoZ95dYK5kvM~q0Czsti8xso3zRx3PyzmC~X9Rabe4BhAXKCt3ykltCKaO1KCDYRxQWgPk0IhU-vf3S12muiFG6~mg2rhQant6Yw04NnpLuEqaVA90J5n6B008J3rPyh3Bf2ktNK9Bt6-N4YJEuanpfqGe6VFYAPtuPHWe2gaAqc8VCLfDOHxFiAF7GRKw2uDXBgLb3~3dJMaqa95pPq53xI2AYw7AJ1bmEVIOxK3JhWt1S8hViOdlBuL2LYe34A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Vascular development and transport of [3H]indole-3-acetic acid (IAA) in lupin and broadbean 18 d after grafting. (A) Photograph of a typical lupin/broadbean (scion/rootstock) wedge graft. (B) Percentage of radiolabel (% CPM, counts per minute) in 1 cm segments of plants 20 h after the application of 37 000 Bq 3H-labelled IAA in 1 μL of ethanol to the intact apex. (C) Longitudinal sections of the graft junctions stained with toluidine blue and safranin O. The graft junctions (yellow line) between the scions (S) and the rootstocks (R) are indicated. (D) Percentages of radiolabel recovered from the scion (excluding the apex and the section immediately above the graft junction), the scion immediately above the graft junction (S), the rootstock immediately below the graft junction (R) and the remainder of the rootstock are shown. Within-tissue values with different letters are significantly different (one way-ANOVA and Tukey’s HSD test, P < 0.05). For (B) and (D), values are the mean ± s.e. (n = 3). Grafts and transport experiments were carried out as described in Foo et al. (2015).
We examined the timing and degree of vascular reconnection in more detail in grafts between pea and lupin (Fig. 4). In the days after grafting, the amounts of new vascular connections between scion and rootstock were similar in homografts and heterografts with the same scion. At 4 d after grafting, 50 % of both homografts and heterografts displayed vascular connections between the scion and stock (Fig. 4A, see Fig. 4C for examples of grafts with no vascular connections). When present, these connections were between four and five cell files in all four graft combinations (two grafts of each type were sectioned). By 7 d after grafting, all homografts and heterografts had visible vascular connections between the scion and stock (Fig. 4B). These connections contained an average of 6.5 cell files in grafts with lupin scions (lupin/lupin and lupin/pea) and 9.5 and ten files in grafts with pea scions (pea/lupin and pea/pea, respectively).
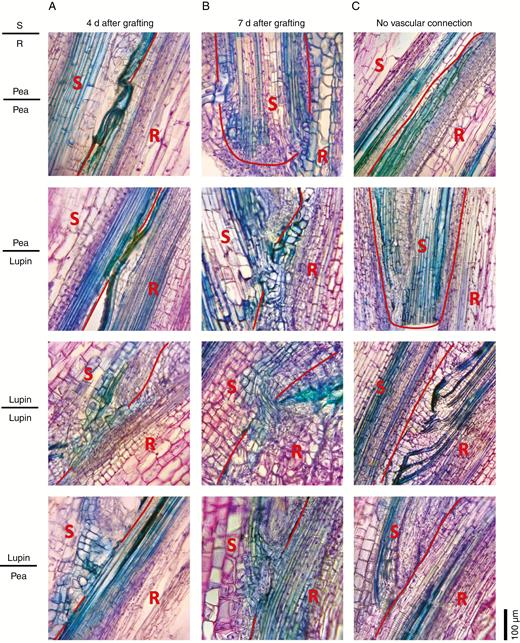
Vascular development in heterografts 4 and 7 d after grafting. Photographs of 30 μm thick longitudinal sections of the graft junction in reciprocal grafts between lupin and pea scions (S) and rootstocks (R). The graft junctions were harvested (A) 4 d or (B) 7 d after grafting. Wedge grafts were carried out with 1- to 2-week-old seedlings as described by Foo et al. (2015). The pea scions and rootstocks were severed in the first or second internode, lupin rootsocks in the epicotyl, and lupin scions in the hypocotyl. Sections were fixed in 4 % paraformaldehyde and embedded in polyethylene glycol (PEG) 1000 (using a method based on Paciorek et al., 2006) prior to sectioning and staining with toluidine blue and safranin O. The red line shows the junction between the scion and stock tissue. (C) Examples of grafts showing regions of no vascular reconnection for comparison.
Collectively, these data suggest that following severing of the stem, a similar degree of vascular tissue can form even in graft combinations with different auxin transport dynamics. This suggests that either auxin transport may not play a driving role in vascular reconnection or that only very minimal auxin transport is required to drive vascular re-connection. These results also suggest the intriguing possibility that re-establishment of auxin transport may be driven by processes in addition to auxin itself. Future studies could explore the timing of reconnection of PAT and vascular development in homografts and heterografts.
A POSSIBLE ROLE FOR OTHER HORMONES IN STEM VASCULAR RECONNECTION
Several other hormones have also been proposed to regulate wound healing and vascular reconnection after wounding, such as cytokinins, gibberellins, ethylene and brassinosteroids (Yoshida et al., 2014; Asahina and Satoh, 2015; Immanen et al., 2016), as well as other candidates such as photoassimilates (Jeffs and Northcote, 1967; Kondo et al., 2016). However, little account has been taken of the fact that grafts have been successfully performed between hormone-deficient or insensitive mutants in a range of species. Indeed, the effect of these hormonal changes has been largely ignored and certainly no systematic analysis has taken place on the effects that these mutants have on graft success. Given the importance of grafting to the horticultural industries and the need for an understanding of cell to cell compatibility in plants, it is appropriate to re-examine the literature for clues that may provide novel insights into the regulation of grafting success by plant hormones other than auxin. In pea, when grafts are performed with mutants disrupted in genes that encode well-characterized proteins required for the biosynthesis or signalling of gibberellins, brassinosteroids, strigolactones or ethylene, no change in the grafting success rates is apparent between mutant and wild-type graft combinations (Table 1). Similarly, no change in grafting success was noted when the pea wilty mutant, which is disrupted in a key abscisic acid biosynthesis enzyme (McAdam et al., 2015, 2016), was used in grafts (S. McAdam pers. comm.). This is the case even where the gene is a single-copy gene (e.g. LH, EIN2 or LK) or is the only member of a gene family expressed in vegetative tissues (NA), ruling out the lack of effect due to genetic redundancy.
Pea mutants that influence auxin responses/levels such as RMS2 (Ligerot et al., 2017) and BSH also fail to influence grafting success (Table 1), consistent with some auxin mutants in arabidopsis discussed above (Melnyk et al., 2015). Similar results with hormone mutants are available in other herbaceous dicots such as tomato, petunia and arabidopsis where grafting has been performed with mutants disrupted in strigolactone, brassinosteroid, abscisic acid or gibberellin levels (e.g. tomato, Holbrook et al., 2002; arabidopsis, Turnbull et al., 2002; Matsuoka et al., 2016). Transgenic disruption of gibberellin biosynthesis in the shoot of apples also does not influence graft success (Bulley et al., 2005), and no change in grafting success was noted in poplar transgenic lines and arabidopsis mutant lines with altered shoot cytokinin levels (Nieminen et al., 2008; Ko et al., 2014). Overall, these data do not provide strong evidence that the classical plant hormones, implicated by others in tissue repair and vascular reconnection as discussed above, are essential for the success of grafting, even though they may play important roles in regulating cambial growth.
Grafting success (expressed as percentage of all grafts performed) between pea (Pisum sativum L.) plant hormone mutants and the parental wild-type line
| . | . | . | Graft type (scion/rootstock) . | . | . | . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Gene . | Action . | WT/WT . | Mutant/Mutant . | Mutant/WT . | WT/Mutant . | n . |
| Gibberellin biosynthesis | NA | ent-Kaurenoic acid oxidase | 67 % | 88 % | 63 % | 67 % | 24 |
| LE | Gibberellin 3-oxidase | 67 % | 58 % | 67 % | 42 % | 12 | |
| LH | ent-Kaurene oxidase | 71 % | 83 % | 56 % | 75 % | * | |
| Brassinosteroid biosynthesis | LK | Steroidal 5α-reductase | 60 % | 40 % | 40 % | 73 % | 15 |
| Strigolactone biosynthesis | CCD8 | Carotenoid cleavage dioxygenase 8 | 44 % | 50 % | 38 % | 38 % | 16 |
| Strigolactone response | MAX2 | F-box protein | 45 % | 35 % | 50 % | 45 % | 20 |
| Ethylene response | EIN2 | N-RAMP metal transporter-like protein | 63 % | 44 % | 57 % | nd | † |
| Auxin response | RMS2 | Putative auxin receptor AFDB4/5 | 40 % | 50 % | 35 % | 45 % | 20 |
| Auxin deficient | BSH | Unknown | 93 % | 93 % | 93 % | 80 % | 15 |
| . | . | . | Graft type (scion/rootstock) . | . | . | . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Gene . | Action . | WT/WT . | Mutant/Mutant . | Mutant/WT . | WT/Mutant . | n . |
| Gibberellin biosynthesis | NA | ent-Kaurenoic acid oxidase | 67 % | 88 % | 63 % | 67 % | 24 |
| LE | Gibberellin 3-oxidase | 67 % | 58 % | 67 % | 42 % | 12 | |
| LH | ent-Kaurene oxidase | 71 % | 83 % | 56 % | 75 % | * | |
| Brassinosteroid biosynthesis | LK | Steroidal 5α-reductase | 60 % | 40 % | 40 % | 73 % | 15 |
| Strigolactone biosynthesis | CCD8 | Carotenoid cleavage dioxygenase 8 | 44 % | 50 % | 38 % | 38 % | 16 |
| Strigolactone response | MAX2 | F-box protein | 45 % | 35 % | 50 % | 45 % | 20 |
| Ethylene response | EIN2 | N-RAMP metal transporter-like protein | 63 % | 44 % | 57 % | nd | † |
| Auxin response | RMS2 | Putative auxin receptor AFDB4/5 | 40 % | 50 % | 35 % | 45 % | 20 |
| Auxin deficient | BSH | Unknown | 93 % | 93 % | 93 % | 80 % | 15 |
Successful grafts are defined as those in which the scion developed through to flowering with active growth of the main stem. Results are mostly from epicotyl/epicotyl grafts, but in some instances involved leafy shoots and are aggregated across more than one experiment. The number of grafts attempted is shown (n). No significant differences in grafting success were observed between graft types for any mutant when tested by contingency χ2. The results are from grafts done over many years including those done for other purposes reported in Reid et al. (1983), Reid and Ross (1989), Beveridge et al. (1996) and Symons et al. (1999) and unpublished results for ein2.
*n = 24 grafts for the self-grafts and 12 for the Mutant/WT and WT/Mutant grafts.
†n = 24 grafts for WT/WT, 16 for Mutant/Mutant and 28 for Mutant/WT.
nd = not done.
Grafting success (expressed as percentage of all grafts performed) between pea (Pisum sativum L.) plant hormone mutants and the parental wild-type line
| . | . | . | Graft type (scion/rootstock) . | . | . | . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Gene . | Action . | WT/WT . | Mutant/Mutant . | Mutant/WT . | WT/Mutant . | n . |
| Gibberellin biosynthesis | NA | ent-Kaurenoic acid oxidase | 67 % | 88 % | 63 % | 67 % | 24 |
| LE | Gibberellin 3-oxidase | 67 % | 58 % | 67 % | 42 % | 12 | |
| LH | ent-Kaurene oxidase | 71 % | 83 % | 56 % | 75 % | * | |
| Brassinosteroid biosynthesis | LK | Steroidal 5α-reductase | 60 % | 40 % | 40 % | 73 % | 15 |
| Strigolactone biosynthesis | CCD8 | Carotenoid cleavage dioxygenase 8 | 44 % | 50 % | 38 % | 38 % | 16 |
| Strigolactone response | MAX2 | F-box protein | 45 % | 35 % | 50 % | 45 % | 20 |
| Ethylene response | EIN2 | N-RAMP metal transporter-like protein | 63 % | 44 % | 57 % | nd | † |
| Auxin response | RMS2 | Putative auxin receptor AFDB4/5 | 40 % | 50 % | 35 % | 45 % | 20 |
| Auxin deficient | BSH | Unknown | 93 % | 93 % | 93 % | 80 % | 15 |
| . | . | . | Graft type (scion/rootstock) . | . | . | . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Gene . | Action . | WT/WT . | Mutant/Mutant . | Mutant/WT . | WT/Mutant . | n . |
| Gibberellin biosynthesis | NA | ent-Kaurenoic acid oxidase | 67 % | 88 % | 63 % | 67 % | 24 |
| LE | Gibberellin 3-oxidase | 67 % | 58 % | 67 % | 42 % | 12 | |
| LH | ent-Kaurene oxidase | 71 % | 83 % | 56 % | 75 % | * | |
| Brassinosteroid biosynthesis | LK | Steroidal 5α-reductase | 60 % | 40 % | 40 % | 73 % | 15 |
| Strigolactone biosynthesis | CCD8 | Carotenoid cleavage dioxygenase 8 | 44 % | 50 % | 38 % | 38 % | 16 |
| Strigolactone response | MAX2 | F-box protein | 45 % | 35 % | 50 % | 45 % | 20 |
| Ethylene response | EIN2 | N-RAMP metal transporter-like protein | 63 % | 44 % | 57 % | nd | † |
| Auxin response | RMS2 | Putative auxin receptor AFDB4/5 | 40 % | 50 % | 35 % | 45 % | 20 |
| Auxin deficient | BSH | Unknown | 93 % | 93 % | 93 % | 80 % | 15 |
Successful grafts are defined as those in which the scion developed through to flowering with active growth of the main stem. Results are mostly from epicotyl/epicotyl grafts, but in some instances involved leafy shoots and are aggregated across more than one experiment. The number of grafts attempted is shown (n). No significant differences in grafting success were observed between graft types for any mutant when tested by contingency χ2. The results are from grafts done over many years including those done for other purposes reported in Reid et al. (1983), Reid and Ross (1989), Beveridge et al. (1996) and Symons et al. (1999) and unpublished results for ein2.
*n = 24 grafts for the self-grafts and 12 for the Mutant/WT and WT/Mutant grafts.
†n = 24 grafts for WT/WT, 16 for Mutant/Mutant and 28 for Mutant/WT.
nd = not done.
CONCLUSIONS
Sachs’ elegant experiments laid the foundation for the study of PAT and its role in vascular development and patterning in a quest to understand this fundamental aspect of plant development. For nearly half a century, researchers have investigated the potential cellular and molecular mechanisms behind Sachs’ canalization hypothesis. A plethora of data has been obtained demonstrating individual aspects of auxin transport and vascular development from studies that visualize auxin transport proteins, observe effects of auxin transport inhibitors and use transgenic plants and mutant analyses. Results from these investigations have, in overall terms, been consistent with Sachs’ concept and have demonstrated that auxin transport and vascularization happen largely in the same tissue and at similar times. The heterografting studies presented here show a disruption of this correlation between auxin transport and vascular reconnection (Figs 3 and 4; Foo et al., 2015). While there is no doubt that auxin plays a role in wound healing, including vascular development, our data indicate that the amount of auxin flow does not necessarily correlate with the extent of vascular strand reconnection.
New approaches to investigate more directly the role of PAT in vascular reconnection after wounding are required. This could include further studies using radiolabelled auxin as presented here or fluorescently labelled auxin analogues (Hayashi et al., 2014). In particular, examination of the timing of PAT re-establishment and vascular reconnection after wounding would be informative. Grafting studies employing mutants that have been directly linked to PAT, such as the pin1 and abcb mutants, would also more directly test the hypothesis. A refined version of the auxin transport inhibitor studies employed by Matsuoka et al. (2016), in which a ring of TIBA is applied above the graft junction, concurrently with auxin transport studies and monitoring of vascular development, may enable improved investigations of vascular reconnection under known auxin conditions. Live-imaging techniques that have been used to visualize cellular changes in callus tissue and explants grown on media (Sugimoto et al., 2010) could also be used to track cellular changes concurrently with auxin transport to ascertain which cells transport auxin and which cells differentiate into vasculature. Furthermore, it is imperative not to exclude the potential that signals in addition to auxin may play a role in vascular development, including photoassimilates (Jeffs and Northcote, 1967; Kondo et al., 2016; Cho et al., 2017). Our aggregation of grafting success rates in mutants disrupted in strigolactone, gibberellin, brassinosteroid, abscisic acid or ethylene synthesis or response (Table 1) indicates that these hormones do not appear to play a major role in vascular reconnection. However, many other signals have been shown to be mobile across graft junctions, including RNA and proteins, and heritable changes in DNA methylation have also been shown to be graft transmissible (for a review, see Wang et al., 2017). It is also important to be mindful that most studies have been centred around a limited number of plant species, primarily the model species arabidopsis, and an understanding of these processes in a larger range of species, especially woody plants and those of commercial significance, would be useful. Indeed, understanding the process of vascular differentiation is not only important for advancing our comprehension of basic plant physiology, but it has far-reaching implications in agriculture and horticulture. Growth patterns, grafting practices, wound healing and disease resistance may all benefit from understanding the cellular and molecular control of vascularization and assist in increasing yield and quality of a varied range of crops used for human consumption, animal feed and industrial processes.
ACKNOWLEDGEMENTS
The authors acknowledge funding from the Australian Research Council for financial support, Shelley Urquhart for technical assistance, and Assistant Professor John Ross for critical reading of the manuscript.



