-
PDF
- Split View
-
Views
-
Cite
Cite
Shuai Yuan, Spencer C H Barrett, Tingting Duan, Xin Qian, Miaomiao Shi, Dianxiang Zhang, Ecological correlates and genetic consequences of evolutionary transitions from distyly to homostyly, Annals of Botany, Volume 120, Issue 5, November 2017, Pages 775–789, https://doi.org/10.1093/aob/mcx098
Close - Share Icon Share
Abstract
The outbreeding floral polymorphism heterostyly frequently breaks down, resulting in the evolution of self-fertilization as a result of homostyle formation. Here, the loss of floral polymorphism in distylous Primula oreodoxa, a sub-alpine species restricted to western Sichuan, China, was examined by investigating the ecological correlates and genetic consequences of mating system transitions. Several key questions were addressed. (1) What are the frequencies, geographical distribution and reproductive characteristics of floral morphs in distylous and homostylous populations? (2) Does increased elevation influence pollinator service and the likelihood of inbreeding in populations? (3) How often has homostyly originated and what are the consequences of the breakdown of distyly for the amounts and distribution of genetic diversity in populations?
Fourteen populations throughout the range of P. oreodoxa were sampled, and morph frequencies and floral characteristics were recorded. Polymorphism at microsatellite loci and chloroplast DNA (cpDNA) variation were used to quantify population genetic structure and genetic relationships among populations. Controlled pollinations and studies of pollen tube growth and fertility were conducted to determine the compatibility status of populations and their facility for autonomous self-pollination. Finally, visitation rates of long- and short-tongued pollinators to distylous and homostylous populations at different elevations were compared to determine if increased elevation was associated with deterioration in pollinator service.
In contrast to most heterostylous species, both distylous and homostylous morphs of P. oreodoxa are highly self-compatible, but only homostyles have the facility for autonomous self-pollination. Homostyles set significantly more fruit and seeds following open pollination than the distylous morphs. Visitation by long-tongued pollinators was significantly lower in homostylous populations, and overall rates of insect visitation decreased with elevation. Genetic diversity was significantly lower in homostylous populations, with evidence of increased inbreeding at higher elevation. Patterns of cpDNA variation were consistent with multiple transitions from distyly to homostyly and limited gene flow among populations.
The results of this study support the hypothesis that the multiple loss of floral polymorphism in distylous P. oreodoxa is associated with unsatisfactory pollinator service, with homostyles benefiting from reproductive assurance as a result of autonomous self-pollination.
INTRODUCTION
The evolutionary breakdown of heterostyly represents a paradigmatic model system for studies of reproductive transitions in flowering plants. In numerous families, the style and stamen polymorphisms that comprise the heterostylous syndrome are lost and are replaced by a monomorphic condition referred to as homostyly (Darwin, 1877; Baker, 1966; Charlesworth and Charlesworth, 1979; Ganders, 1979; Barrett, 1989; Weller, 1992; Richards, 1997; Chen et al., 2013). This change in floral biology and population morph structure has an important influence on the mating system of populations. Outbreeding in heterostylous populations is replaced by high levels of self-fertilization owing to the capacity of homostyles to set seed from autonomous self-pollination (Piper et al., 1984; Ganders et al., 1985; Barrett et al., 1989; Belaoussoff and Shore, 1995). In common with mating system transitions in non-heterostylous lineages (reviewed in Stebbins, 1974; Levin, 2012; Igic and Busch, 2013; Wright et al., 2013; Barrett et al., 2014), the shift to selfing can have important influences on the ecology, demography, genetics and evolution of populations. Therefore, identifying the environmental conditions favouring transitions to selfing and determining their population genetic consequences can provide insights into how often and why selfing evolves in heterostylous lineages.
The most widely invoked selective mechanism to explain why selfing evolves from outcrossing is Darwin’s reproductive assurance hypothesis (Darwin, 1876). Unsatisfactory pollinator service and/or a scarcity of mates can result in pollen limitation of outcrossed seed set and conditions favouring increased levels of self-pollination (Eckert et al., 2006; Moeller, 2006; Busch and Delph, 2012). Under persistent outcrossed pollen limitation, floral variants with a well-developed capacity for autonomous self-pollination are favoured by fertility selection, although other potential reproductive options may occur (see Harder and Aizen, 2010). Identifying the demographic conditions in which selfing is favoured most often focuses on conditions of low density (allele effects), such as occurs during colonizing episodes, including island colonization, or in geographically marginal populations (Baker, 1955; Dornier et al., 2008; Levin, 2012; Pannell, 2015). However, insufficient and/or inefficient pollinator service resulting in floral mechanisms promoting reproductive assurance can also be selected in diverse ecological contexts including tropical forests (Fan et al., 2012), deserts (Yin et al., 2016) and alpine environments (de Vos et al., 2012). Investigations of pollinator service along environmental gradients may be especially useful for examining the ecological context in which mating system transitions occur, although this has not been examined for the evolutionary breakdown of heterostyly to homostyly.
The evolution of selfing has profound consequences for the genetic structure of populations, including the amounts and partitioning of genetic diversity within and among populations. Inbreeding decreases heterozygosity, and persistent selfing results in populations that are less diverse and more differentiated from one another than outcrossing populations (Allard et al., 1968; Jain, 1976; Charlesworth and Pannell, 2001). Increased selfing rates also reduce effective population size, causing populations to lose genetic diversity owing to stochastic forces including drift and founder events (Wright, 1931; Nordborg, 2000; Barrett et al., 2014). Comparative surveys of population genetic structure using genetic markers in numerous outbreeding and selfing plant species have provided strong empirical support for these general expectations (Schoen and Brown, 1991; Hamrick and Godt, 1996; Charlesworth and Wright, 2001). Intraspecific studies of the influence of the mating system on population genetic structure can be especially informative (e.g. Rick et al., 1977; Barrett et al., 1989; Holtsford and Ellstrand, 1989). This is because they are less likely to be confounded with species-level contrasts in traits influencing genetic diversity and differences in the evolutionary history of lineages. Occasional heterostylous species are reported that maintain both heterostylous and homostylous populations, and these can provide valuable systems for investigating the influence of mating patterns on population genetic structure (reviewed in Barrett, 1989; Weller, 1992). The few studies that have been undertaken are consistent with species-wide surveys in showing reduced genetic variation in derived selfing homostylous populations (Barrett and Husband, 1990; Perusse and Schoen, 2004; Ness et al., 2010; Zhou et al., 2017). One of the goals of this study is to investigate a distylous species in which the distyly has broken down to homostyly and examine the genetic consequences of this transition.
Primula (Primulaceae) is the best known heterostylous genus and has received sustained attention since Darwin described reciprocal stigma and anther heights in Primula vulgaris and P. veris, and experimentally determined the fundamental crossing relationships that characterize dimorphic incompatibility (Darwin, 1862, 1877). Since then, theoretical studies (Charlesworth and Charlesworth, 1979) and empirical work on the reproductive ecology (Ornduff, 1979; Piper et al., 1984; Keller et al., 2014), inheritance patterns (Mather, 1950; Dowrick, 1956; Bodmer, 1960), phylogeography (Guggisberg et al., 2006; Zhou et al., 2017), systematics and comparative biology (Richards, 2003; Mast et al., 2006) and genetic architecture (Hu et al., 2016; Li et al., 2016; Burrows and McCubbin, 2017) of distyly and homostyly have provided a valuable framework for understanding the repeated loss of floral polymorphism in the genus. Primula is comprised of approx. 430 species of which 95 % are distylous and 45 species are homostylous, with homostyles distributed across 19 of the 38 sections, virtually all of which also contain distylous species (Richards, 2003; Mast et al., 2006; de Vos et al., 2014). Phylogenetic reconstructions in Primula indicate that homostyly has had multiple independent origins from distyly in the genus (Mast et al., 2006), and studies on species with intraspecific variation in floral polymorphism have provided convincing evidence that homostyles in distylous species are evolutionarily derived from heterostylous morphs (Crosby, 1949; Charlesworth and Charlesworth, 1979; Zhou et al., 2017). These evolutionary transitions to homostyly represent a remarkable example of the convergent loss of floral polymorphism, raising important questions concerning the ecological and genetic mechanisms involved.
Although the majority of Primula species are either distylous or homostylous, a few are known that are comprised of a mixture of the two floral conditions (Charlesworth and Charlesworth, 1979; Richards, 1997). For example, in the widespread P. vulgaris, homostylous plants are reported from two small areas within the UK, where they coexist in populations with distylous morphs at varying frequencies (Crosby, 1949; Curtis and Curtis, 1985). Populations in other parts of the UK and mainland Europe appear to be largely distylous. In contrast, in the Chinese endemic P. chungensis, most populations in western Sichuan and the Tibetan plateau are homostylous, and purely distylous populations are restricted in distribution (Zhou et al., 2017). In P. floribunda, there is some evidence, based on a survey of herbarium specimens, that the frequency of homostyly in the western Himalayas is associated with increased elevation, and it has been suggested that homostyly is favoured at higher elevations owing to the benefits of autonomous self-pollination in environments with an absence of insect visits (Richards, 1997, p. 266). These earlier findings motivated us to investigate the reproductive ecology and genetics of P. oreodoxa, a species with distylous and homostylous populations that occurs in sub-alpine habitats of western Sichuan to examine the potential role of elevation on the pollination biology and degrees of inbreeding in different populations.
Primula oreodoxa is a little-known taxon described as possessing heteromorphic flowers and has been assumed to be distylous (Richard, 2003). However, our preliminary observations revealed a more complex pattern of floral variation including distylous populations, comprised of the long-styled (L-morph) and short-styled (S-morph) morphs, homostylous populations monomorphic for homostylous plants (H-morph), with long styles and long-level anthers [‘long homostyly’, see Charlesworth and Charlesworth (1979) and Barrett and Shore (2008) for discussion of the forms of homostyly], and populations containing a mixture of all three floral morphs. This discovery led us to investigate P. oreodoxa in more detail to address a series of questions concerning the potential ecological mechanisms and genetics consequences of the loss of floral polymorphism in the species. Despite the extensive literature on the distyly–homostyly system in Primula, understanding of the environmental conditions associated with shifts to selfing is poorly understood. Moreover, only a single study (see Zhou et al., 2017) has examined the population genetic consequences of the breakdown of distyly to homostyly in Primula.
We began by surveying populations of P. oreodoxa throughout its range in western Sichuan to determine their population morph structure and the frequencies of style morphs. Next, we examined features of their floral biology related to distyly and homostyly. In particular, through controlled pollinations and studies of pollen tube growth and fertility we determined whether the floral morphs were self-incompatible or self-compatible. Having established that distylous and homostylous plants were both thoroughly self-compatible, we then determined the extent to which the floral morphs had the capacity for autonomous self-pollination under field conditions, an important pre-condition for reproductive assurance. We next investigated whether the elevation of populations influenced the frequency and type of pollinators visiting flowers, predicting that pollinator service might decline with elevation, owing to the mountainous terrain that the species occupies. Because increased selfing owing to homostyle evolution is expected to reduce genetic variation, we then used polymorphism at microsatellite markers to compare population genetic parameters and levels of inbreeding in distylous and homostylous populations. Finally, using chloroplast DNA (cpDNA) variation, we investigated the genetic relationships among populations and whether there was evidence of multiple independent transitions from distyly to homostyly. Our studies indicate that elevation gradients have a significant effect on the pollination biology and mating system of populations, with important influences on their patterns of genetic variation.
MATERIALS AND METHODS
Study species
Primula oreodoxa Franchet (subgenus Auganthus, section Obconicolisteri; Richards, 1993) is a herbaceous perennial, restricted in distribution to western Sichuan province, China (102°–104°E, 28°–31°N). Populations grow at the margins of mixed woodlands and alongside streams in open sun from 1054 to 2448 m. Flowering individuals usually bear 1–8 inflorescences with 1–15 rose purple flowers in an umbel (Fig. 1); blooming occurs from March to May and flowers senesce after 6–9 d from the beginning of anthesis. Fruits mature from late May to early June, after which capsules dehisce and release the small, light seeds (thousand seed weight: 52.4 ± 8.2 mg).

Flowers of Primula oreodoxa with diverse insect visitors. (A) Flowering plant; (B) habitat of population TTS; (C) Pieris melete (Pieridae); (D) Bombus (Bombidae); (E) Syrphidae; (F) Bombyliidae.
Population sampling, floral traits and chromosome counts
We conducted field studies during the flowering seasons of 2013 and 2014. We determined the floral morph composition of 14 populations across the known distributional range of the species. In populations of <150 plants, we sampled all flowering individuals but, in three large populations (WWS, HZG and TTS), plants were sampled at 1–2 m intervals along transects. Estimates of population size were made in all populations by direct count or in the three large populations by estimating the distributional area and density of plants per area. We used G-tests (Sokal and Rohlf, 1995) to determine whether the frequencies of the L- and S-morphs in distylous populations deviated from the expected equilibrium ratio of 1:1. We conducted detrended correspondence analysis (DCA) to identify the dominant axes of variability in floral morph ratio among populations and to examine correlations between axis values and population elevation (ter Braak, 1995). We sampled leaves from an average of 28 (range 21–30) plants in each population, and these were then dried in silica gel for subsequent sequencing and genotyping.
To compare variation in floral traits among populations and floral morphs, we randomly sampled an average of 18 (range 10–22) fully mature flowers from 15 plants of each style morph in 12 populations. We measured stigma and anther heights from the base of the ovary in all populations, and corolla size and floral tube length in selected distylous (DWS, JCC and WWS), homostylous (CSQ, LWP, QCS, TTS and XXC) and mixed (HZG) populations. All measurements were conducted on fresh flowers using electronic calipers (573-S; Mitutoyo, Kawasaki, Japan). Sample sizes are provided in Supplementary Data Table S1. We compared the corolla width, floral tube length and sigma–anther separation among morphs using generalized linear mixed models (GLMMs) (Pinheiro and Bates, 2000; Zuur et al., 2009) with a Gaussian distribution and restricted maximum likelihood (REML). The measurements of corolla width, floral tube length and stigma–anther separation were response variables with floral morph as a fixed effect and population as a random variable (packages ‘lme4’; Bates et al., 2012).
To investigate variation in pollen size in P. oreodoxa, we sampled pollen from one distylous populations (JCC), two homostylous populations (CSQ and QCS) and one mixed population (ELS). Pollen grains were collected from large mature buds, which were fixed in 70 % FAA (18:1:1 = 70 % ethanol:glacial acetic acid:formaldehyde solution). In common with most distylous species, we predicted that pollen from short-level anthers of the L-morph would be significantly smaller in size than pollen from long-level anthers of the S-morph (Dulberger, 1992). If this were true, we would expect that pollen from long-level anthers of long homostyles would be of a similar size to pollen of the S-morph and significantly larger than pollen of the L-morph (see Zhou et al., 2017). To test these hypotheses, pollen grains were photographed under a scanning electron microscope (Philips XL 30) and their polar and equatorial axes were measured. We then used GLMMs with a Gaussian distribution to compare pollen size variation among the floral morphs, with the polar and equatorial axes as response variables, floral morph as a fixed effect, and population as a random variable (packages ‘lme4’; Bates et al., 2012).
Because of the potential association between polyploidy and homostyly (see Naiki, 2012), we examined variation in chromosome number by collecting individuals from one distylous (JCC), three homostylous (CSQ, LWP and QCS) and one mixed (ELS) population from the field and transplanted them to an alpine glasshouse at South China Botanical garden (SCBG), Chinese Academy of Sciences. From plants, root tips were excised and incubated in 0.1 % colchicine for 3.5 h before fixation in Carnoy’s solution (glacial acetic acid:absolute ethanol = 1:3), then macerated in a 1:1 mixture of 45 % acetic acid and 1 m HCl at 37 °C for 45 min, stained with Carbol fuchsin for 10–30 min, and finally squashed gently. We counted chromosome numbers under an optical microscope (BX41; Olympus).
Compatibility and fertility relationships
We investigated the compatibility/incompatibility status of distylous and homostylous populations of P. oredoxa by controlled crosses and measurements of fruit and seed set in a glasshouse, and by examining pollen tube growth following controlled pollinations conducted in the field. We transplanted individuals from the distylous population JCC and the homostylous population CSQ into a glasshouse at the Biological Resources Research Station at E’mei Mountain (altitude 800 m). Controlled hand pollinations involved self- and intermorph crosses in the distylous population, and self- and cross-pollination in the homostylous population. An average of ten plants (range 8–12) and 50 flowers (range 36–62) were pollinated for each pollination treatment using flowers on the second or third day of anthesis. All flowers were caged before and after pollinations, and for cross-pollinations flowers were emasculated prior to anther dehiscence. Pollinations were conducted in March and fruits were harvested 6–8 weeks later when mature, and fruit and seed set per capsule were recorded. We compared fruit and seed set among treatments for each floral morph using t-tests in R 3.2.1 (R Development Core Team, 2015).
For studies of pollen tube growth, the following pollination treatments were used: (1) intramorph and intermorph crosses in the distylous population (JCC); and (2) self- and cross-pollinations in the homostylous population (CSQ). Each pollination type involved 20 flowers per morph with five flowers fixed in 50 % FAA after 4, 24, 48 and 72 h. After rinsing with water, we cleared the fixed pistils in 1 m NaOH until tissues became transparent and they were then washed in distilled water and stained with aniline blue according to Teng et al. (2006). We measured the length of pollen tubes in cleared pistils using a fluorescence microscope (BX41; Olympus) with three pollen tubes measured in each flower. We then compared pollen tube lengths among treatments at each time interval using t-tests in R 3.2.1 (R Development Core Team, 2015).
Using the same approach, we also investigated pollen tube growth in crosses between homostyles of P. oreodoxa and the distylous morphs of the related P. obconica. We conducted these pollinations to validate our assumption that homostyles of P. oreodoxa are long homostyles that combine the female organs of the L-morph with the male organs of the S-morph (see Barrett and Shore, 2008, fig 1.4). If this assumption is correct, we expect inhibition of pollen tubes when pollen of short-level anthers of the L-morph of P. obconica is applied to stigmas of P. oreodoxa homostyles and inhibition of homostylous pollen of P. oreodoxa when applied to short-level stigmas of the S-morph of P. obconica. In contrast, the remaining crossing combinations should result in compatible pollen tube growth. These interspecific crosses were conducted assuming that the homostyles of P. oreodoxa are derived from the heterostylous morphs and that they still retain some residual dimorphic incompatibility affecting pollen tube growth.
To determine the facility for self-pollination and the natural levels of open-pollinated fruit and seed set, we carried out a manipulative field experiment in three homostylous (TTS, XXC and LWP) and one distylous (JCC) population. Four treatments were applied to an average of 32 plants and 172 flowers per population (see Supplementary Data Table S7). The treatments were: (1) caged plants; (2) emasculated flowers; (3) emasculation + caging; and (4) open pollination. Caging of plants excluded pollinators, and fruit set can only be achieved by autonomous self-pollination. Any fruit set in emasculated uncaged flowers results from either geitonogamous or outcrossed pollination. Caged and emasculated flowers can only set fruit asexually by apomixis. The open-pollinated treatment assessed natural levels of fruit and seed set. To investigate more widely if natural levels of fruit set differed between distylous and homostylous populations, we measured the percentage fruit set in an additional three distylous (HZS, WWS and DWS), two homostylous (CSQ and QCS) and two mixed (ELS and HZG) populations. In ELS, we also compared the seed set of the L-, S- and H-morphs. First, we compared the fruit and seed set between treatments 1, 2 and 4 in each homostylous population using GLMMs with a Gaussian distribution for fruit set and a Poisson distribution and REML for seed set. Pollination treatments were fixed effects in each model, and population and plant identity nested within populations were random effects. Secondly, we employed additional GLMMs with a Gaussian distribution to examine differences in open-pollinated fruit set between homostylous and distylous plants with morph as a fixed effect and population as a random effect. A comparison of seed set in three morphs of ELS was done using analysis of variance (ANOVA). All analyses were performed in R. 3.2.1 (packages ‘lme4’; Bates et al., 2012).
Insect visitors to flowers
We conducted observations of insects visiting the flowers of P. oreodoxa during the flowering seasons of 2013–2015 in ten populations with elevations ranging from 1045 to 2448 m. We verified that all insects we recorded were pollinators, based on their feeding behaviour and presence of pollen on their bodies and contact with sex organs of flowers. In each population, we established a 5 × 3 m plot in an area of high flowering density and on sunny days from 9.00 to 17.00 h recorded all pollinators visiting flowers, and for each foraging bout we recorded the number of flowers visited. Visitors were photographed, collected and returned to the Laboratory of Pollination Biology, SCBG where they were divided into two groups based on proboscis length: long- and short-tongued insects. To calculate visitation frequency in each population, we divided the number of flowers visited by pollinators by the number of flowers in plots. We used the Mann–Whitney U-test to compare visitation frequencies of long- and short-tongued insects in distylous + mixed populations vs. homostylous populations, and linear regression analysis to investigate the relationships of these two classes of pollinators to population elevation, with visitation frequency as a response variable and elevation as a quantitative predictor. Both analyses were conducted in R 3.2.1 (R Development Core Team, 2015).
Population genetic structure inferred from microsatellite markers
We tested for polymorphism using 120 microsatellite markers developed for P. sieboldii (Ueno et al., 2009) and chose four highly polymorphic primer pairs (ga0047, ga0948, ga1041 and ga1251) for our study. We extracted genomic DNA using a modified cetyltrimethylammonium bromide (CTAB) protocol (Doyle, 1991) from 371 individuals from 13 diploid populations. The tetraploid population QCS was not included in the genetic analysis. The amplified 10 μL mixture for simple sequence repeats (SSRs) included 5 μL of Master Mix (Generay, Guangzhou, China), 0.4 mm of each primer pair, 3.2 μL of deionized water and 30–50 ng of genomic DNA. We ran PCRs with initial denaturation for 4 min at 94 °C, followed by ten cycles of 94 °C for 35 s, 35 s at 60 °C with an increment of –1 °C per cycle, 45 s at 72 °C, then followed by 28 cycles of 94 °C for 35 s, 35 s at 50 °C, 45 s at 72 °C, and ending with an extra extension of 10 min at 72 °C. We scanned PCR products using an ABI PRISM 3100 Genetic Analyser (Invitrogen) with an internal size standard GeneScan™ 500 LIZ. We conducted allele binning and calling using GeneMarker version 2.4.0 (SoftGenetics LLC, State College, Pennsylvania, USA).
We estimated the number of alleles (NA), expected heterozygosity (HE), allelic richness (AR) and inbreeding index (FIS) using FSTAT version 2.9.3 (Goudet, 2001). The proportion of individuals resulting from selfing (s), was estimated with the equilibrium equation FIS=s/[2 – s] (Hedrick, 2000). To investigate whether the floral morph composition of populations was correlated with the above genetic parameters, we compared these parameters between distylous and homostylous groups by permuting populations 1000 times in FSTAT version 2.9.3. We also used a Mann–Whitney U-test to compare s values between distylous and homostylous groups. In addition, we calculated pairwise FST between populations and overall FST values in FSTAT. We tested the relationships between elevation and HE, AR, FIS and s, respectively, by linear regression.
Genetic relationships among populations
To investigate the genetic relationship among distylous and homostylous populations, we sequenced two chloroplast intergenic spacer regions, trnD–trnT and ropB–trnC, from a total of 132 individuals from 14 populations, including the tetraploid population QCS, and two outgroup samples. The appropriate primers were designed online (www.yeastgenome.org/cgi-bin/web-primer) according to the complete chloroplast genome of Ardisia polysticta (Ku et al., 2013). The sequences of the primers were: trnD(GUC)F (AGG GCG GTA CTC TAA CCA A), trnT(GGU)R (CGA TGA CTT ACG CCT TAC CA), rpoB F (CAA GCC CTG ATC AAT GAA CCT) and trnC R (G AT TTG AAC TGG GGA AAA AGG). The target regions were amplified in 25 μL mixtures as follows: 12.5 μL of Master Mix (Generay), 0.4 mm of each primer pair, 10.5 μL of deionized water and 30–50 ng of genomic DNA. We ran PCRs, the same as for the microsatellites mentioned above. Sequencing reactions were carried out on an ABI 3700 automated sequencer (Invitrogen, San Diego, CA, USA). Sequences of two markers were aligned manually with the assistance of Bioedit 7.0.5.0 (Hall, 1999). We treated insertions and deletions (indels) as single mutations. We estimated overall and pairwise genetic differentiation among populations (FST) using the program Arlequin 3.5 (Excoffier and Lischer, 2010).
To determine the relatedness among cpDNA haplotypes, we conducted a network analysis of haplotypes using the TCS program version 1.21 (Clement et al., 2000). The TCS program collapses sequences into haplotypes and calculates the frequencies of the haplotypes in the samples. The probability of parsimony is calculated for pairwise differences until the probability exceeds 0.95. In the TCS analysis, indels were treated as a single character resulting from one mutation event.
We also inferred the phylogenetic relationships among cpDNA haplotypes using the maximum parsimony (MP) method and Bayesian inference (BI) employing P. heucherifolia as an outgroup. We conducted MP analyses with PAUP* version 4.0b (Swofford, 2002) using heuristic searches with 1000 addition sequence replicates, tree bisection–reconnection (TBR) branch swapping, steepest descent off, and keeping all most parsimonious trees. Gaps were treated as missing data. We calculated bootstrap values using 1000 replicates of the original data set, and ten random addition sequences were performed in each replicate. We performed BI analyses in MrBayes version 3.1.2 (Ronquist and Huelsenbeck, 2003). Three incrementally heated chains and a cold chain of Markov Chain Monte Carlo (MCMC) were run for 10 000 000 generations, starting from random trees and sampling one tree out of every 1000 generations. The first 2 000 000 generations were discarded as ‘burn in’ after stationarity was reached, with the value of average s.d. <0.01. We used the remaining trees to construct the Bayesian consensus tree. Internodes with posterior probabilities (PP) ≥95 % were considered highly supported.
RESULTS
Morph ratios and chromosome numbers
Surveys of 14 populations of P. oreodoxa revealed a wide range of population morph structures (Fig. 2). Four populations (JCC, WWS, DWS and HZS) were distylous, with the S-morph in two of the four populations at a significantly higher frequency than the L-morph (average frequency ± s.e., L-morph = 0.41 ± 0.04; S-morph = 0.59 ± 0.04). Floral morph ratios exhibited significant deviation from the expected 1:1 isoplethic equilibrium (Gtotal = 16.16, d.f. = 4, P < 0.01; Gpooled = 11.92, d.f. = 1, P < 0.001; Supplementary Data Table S2). Three populations (ELS, HZG and QLP) were comprised of mixtures of the L-morph, S-morph and homostylous morph, with no consistent pattern in their relative frequencies. One population (LB) was composed primarily of homostyles but with five long-styled plants, and the remaining six populations (CSQ, DFD, LWP, QCS, TTS and XXC) were composed exclusively of homostylous plants (Table 1). DCA revealed gradients of floral morph frequency among populations, with the first axis negatively correlated with elevation (r = 0.618, P < 0.05), owing to a tendency for homostylous individuals to occur in populations at higher elevations.

(A) Geographical distribution of 14 populations of Primula oreodoxa investigated in this study, illustrating floral morph frequencies and molecular variation. Small black circles indicate the location of populations; coloured circles represent the distribution of cpDNA (trnD–trnT and rpoB–trnC) haplotypes (H1–21) within each population; white–grey–black circles indicate floral morph frequencies in each population. (B) The 95 % confidence network of 21 cpDNA haplotypes. The size of circles corresponds to the frequency of each haplotype in the sample. Each solid line represents one mutational step that interconnects two haplotypes for which parsimony is supported at the 95 % level. The small open circles indicate hypothetical missing haplotypes. The red zone in the map at the right bottom indicates the sampled region in China.
Summary information and statistics for the 14 Primula oreodoxa populations from western Sichuan
| Population code . | Location . | Elevation . | Latitude (N), longitude (E) . | Morph frequency . | Sample size/ population size . | FIS . | s . | Haplotypes . | cpDNA/SSR . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | L . | S . | H . | . | . | . | . | . |
| CSQ | E’mei | 1702 | 29.57°, 103.38° | 0 | 0 | 1.00 | 128/approx. 150 | 0.45 | 0.62 | H1(8); H2(1); H3(1) | 10/30 |
| DFD | Mabian | 1961 | 28.68°, 103.37° | 0 | 0 | 1.00 | 58/approx. 100 | 0.60 | 0.75 | H4(8); H5(2) | 10/30 |
| DWS | Jinkouhe | 1887 | 29.37°, 103.02° | 0.48 | 0.52 | 0 | 29/approx. 100 | 0.31 | 0.48 | H6 | 7/29 |
| ELS | Tianquan | 1729 | 29.88°, 102.35° | 0.18 | 0.34 | 0.48 | 130/approx. 200 | 0.77 | 0.87 | H7(8); H8(2) | 10/30 |
| HZG | E’bian | 1793 | 29.02°, 103.05° | 0.34 | 0.52 | 0.14 | 229/approx. 500 | 0.47 | 0.64 | H9 | 10/30 |
| HZS | Ya’an | 1054 | 29.74°, 103.01° | 0.29 | 0.71 | 0 | 51/approx. 60 | 0.24 | 0.38 | H10(3); H11(6) | 9/21 |
| JCC | E’mei | 1668 | 29.46°, 103.22° | 0.45 | 0.55 | 0 | 100/approx. 120 | 0.65 | 0.79 | H4(1); H12(3); H13(5); H14(1) | 10/30 |
| LB | Leibo | 1904 | 28.34°, 103.47° | 0.06 | 0 | 0.94 | 84/approx. 100 | 0.71 | 0.83 | H15 | 8/30 |
| LWP | E’mei | 2448 | 29.55°, 103.35° | 0 | 0 | 1.00 | 64/approx. 100 | 0.84 | 0.91 | H16 | 10/30 |
| QCS | Dujiangyan | 1131 | 30.90°, 103.55° | 0 | 0 | 1.00 | 51/approx. 60 | – | – | H17 | 9/- |
| QLP | Hongya | 1820 | 29.50°, 103.25° | 0.17 | 0.32 | 0.51 | 120/approx. 150 | 0.59 | 0.75 | H12(7); H18(2) | 9/30 |
| TTS | Pengzhou | 1759 | 31.24°, 103.83° | 0 | 0 | 1.00 | 142/approx. 500 | 0.4 | 0.57 | H19 | 10/30 |
| WWS | Hongya | 1224 | 29.68°, 102.95° | 0.41 | 0.59 | 0 | 210/approx. 1000 | 0.41 | 0.58 | H20 | 10/30 |
| XXC | E’mei | 2099 | 29.50°, 103.33° | 0 | 0 | 1.00 | 78/approx. 100 | 0.77 | 0.87 | H16(5); H21(5) | 10/21 |
| Population code . | Location . | Elevation . | Latitude (N), longitude (E) . | Morph frequency . | Sample size/ population size . | FIS . | s . | Haplotypes . | cpDNA/SSR . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | L . | S . | H . | . | . | . | . | . |
| CSQ | E’mei | 1702 | 29.57°, 103.38° | 0 | 0 | 1.00 | 128/approx. 150 | 0.45 | 0.62 | H1(8); H2(1); H3(1) | 10/30 |
| DFD | Mabian | 1961 | 28.68°, 103.37° | 0 | 0 | 1.00 | 58/approx. 100 | 0.60 | 0.75 | H4(8); H5(2) | 10/30 |
| DWS | Jinkouhe | 1887 | 29.37°, 103.02° | 0.48 | 0.52 | 0 | 29/approx. 100 | 0.31 | 0.48 | H6 | 7/29 |
| ELS | Tianquan | 1729 | 29.88°, 102.35° | 0.18 | 0.34 | 0.48 | 130/approx. 200 | 0.77 | 0.87 | H7(8); H8(2) | 10/30 |
| HZG | E’bian | 1793 | 29.02°, 103.05° | 0.34 | 0.52 | 0.14 | 229/approx. 500 | 0.47 | 0.64 | H9 | 10/30 |
| HZS | Ya’an | 1054 | 29.74°, 103.01° | 0.29 | 0.71 | 0 | 51/approx. 60 | 0.24 | 0.38 | H10(3); H11(6) | 9/21 |
| JCC | E’mei | 1668 | 29.46°, 103.22° | 0.45 | 0.55 | 0 | 100/approx. 120 | 0.65 | 0.79 | H4(1); H12(3); H13(5); H14(1) | 10/30 |
| LB | Leibo | 1904 | 28.34°, 103.47° | 0.06 | 0 | 0.94 | 84/approx. 100 | 0.71 | 0.83 | H15 | 8/30 |
| LWP | E’mei | 2448 | 29.55°, 103.35° | 0 | 0 | 1.00 | 64/approx. 100 | 0.84 | 0.91 | H16 | 10/30 |
| QCS | Dujiangyan | 1131 | 30.90°, 103.55° | 0 | 0 | 1.00 | 51/approx. 60 | – | – | H17 | 9/- |
| QLP | Hongya | 1820 | 29.50°, 103.25° | 0.17 | 0.32 | 0.51 | 120/approx. 150 | 0.59 | 0.75 | H12(7); H18(2) | 9/30 |
| TTS | Pengzhou | 1759 | 31.24°, 103.83° | 0 | 0 | 1.00 | 142/approx. 500 | 0.4 | 0.57 | H19 | 10/30 |
| WWS | Hongya | 1224 | 29.68°, 102.95° | 0.41 | 0.59 | 0 | 210/approx. 1000 | 0.41 | 0.58 | H20 | 10/30 |
| XXC | E’mei | 2099 | 29.50°, 103.33° | 0 | 0 | 1.00 | 78/approx. 100 | 0.77 | 0.87 | H16(5); H21(5) | 10/21 |
L, S, H = long-styled, short-styled and homostylous morphs; FIS, inbreeding index; s, proportion of selfing; cpDNA/SSR, sample number for cpDNA and SSR analysis; –, not available, because the tetraploid population QCS was not included in the genetic analysis of microsatellites.
Summary information and statistics for the 14 Primula oreodoxa populations from western Sichuan
| Population code . | Location . | Elevation . | Latitude (N), longitude (E) . | Morph frequency . | Sample size/ population size . | FIS . | s . | Haplotypes . | cpDNA/SSR . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | L . | S . | H . | . | . | . | . | . |
| CSQ | E’mei | 1702 | 29.57°, 103.38° | 0 | 0 | 1.00 | 128/approx. 150 | 0.45 | 0.62 | H1(8); H2(1); H3(1) | 10/30 |
| DFD | Mabian | 1961 | 28.68°, 103.37° | 0 | 0 | 1.00 | 58/approx. 100 | 0.60 | 0.75 | H4(8); H5(2) | 10/30 |
| DWS | Jinkouhe | 1887 | 29.37°, 103.02° | 0.48 | 0.52 | 0 | 29/approx. 100 | 0.31 | 0.48 | H6 | 7/29 |
| ELS | Tianquan | 1729 | 29.88°, 102.35° | 0.18 | 0.34 | 0.48 | 130/approx. 200 | 0.77 | 0.87 | H7(8); H8(2) | 10/30 |
| HZG | E’bian | 1793 | 29.02°, 103.05° | 0.34 | 0.52 | 0.14 | 229/approx. 500 | 0.47 | 0.64 | H9 | 10/30 |
| HZS | Ya’an | 1054 | 29.74°, 103.01° | 0.29 | 0.71 | 0 | 51/approx. 60 | 0.24 | 0.38 | H10(3); H11(6) | 9/21 |
| JCC | E’mei | 1668 | 29.46°, 103.22° | 0.45 | 0.55 | 0 | 100/approx. 120 | 0.65 | 0.79 | H4(1); H12(3); H13(5); H14(1) | 10/30 |
| LB | Leibo | 1904 | 28.34°, 103.47° | 0.06 | 0 | 0.94 | 84/approx. 100 | 0.71 | 0.83 | H15 | 8/30 |
| LWP | E’mei | 2448 | 29.55°, 103.35° | 0 | 0 | 1.00 | 64/approx. 100 | 0.84 | 0.91 | H16 | 10/30 |
| QCS | Dujiangyan | 1131 | 30.90°, 103.55° | 0 | 0 | 1.00 | 51/approx. 60 | – | – | H17 | 9/- |
| QLP | Hongya | 1820 | 29.50°, 103.25° | 0.17 | 0.32 | 0.51 | 120/approx. 150 | 0.59 | 0.75 | H12(7); H18(2) | 9/30 |
| TTS | Pengzhou | 1759 | 31.24°, 103.83° | 0 | 0 | 1.00 | 142/approx. 500 | 0.4 | 0.57 | H19 | 10/30 |
| WWS | Hongya | 1224 | 29.68°, 102.95° | 0.41 | 0.59 | 0 | 210/approx. 1000 | 0.41 | 0.58 | H20 | 10/30 |
| XXC | E’mei | 2099 | 29.50°, 103.33° | 0 | 0 | 1.00 | 78/approx. 100 | 0.77 | 0.87 | H16(5); H21(5) | 10/21 |
| Population code . | Location . | Elevation . | Latitude (N), longitude (E) . | Morph frequency . | Sample size/ population size . | FIS . | s . | Haplotypes . | cpDNA/SSR . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | L . | S . | H . | . | . | . | . | . |
| CSQ | E’mei | 1702 | 29.57°, 103.38° | 0 | 0 | 1.00 | 128/approx. 150 | 0.45 | 0.62 | H1(8); H2(1); H3(1) | 10/30 |
| DFD | Mabian | 1961 | 28.68°, 103.37° | 0 | 0 | 1.00 | 58/approx. 100 | 0.60 | 0.75 | H4(8); H5(2) | 10/30 |
| DWS | Jinkouhe | 1887 | 29.37°, 103.02° | 0.48 | 0.52 | 0 | 29/approx. 100 | 0.31 | 0.48 | H6 | 7/29 |
| ELS | Tianquan | 1729 | 29.88°, 102.35° | 0.18 | 0.34 | 0.48 | 130/approx. 200 | 0.77 | 0.87 | H7(8); H8(2) | 10/30 |
| HZG | E’bian | 1793 | 29.02°, 103.05° | 0.34 | 0.52 | 0.14 | 229/approx. 500 | 0.47 | 0.64 | H9 | 10/30 |
| HZS | Ya’an | 1054 | 29.74°, 103.01° | 0.29 | 0.71 | 0 | 51/approx. 60 | 0.24 | 0.38 | H10(3); H11(6) | 9/21 |
| JCC | E’mei | 1668 | 29.46°, 103.22° | 0.45 | 0.55 | 0 | 100/approx. 120 | 0.65 | 0.79 | H4(1); H12(3); H13(5); H14(1) | 10/30 |
| LB | Leibo | 1904 | 28.34°, 103.47° | 0.06 | 0 | 0.94 | 84/approx. 100 | 0.71 | 0.83 | H15 | 8/30 |
| LWP | E’mei | 2448 | 29.55°, 103.35° | 0 | 0 | 1.00 | 64/approx. 100 | 0.84 | 0.91 | H16 | 10/30 |
| QCS | Dujiangyan | 1131 | 30.90°, 103.55° | 0 | 0 | 1.00 | 51/approx. 60 | – | – | H17 | 9/- |
| QLP | Hongya | 1820 | 29.50°, 103.25° | 0.17 | 0.32 | 0.51 | 120/approx. 150 | 0.59 | 0.75 | H12(7); H18(2) | 9/30 |
| TTS | Pengzhou | 1759 | 31.24°, 103.83° | 0 | 0 | 1.00 | 142/approx. 500 | 0.4 | 0.57 | H19 | 10/30 |
| WWS | Hongya | 1224 | 29.68°, 102.95° | 0.41 | 0.59 | 0 | 210/approx. 1000 | 0.41 | 0.58 | H20 | 10/30 |
| XXC | E’mei | 2099 | 29.50°, 103.33° | 0 | 0 | 1.00 | 78/approx. 100 | 0.77 | 0.87 | H16(5); H21(5) | 10/21 |
L, S, H = long-styled, short-styled and homostylous morphs; FIS, inbreeding index; s, proportion of selfing; cpDNA/SSR, sample number for cpDNA and SSR analysis; –, not available, because the tetraploid population QCS was not included in the genetic analysis of microsatellites.
Chromosome counts from five populations (CSQ, ELS, JCC, LWP and QCS) including distylous, mixed and homostylous populations revealed that all were diploid (2n = 24 chromosomes) except homostylous population QCS which was tetraploid (2n = 48). Thus, homostylous populations included both diploid and tetraploid plants. Mitotic chromosome counts at metaphase are illustrated in Supplementary Data Fig. S1.
Floral variation
As expected for a distylous species, stigma and anther height in L- and S-morphs displayed a clear bimodal distribution, whereas these organs exhibited a unimodal distribution in homostyles (Fig. 3). Average stigma–anther separation in L-morphs did not differ significantly from the S-morphs (L-morph = 3.97 ± 0.95 mm; S-morph = 3.88 ± 0.77 mm; t = 0.57, P = 0.572) (Supplementary Data Table S1 and Fig. S3). However, stigma–anther separation in homostyles was significantly smaller than in the distylous morphs (0.39 ± 0.40 mm, F = 575.93, P < 0.001) (Supplementary Data Table S1 and Fig. S3).
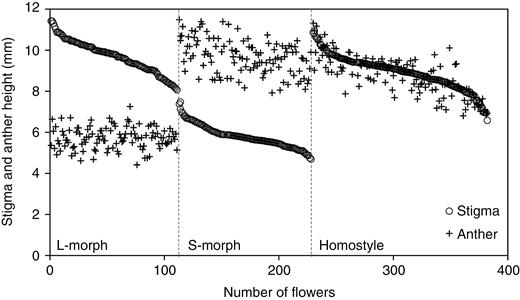
Variation in stigma and anther height in the floral morphs of Primula oreodoxa (n = 378 flowers). The flowers measured are ranked by style length to illustrate the relative stigma and anther positions in the long-styled, short-styled and homostyled morphs. Flowers plotted are from 12 populations (DWS, JCC, WWS, ELS, QLP, HZG, CSQ, DFD, TTS, QCS, XXC and LWP).
The GLMMs revealed significant differentiation in corolla width and floral tube length among the morphs (F254 = 17.39, P < 0.001; F112 = 17.84, P < 0.001). Corolla width was significantly smaller in the homostylous morph compared with the L-morph (t = 5.69, P < 0.001) and the S-morph (t = 5.64, P < 0.001) (Supplementary Data Fig. S2A). Floral tube length was significantly longer in the S-morph than the L-morph (t = 5.83, P < 0.001) and the homostylous morph (t = 3.12, P < 0.005) (Fig. S2B).
In agreement with our expectations for a distylous species, pollen size differed significantly between the L- and S-morphs, with both the equatorial (t = 27.78, P < 0.001) and polar axes (t = 20.57, P < 0.001) larger in the S-morph (Supplementary Data Fig. S4). The equatorial and polar axes of pollen of long-level anthers of the homostylous morph were not significantly different in size compared with pollen from long-level anthers of the S-morph (t = 0.30, P = 0.76; t = 1.12, P = 0.27; Supplementary Data Fig. S4).
Compatibility status and patterns of pollen tube growth
Controlled hand pollinations of P. oreodoxa demonstrated that the floral morphs in both distylous and homostylous populations were fully self-compatible. In the L- and S-morph, there was no significant difference (P > 0.05) in fruit or seed set following self- and intermorph pollinations; similarly, in the H-morph, there was also no significant difference (P > 0.05) in the fertility of self- and cross-pollinations (Supplementary Data Tables S3 and S4).
Investigations of pollen tube growth after various time intervals revealed only minor differences in the length of pollen tubes between intramorph and intermorph pollinations in distylous morphs, and between self- and cross-pollinations in homostylous populations (Fig. 4; Supplementary Data Fig. S5 and Table S5). Pollen germinated within 4 h in all pollination treatments in each floral morph and arrived at the ovary within 72 h in the L-morph, 48 h in the S-morph and 72 h in the homostylous morph. Thus, the patterns of pollen tube growth we observed were consistent with the self-compatible status of P. oreodoxa, as revealed from controlled pollination studies described above.
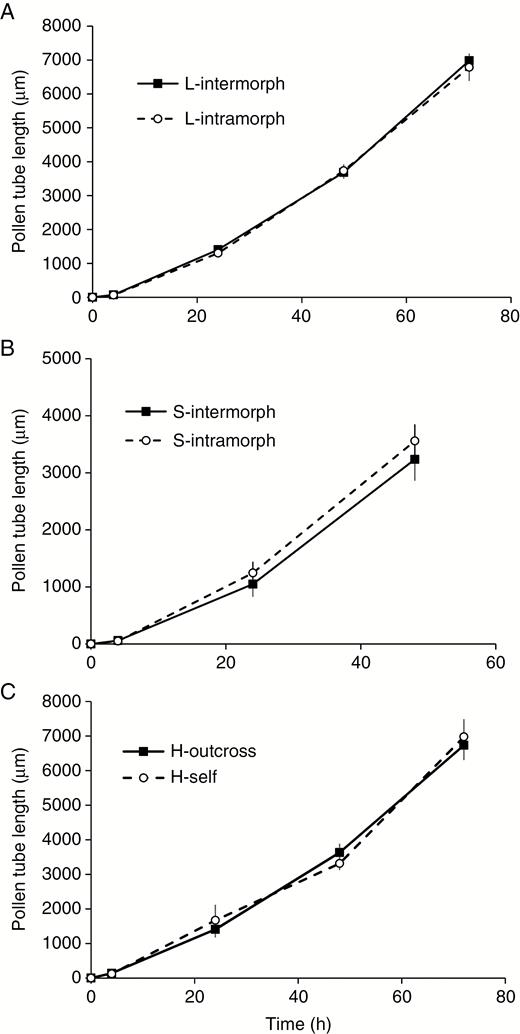
Pollen tube length following controlled hand pollinations of Primula oreodoxa conducted in the field. Data are means, and vertical bars are the standard deviation.
Interspecific crosses between P. oreodoxa homostyles and distylous morphs of P. obconica revealed patterns of pollen tube growth consistent with the long homostylous nature and the occurrence of residual dimorphic incompatibility in this morph (Supplementary Data Fig. S5 and Table S6). The styles of homostyles behaved as long styles, rejecting pollen from short-level anthers of the L-morph of P. obconica and accepting pollen from long-level anthers of the S-morph. Similarly, pollen from long-level anthers of P. oreodoxa homostyles was rejected by the style of the S-morph of P. obconica but was accepted by long styles of the L-morph.
Capacity for autonomous self-pollination and open-pollinated fertility
Caged and emasculated flowers set no fruit, indicating that P. oreodoxa has no capacity for apomictic seed production. There were no significant differences in fruit set between caged and open-pollinated flowers in the three homostylous populations (t = –0.99, P = 0.32; Fig. 5A; Supplementary Data Table S7) indicating a well-developed capacity for autonomous self-pollination, as would be predicted because of the close proximity of sexual organs. In contrast, in the distylous population (JCC) fruit set was dramatically reduced in caged flowers compared with the open-pollinated treatment as a result of the well-developed herkogamy typical of distylous morphs (L-morph, t = –18.17, P < 0.001; S-morph, t = –5.09, P < 0.001; Fig. 5A; Table S7).
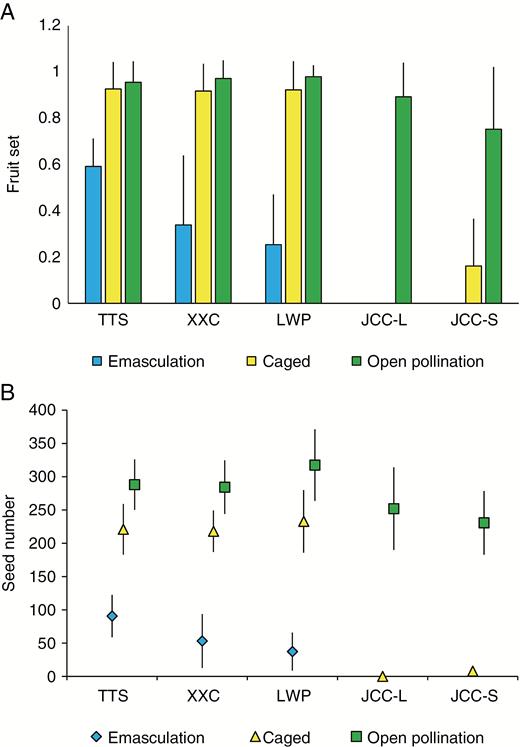
Fruit set (A) and seed set per fruit (B) following three pollination treatments (emasculated flowers, caged flowers and open-pollinated flowers) in three homostylous (TTS, XXC and LWP) and one distylous (JCC) population of Primula oreodoxa. In the emasculated treatment, only outcrossing and geitnogamy were possible and in the caged treatment only autonomous self-pollination was possible. Open-pollinated flowers can receive a mixture of cross- and self-pollen. Error bars are ±95 % confidence intervals; JCC-L = L-morph; JCC-S = S-morph. The remaining bars are homostylous individuals.
Our survey of open-pollinated flowers revealed that percentage fruit set was generally high in populations (range 0.82–1.00), although GLMMs indicated that overall homostyles had significantly higher fruit set than distylous morphs (F = 5.51, P < 0.01; Table S8). In the only population (ELS) in which we investigated seed set per fruit, there were striking differences among the floral morphs in maternal fertility, with homostyles setting substantially more seeds per fruit than the S-morph, and the L-morph exhibiting the lowest seed set (L-morph = 107.8 ± 7.91; S-morph = 173.0 ± 12.70; H-morph = 273.9 ± 12.69; F = 64.6, P < 0.001). These differences were not the result of morph-specific differences in ovule number. Comparisons of ovule number indicated no significant differences among the floral morphs in ELS, a pattern also evident in a comparison among the floral morphs sampled from 11 populations (unpublished data).
Insect visitation to flowers
Flowers of P. oreodoxa were visited by a wide range of insects foraging for pollen and nectar (Fig. 1). However, only a sub-set of pollinators with long tongues was capable of transferring pollen to stigmas of the S-morph; these included the bees Bombus (Bombidae) and Anthophora (Anthophoridae), flies (Bombyliidae) and a butterfly (Pieris melete). The remaining pollinators, including halictid bees and syrphid flies, possess short tongues and were only capable of pollinating the L- and H-morphs, as well as transferring pollen from long-level anthers of the S-morph to other plants. The visitation frequency of long-tongued pollinators was significantly higher in distylous (including mixed populations) than homostylous populations (W = 25, P < 0.01; Fig. 6). In contrast, there was no significant difference in the visitation frequencies of short-tongued pollinators to distylous and homostylous populations (W = 18, P = 0.31; Fig. 6). The visitation frequency of long-tongued pollinators was negatively correlated with the elevation of population (r = –0.79, P < 0.01; Fig. 7A); however, there was no correlation between elevation and the visitation frequency of short-tongued pollinators (r = 0.05, P = 0.89; Fig. 7A).
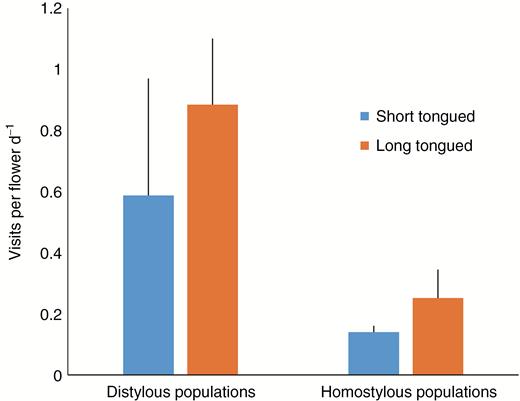
Mean ± s.e. visitation frequencies of long- and short-tongued pollinators in distylous vs. homostylous populations of Primula oreodoxa. Mixed populations were treated as distylous populations.

Relationships between elevation and (A) long- and short-tongued pollinator visitation to flowers; (B) FIS and s, among populations of Primula oreodoxa in western Sichuan. Plotted lines are linear regressions for both relationships.
Population genetic structure, inbreeding and relationships to elevation
Populations of P. oreodoxa maintained considerable genetic diversity at microsatellite loci (Supplementary Data Table S9) with average values for NA = 5.55 ± 2.58 (range 2.0–9.75), HE = 0.55 ± 0.21 (range 0.04–0.81) and AR = 4.25 ± 1.78 (range 1.39–7.14). Overall, distylous and mixed populations maintained significantly more genetic diversity and were on average more heterozygous than homostylous populations. Populations were significantly differentiated from one another (FST = 0 .393, P < 0.05) and the FIS ranged from 0.24 to 0.84 with an average of 0.55 ± 0.05 (Table 1), and values of s ranged from 0.38 to 0.91. Although there was no significant difference in values of FIS (P = 0.43; Table 2) or s (W = 16, P = 0.27) between distylous and homostylous populations, we detected a significant positive relationship between FIS and elevation (r = 0.71, P < 0.01) and between elevation and s (r = 0.71, P < 0.01), indicating that populations at higher elevations were more susceptible to inbreeding than were those at lower elevations (Fig. 7B).
Comparison of genetic diversity and differentiation among distylous (n = 7) and homostylous (n = 6) populations of Primula oreodoxa
| Parameters . | Distylous . | Homostylous . | P-value . |
|---|---|---|---|
| AR | 6.007 | 3.197 | 0.014 |
| HE | 0.670 | 0.414 | 0.012 |
| FIS | 0.509 | 0.657 | 0.431 |
| FST | 0.261 | 0.533 | 0.036 |
| Parameters . | Distylous . | Homostylous . | P-value . |
|---|---|---|---|
| AR | 6.007 | 3.197 | 0.014 |
| HE | 0.670 | 0.414 | 0.012 |
| FIS | 0.509 | 0.657 | 0.431 |
| FST | 0.261 | 0.533 | 0.036 |
Mixed populations were treated as distylous populations.
The two-sided P-values were obtained after 1000 permutations.
AR, allele richness; HE, expected heterozygosity; FIS, inbreeding index; FST, overall genetic differentiation among populations.
Comparison of genetic diversity and differentiation among distylous (n = 7) and homostylous (n = 6) populations of Primula oreodoxa
| Parameters . | Distylous . | Homostylous . | P-value . |
|---|---|---|---|
| AR | 6.007 | 3.197 | 0.014 |
| HE | 0.670 | 0.414 | 0.012 |
| FIS | 0.509 | 0.657 | 0.431 |
| FST | 0.261 | 0.533 | 0.036 |
| Parameters . | Distylous . | Homostylous . | P-value . |
|---|---|---|---|
| AR | 6.007 | 3.197 | 0.014 |
| HE | 0.670 | 0.414 | 0.012 |
| FIS | 0.509 | 0.657 | 0.431 |
| FST | 0.261 | 0.533 | 0.036 |
Mixed populations were treated as distylous populations.
The two-sided P-values were obtained after 1000 permutations.
AR, allele richness; HE, expected heterozygosity; FIS, inbreeding index; FST, overall genetic differentiation among populations.
Genetic relationships among distylous and homostylous populations
For the plastid DNA data set, rpoB–trnC and trnD–trnT sequences were combined and aligned with a consensus length of 1812 bp. A total of 21 haplotypes were identified from the 66 polymorphisms. The geographical distribution of the haplotypes and their frequencies within populations are illustrated in Fig. 2A. Nucleotide sequences for the two cpDNA regions have been deposited in GenBank (accession nos. MF590428-MF590695). Population differentiation indicated by analysis of molecular variance (AMOVA) was high (FST = 0.898, P < 0.01), and relatively few haplotypes were shared among populations (Table 1; Fig. 2). Three haplotypes (H4, 12 and 16) were shared between two populations (JCC and DFD, JCC and QLP, and XXC and LWP, respectively), whereas all other haplotypes were unique to a single population (CSQ, DWS, ELS, HZG, HZS, LB, QCS, TTS and WWS), three of which were homostylous.
Network analysis revealed that haplotypes were organized into three groups (Fig. 2B). H17 from tetraploid population QCS formed a single group genetically distinct from the other haplotypes. H4, 5, 8, 10, 11, 18 and 20 formed a second group connected to each other by 1–7 mutational steps, and the remaining 14 haplotypes formed a third group connected to each other by <15 mutational steps. Phylogenetic trees constructed using MP and Bayesian methods were largely congruent in topology with that of network analysis (Supplementary Data Fig. S6). Posterior probabilities and bootstrap values are presented in the strict consensus tree. All haplotypes clustered into three clades each containing homostyles.
DISCUSSION
Our investigations of Primula oreodoxa have revealed several features that differ from classic textbook descriptions of heterostyly based on P. vulgaris (e.g. Roughgarden, 1979; Silvertown and Charlesworth, 2001; Charlesworth and Charlesworth, 2010). Our controlled crosses of the distylous morphs revealed the absence of a strong diallelic incompatibility system; the plants we tested were thoroughly self-compatible (Supplementary Data Tables S3 and S4). Also, in contrast to most Primula species which are usually either distylous or homostylous, populations of P. oreodoxa exhibited a wide range of floral morph structures, including distylous populations with varying morph frequencies, mixtures of distylous and homostylous morphs and pure homostylous populations (Fig. 2). Based on general trends in Primula indicating multiple evolutionary origins of homostyly from heterostyly (Mast et al., 2006), and theoretical (Charlesworth and Charlesworth, 1979) and empirical studies of the breakdown process in other Primula species (e.g. Crosby, 1949; Bodmer, 1960; Zhou et al., 2017), the variation in floral morph structure of P. oreodoxa populations is best interpreted as representing stages in the shift from outbreeding to inbreeding as a result of homostyle evolution. Evidence reviewed below supports this hypothesis. Our discussion commences with an evaluation of the salient features of the reproductive biology of distylous and homostylous populations and their influence on mating patterns and population genetic structure. We then consider ecological mechanisms that may have resulted in the dissolution of floral polymorphism, and conclude by identifying several unresolved issues concerning the evolution of homostyly.
Salient features of distyly and homostyly and their mating consequences
Our measurements of variation in sex organ traits and pollen size in distylous populations of P. oreodoxa revealed typical expressions of the floral polymorphisms comprising the distylous syndrome. Stigma and anther heights were reciprocally positioned in the L- and S-morph (Fig. 3), and pollen size relationships were those commonly reported, with the L-morph possessing significantly smaller pollen than the S-morph (Supplementary Data Fig. S4). Measurements of sex organ position in homostylous populations (Fig. 3) confirmed that plants possessed stigmas and anthers of similar height, consistent with their status as long homostyles, the predominant form of homostyly in Primula (Charlesworth and Charlesworth, 1979; de Vos et al., 2014; Zhou et al., 2017). The pollen of P. oreodoxa homostyles was equivalent in size to pollen of long-level anthers of the S-morph, a pattern also consistent with long homostyly (Fig. S4). Thus, in these aspects of floral biology, P. oreodoxa resembles the distyly and homostyly first described in Primula vulgaris and several other Primula species (Darwin, 1877).
Most distylous Primula species examined experimentally possess a diallelic incompatibility system, although its strength may vary between the floral morphs (Wedderburn and Richards, 1990). In contrast, our controlled pollinations and measurements of pollen tube growth revealed that P. oreodoxa was thoroughly self-compatible. Seed set was equivalent from self- and intermorph pollinations (Supplementary Data Table S4), and pollen tube growth in styles was similar following intramorph vs. intermorph pollinations (Fig. 4). Unexpectedly, crosses with the related P. obconica revealed the existence of a residual dimorphic incompatibility system in long homostyles (Supplementary Data Table S6). This suggests that the current self-compatible status of P. oreodoxa is most probably a derived condition owing to weakening of self-incompatibility, as often occurs in heterostylous taxa (reviewed in Barrett and Cruzan, 1994). Unfortunately, our crosses with P. obconica did not involve the distylous morphs of P. oreodoxa; therefore, it is not known if such crosses would also reveal evidence of residual incompatibility in these morphs.
The occurrence of a high degree of self-fertility in P. oreodoxa has potentially important consequences for the mating system of populations. In species with strong diallelic incompatibility, enforced mating between floral morphs (disassortative mating) results in an isoplethic equilibrium of 1:1 as a result of negative frequency-dependent selection. However, if self and intramorph (assortative) mating are permitted, floral morph ratios can deviate significantly from isoplethy, depending on the relative amounts of assortative mating in populations (Baker et al., 2000). Although floral morphology functioning in the absence of intramorph incompatibility promotes some degree of disassortative mating in species with stylar polymorphisms (Kohn and Barrett, 1990; Simón-Porcar et al., 2014; Zhou et al., 2015), deviations from symmetrical disassortative mating can lead to a wide range of floral morph frequencies (reviewed in Barrett and Hodgins, 2006). Significantly, in our study, morph ratios in each of the four distylous populations of P. oreodoxa deviated from isoplethy, with two exhibiting a significant excess of the S-morph, perhaps owing to significant amounts of selfing in this morph (Supplementary Data Table S2). Morph-specific differences in selfing and intramorph mating may contribute to the biased morph ratios observed in distylous populations. A recent study of P. chungensis, a montane species endemic to the Tibetan plateau and Sichuan province of China, also reports similar patterns of morph frequency variation including distylous, homostylous and mixed populations (Zhou et al., 2017). Significantly, these authors also report that P. chungensis is strongly self-compatible. In heterostylous species lacking strong self and intramorph incompatibility, mating patterns will be particularly sensitive to demographic and environmental factors influencing outcross pollen dispersal.
Our comparison of floral traits in distylous and homostylous flowers revealed several functionally significant differences likely to influence the pollination and mating biology of the floral morphs. For example, there was a major difference in the degree of herkogamy between distylous and homostylous flowers. Stigmas and anthers were separated by similar distances (approx. 4 mm) in the L- and S-morphs, whereas herkogamy was much reduced (<0.4 mm) in the H-morph (Supplementary Data Fig. S3). Variation in herkogamy is an important predictor of the mating system in self-compatible species (Breese, 1959; Ganders et al., 1985; Belaoussoff and Shore, 1995; Takebayashi et al., 2006) as it largely determines the facility for autonomous self-pollination (Barrett and Shore, 1987; Brunet and Eckert, 1998; de Vos et al., 2012). Our field experiments using caged flowers demonstrated striking differences in the capacity of distylous vs. homostylous flowers to set seed by autonomous self-pollination (Fig. 5). Caged distylous flowers set virtually no fruit, whereas the fertility of homostylous flowers was only marginally reduced compared with open pollination. Under ecological conditions in which pollinator service is unreliable, the homostylous morph of P. oreodoxa should have a reproductive advantage over distylous morphs, with autonomous self-pollination providing reproductive assurance.
Increased inbreeding in homostyles of P. oreodoxa may also be associated with their reduced corolla size compared with flowers from distylous populations (Supplementary Data Fig. S2). Transitions to homostyly in heterostylous groups are frequently accompanied by reductions in flower size (e.g. Li and Johnston, 2001; de Vos et al., 2014), a pattern that is commonly correlated with the evolution of increased selfing rates in angiosperms (Sicard and Lenhard, 2011). However, the degree of flower size reduction accompanying transitions to homostyly in Primula is determined by a variety of factors including the age of homostyles, the history and intensity of inbreeding, and the opportunities for outcrossing in habitats that homostyles occupy (de Vos et al., 2014; Zhou et al., 2017). The relatively small reduction in flower size in P. oreodoxa that we report suggests that homostylous populations may also benefit from some degree of outcrossing when pollinators are available in populations.
Ecological conditions favouring homostyle evolution
Alpine and sub-alpine environments are generally characterized by unpredictable climatic conditions, shorter flowering seasons and a pollinator fauna that is often depauperate compared with lowland elevations (Arroyo et al., 1985, 2006; Warren et al., 1988; Korner, 2003). Such conditions favour floral mechanisms that provide reproductive assurance when unsatisfactory pollinator service limits outcrossed seed set. Indeed, it has been suggested that in Primula homostyly may be favoured over distyly in alpine and arctic environments because of the ability of homostyles to self-pollinate when pollinator service limits seed production (Richard, 2003; Guggisberg et al., 2006; Carlson et al., 2008; de Vos et al., 2012). Our investigations of insect visitation to populations of P. oreodoxa situated at varying elevations provided some support for the hypothesis that pollination conditions deteriorate with increased elevation. We found that there was a significant decline in visits of long-tongued pollinators (Bombus, Anthophora, Bombyliidae and Pieris melete) to flowers at higher elevation; whereas visitation by short-tongued pollinators (halictid bees and syrphid flies) was unaffected by the elevation of populations (Fig. 7). In addition, both types of pollinators were significantly less abundant in homostylous populations compared with distylous populations (Fig. 6). It is unclear why pollinator activity was significantly lower in homostylous populations compared with distylous populations. Among the ten populations chosen for pollinator observations, there was no evidence that the size or elevation of the two classes of population differed substantially.
Long-tongued pollinators are generally considered more effective pollinators of heterostylous plants. This is because they are usually capable of contacting both long- and short-level sex organs, thus mediating disassortative pollen transfer, a requirement for maintaining the floral polymorphism. Moreover, the stereomorphic, depth-probed, tubular flowers with concealed nectar at their base that characterize most heterostylous species, including Primula, restrict insect feeding posture, and the path followed by the pollinator’s probe results in more precise pollen transfer between anthers and stigmas (Lloyd and Webb, 1992, p. 200, fig. 1; Stone and Thomson, 1994; Washitani et al., 1995; Santos-Gally et al., 2013). Therefore, the loss of ‘high quality’ long-tongued pollinators at higher elevations has the potential to influence mating patterns and fertility in distylous populations. Transplant studies of the floral morphs of P. oreodoxa and measurements of pollination, fertility and mating along elevational gradients would be required to susbtantiate whether homostyles have a reproductive advantage at sites where distylous morphs are absent.
Our estimates of inbreeding, based on FIS and s calculated from microsatellite data indicated that both measures were postively associated with elevation. These results are consistent with the hypothesis that increased elevation is associated with greater inbreeding in P. oreodoxa populations. These results would be predicted by the hypothesis that homostyles are more likely to self-pollinate if pollinator service is unreliable, and that their frequency increases with the elevation of populations. However, there are several problems with this simple cause and effect explanation. First, although there were significant differences between distylous and homostylous populations in the amounts and distribution of genetic diversity (Table 2), a result consistent with greater inbreeding in homostylous populations, we detected no significant differences between the two groups of populations in FIS and s, an unexpected result if there were substantial differences in the selfing rates of populations. Secondly, although there was a general tendency for increased homostyle frequency with elevation, based on our DCA, inspection of Fig. 2 and Table 1 indicates that both distylous and homostylous populations are scattered throughout the range of elevations sampled, and there is no simple elevational segregation of the mating system morphs. The absence of clear-cut differences in FIS and s between distylous and homostylous populations could be because homostyles at lower elevation sites where pollinators are more abundant outcross to varying degrees and, similarly, distylous populations owing to their self-compatible status may experience pollinator-mediated intraflower and geitonogamous pollination at higher elevations where long-tongued pollinators are less abundant. In addition, our measures of selfing based on FIS should be treated with some caution because they represent relatively coarse and indirect estimates of the true amount of self-fertilization in populations. Other factors, including biparental inbreeding and Wahlund effects caused by population sub-division, also influence estimates of FIS in populations. Ideally, direct marker-based measurements of the selfing rate, based on open-pollinated families, would be necessary to estimate mating patterns in populations more accurately.
Phylogeographic patterns and origins of homostyly
Among the 14 populations of P. oreodoxa, we detected a total of 21 cpDNA haplotypes clustered into three groups, with most haplotypes restricted to a single population (Fig. 2A, B; Supplementary Data Fig. S6). H17 formed a distinct group representing the single tetraploid, homostylous population (QCS). This haplotype could not be connected to other haplotypes in the network analysis because of its distinct genetic distance. This is not unexpected as gene flow between tetraploid and diploid populations is likely to be infrequent. The remaining diploid individuals were split between two major lineages both of which were comprised of distylous and homostylous populations (Fig. 2B; Fig. S6). Thus, homostylous populations occur at both diploid and tetraploid levels, and there is no evidence of a clear association between tetraploidy and the breakdown of distyly (see also Guggisberg et al., 2006; Naiki, 2012). The dispersed distribution of homostyles among three clades of P. oreodoxa suggests that homostyly has probably originated from distyly on at least three occasions.
Both the network and tree topologies we obtained (Fig. 2B; Supplementary Data Fig. S6) showed little geographical clustering of cpDNA haplotypes, and no obvious population structure was evident. For example, DFD contained H4 and 5 from one of the large clades, but JCC also contained H4, and three haplotypes from the other large clade. QLP possessed two haplotypes each from different clades (Fig. S6). Two scenarios could explain these patterns. The first is long-distance seed dispersal, with self-compatibility aiding establishment (Baker, 1955). However, seeds of P. oreodoxa are predominantly dispersed by gravity and occasionally by flowing streams. The rugged mountainous terrain of the region occupied by P. oreodoxa may limit extensive long-distance dispersal, and the occurrence of many haplotypes each isolated to a single population supports limited gene flow. The relatively high level of genetic differentiation among populations (FST = 0.898) is also consistent with restricted gene flow. A more probable explanation for the patterns we observed is in situ preservation of haplotype diversity, probably following range contraction or fragmentation of a previously wider distribution. Thus, climate oscillations during glacial periods and the complex local topography of the ‘Sichuan Basin’ located just east of the Tibetan (Qinghai–Xizang) Plateau may be largely responsible for the phylogeographic patterns we detected in P. oreodoxa, as has also been proposed for P. ovalifolia (Xie et al., 2012) and P. obconica (Yan et al., 2012).
A final question that arises from our study concerns the genetic mechanism(s) responsible for homostyle evolution. Until recently, it has been assumed that homostyles in Primula arise from rare recombination events, within the S-locus linkage group (supergene) governing distyly (see Charlesworth and Charlesworth 1979), where the L-morph is homozygous recessive (ss) and the S-morph is governed by a dominant S-allele (Ss). According to the recombination hypothesis, long homostyles combine, in a single phenotype, the long styles of the L-morph and the long stamens of the S-morph, and are therefore fully self-compatible (Ernst, 1955; Dowrick, 1956; Barrett and Shore, 2008). Theoretical models provide a satisfying explanation as to why long homostyles are much more frequent than short homostyles in Primula and other heterostylous groups (Charlesworth and Charlesworth, 1979). As a result of the dominance and compatibility relationships of outcrossing and selfing morphs, long homostyles have a genetic transmission advantage through male function compared with short homostyles and should therefore spread preferentially when they arise.
Recent studies of the genetic architecture of the distyly linkage group in Primula indicate that the S-locus supergene is composed of a tightly linked cluster of S-morph-specific genes that are absent in the L-morph. Thus, S-morph plants are hemizygous not heterozygous at the S-locus (Huu et al., 2016; Li et al., 2016; Burrows and McCubbin, 2017). This finding suggests that long homostyles are more likely to have arisen by mutation of component genes in the linkage group rather than recombination (Li et al., 2016; Burrows and McCubbin, 2017). It is conceivable, given the large size of the genus Primula (approx. 430 species), that different genetic mechanisms are responsible for homostyle origins in different Primula species, although phylogenetic reconstructions support a single origin of distyly (Mast et al., 2006). Clearly, further comparative molecular work on the Primula supergene is needed to investigate this problem. With its range of population morph structures and reproductive phenotypes, P. oreodoxa offers a valuable experimental system for investigating both the ecological and the genetic mechanisms driving transitions from outcrossing to selfing.
SUPPLEMENTARY DATA
Supplementary data are available online at https://dbpia.nl.go.kr/aob and consist of the following. Table S1: variation in floral traits of Primula oreodoxa pooled across morph structures. Table S2: G-tests of goodness-of-fit of morph ratios in four distylous populations of Primula oreodoxa. Table S3: mean percentage fruit set and 95 % confidence intervals following controlled hand pollinations of floral morphs in a distylous and homostylous population of Primula oreodoxa. Table S4: mean seed set per fruit and 95 % confidence intervals following controlled hand pollinations of floral morphs in a distylous and homostylous population of Primula oreodoxa. Table S5: comparisons of mean pollen tube (± s.d.) length (μm) between intermorph and intramorph pollinations in distylous plants, and between outcrossing and selfing in homostylous plants of Primula oreodoxa after four time intervals (4, 24, 48 and 72 h). Table S6: mean pollen tube length (± s.d.) (μm) in Primula oreodoxa × P. obconica, P. oreodoxa × P. oreodoxa and P. obconica × P. obconica crosses 72 h after pollination. Table S7: mean fruit set and seed set (± s.d.) of pollination treatments in three homostylous and one distylous population of Primula oreodoxa. Table S8: mean fruit set and seed set (± s.d.) following open pollination in seven populations of Primula oreodoxa. Table S9 The genetic diversity (± s.d.) of 13 Primula oreodoxa populations in western Sichuan based on polymorphism at SSR loci. Figure S1: chromosomes of mitotic metaphase in Primula oreodoxa. Figure S2: (A) Variation in corolla size and (B) floral tube length among the floral morphs of Primula oreodoxa. Figure S3: stigma–anther separation (herkogamy) of the floral morphs of Primula oreodoxa. Figure S4: distribution of the equatorial axis and polar axis of pollen in the three floral morphs of Primula oreodoxa. Figure S5: pollen tube growth following intramorph, intermorph, cross- and self-pollination in Primula oreodoxa and P. obconica. Figure S6: strict consensus tree of the 21 cpDNA haplotypes of Primula oreodoxa with Primula heucherifolia used as the outgroup.
ACKNOWLEDGEMENTS
This study was financially supported by the National Natural Science Foundation of China (grants U1202261, U1603231 to D.X.Z., 31400209 to M.M.S.). S.C.H.B.’s collaborations in China were funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada. We thank Professor Xiaoli Tong (South China Agricultural University) for identifying insects, Ms Peixing Li, Mr Fei Cui, Mr Lianxuan Zhou, Ms Yuanqing Xu and Dr Yuan Xu (SCBG) for field assistance and/or helping with sample collections, Dr Ming Tang (SCBG) for assistance in chromosome counts, and Dr Zhonglai Luo (SCBG) for useful discussion.



