-
PDF
- Split View
-
Views
-
Cite
Cite
Kathryn Richardson, Katharina Mattishent, Yoon K Loke, Nicholas Steel, Chris Fox, Carlota M Grossi, Kathleen Bennett, Ian Maidment, Malaz Boustani, Fiona E Matthews, Phyo K Myint, Noll L Campbell, Carol Brayne, Louise Robinson, George M Savva, History of Benzodiazepine Prescriptions and Risk of Dementia: Possible Bias Due to Prevalent Users and Covariate Measurement Timing in a Nested Case-Control Study, American Journal of Epidemiology, Volume 188, Issue 7, July 2019, Pages 1228–1236, https://doi.org/10.1093/aje/kwz073
Close - Share Icon Share
Abstract
Previous estimates of whether long-term exposure to benzodiazepines increases dementia risk are conflicting and are compromised by the difficulty of controlling for confounders and by reverse causation. We investigated how estimates for the association between benzodiazepine use and later dementia incidence varied based on study design choices, using a case-control study nested within the United Kingdom’s Clinical Practice Research Datalink. A total of 40,770 dementia cases diagnosed between April 2006 and July 2015 were matched on age, sex, available data history, and deprivation to 283,933 control subjects. Benzodiazepines and Z-drug prescriptions were ascertained in a drug-exposure period 4–20 years before dementia diagnosis. Estimates varied with the inclusion of new or prevalent users, with the timing of covariate ascertainment, and with varying time between exposure and outcome. There was no association between any new prescription of benzodiazepines and dementia (adjusted odds ratio (OR) = 1.03, 95% confidence interval (CI): 1.00, 1.07), whereas an inverse association was observed among prevalent users (adjusted OR = 0.91, 95% CI: 0.87, 0.95), although this was likely induced by unintentional adjustment for colliders. By considering the choice of confounders and timing of exposure and covariate measurement, our findings overall are consistent with no causal effect of benzodiazepines or Z-drugs on dementia incidence.
Dementia prevention is a public health priority. More than 152 million people are expected to be living with dementia by 2050. Dementia is recognized as a leading cause of disability, is the fifth most important cause of death, and has a global economic cost of US$1 trillion (1, 2). There is no curative or disease-modifying treatment for dementia, increasing the importance of identifying its risk factors (3). Authors of several studies have suggested that long-term benzodiazepine use could increase dementia risk (4). If true, this is an important opportunity to prevent dementia, because 9% of older US adults currently use benzodiazepines, with 31% of these being long-term users (5, 6).
Benzodiazepines, including diazepam (Valium; Hoffmann-La Roche Inc, Little Falls, New Jersey) and alprazolam (Xanax; Pfizer Inc, New York, New York) are the most commonly prescribed sedatives and are typically used for insomnia or anxiety. Despite years of guidance advising against long-term benzodiazepine use, because of adverse effects, addiction, and tolerance (7), there has been no decline in their use in the past decade in the United States (8–10), whereas a small decline in the United Kingdom has been accompanied by greater use of benzodiazepine-related drugs, including zopiclone, (e.g., Lunesta; Sunovion Pharmaceuticals Inc. Marlborough, Massachusetts), zolpidem (e.g., Ambien; Sanofi-Aventis U.S. LLC, Bridgewater, New Jersey), and zaleplon (e.g., Sonata; Wyeth Pharmaceuticals Inc., Philadelphia, Pennsylvania), collectively known as Z-drugs (11).
Benzodiazepines and Z-drugs have dose-related effects on memory and other aspects of cognitive function (12, 13). However, no biological mechanism has been demonstrated to underlie any link to dementia incidence. Although increased risks of dementia with long-term benzodiazepine use (14–16) have been suggested on the basis of studies using insurance records and epidemiologic cohort studies, no association was noted in other recent studies (17, 18). These conflicting results may reflect genuine differences across populations, or different study designs, availability and use of covariate data, or analysis parameters, such as minimum time lag between exposure and outcomes (19, 20).
It is not practical or ethical to randomly assign patients to receive benzodiazepine treatment to estimate harms; therefore, observational studies are central to addressing this important question. Individual, patient-level data sets exist that include detailed histories of benzodiazepine use going back years or decades, details of diagnoses and treatment for cognitive disorders, and records of many possible confounding variables for this relationship. However, several factors complicate any analysis. Benzodiazepine treatment often is initiated before records for a patient begin, precluding the use of the “new-user” design (21). This is particularly true for those with very long-term use, who may be most at risk (22). Second, the main indications for benzodiazepines—anxiety and sleep disturbance—are both risk factors for and prodromal symptoms of neurodegenerative disease that may occur many years before dementia diagnosis, necessitating a lag period to avoid protopathic bias (4). Furthermore, dates associated with diagnoses in electronic health records may reflect the time of the underlying event. Together, these factors make the theoretical identification of confounding from mediating or colliding variables, as is often suggested (23, 24), difficult. This is important because valid causal inference relies on the correct identification of and control for confounders (i.e., variables that are common causes of both the exposure and the outcome), but conditioning on mediators (i.e., variables on the causal pathway from exposure to outcome) or on colliders (i.e., common consequences of the exposure and the outcome) will introduce bias rather than reduce it (25).
Case-control studies, in which exposures within an exposure period are compared between cases of a disease and matched controls, are often used for estimating the associations between multiple complex exposures and a single outcome. Case-control studies are used particularly when tackling rare adverse events or adverse events that may only become apparent after long-term exposures. However, selection based on outcome rather than exposure status further complicates the ascertainment of confounders. Clearly, it is optimal to measure potential confounders at treatment initiation (20), but because cases and controls are not matched on exposure, the presence of treatment or time of treatment initiation will vary within a matched set. Hence, it is difficult to know when to optimally ascertain and encode covariates. Measuring covariates recorded only up to the start of an exposure window (possibly years before exposure) risks missing confounders and omitted variable bias, whereas including covariates recorded during or after the exposure window (hence, after the exposure) risks underestimation through unintended adjustment for mediators or colliders (26).
We conducted a case-control study nested within an electronic health record data set as part of a wider project estimating the associations of drug use on dementia incidence (27), and we have explored several of these issues. We present estimates for the association between benzodiazepine and Z-drug prescription and dementia incidence, and explore how these depend on 1) the inclusion or exclusion of prevalent users, 2) the timing of covariate ascertainment, and 3) the minimum lag between treatment and dementia incidence. Finally, we explore the role of specific covariates and implications for the conduct and interpretation of future similar studies.
METHODS
The study was approved by the Independent Scientific Advisory Committee for the Clinical Practice Research Datalink (CPRD) research (protocol no. 15_056R) and was registered on the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance e-register of studies (register no. EUPAS8705). This manuscript has been prepared according to the Reporting of Studies Conducted Using Observational Routinely Collected Data guidelines (28). Code lists for the outcome and covariates are available on request.
Study population and data
CPRD consists of anonymized electronic health records of 17 million patients from 719 general practices and is representative of age, sex, and ethnicity of the United Kingdom population (29). Available data include basic demographics and coded details of consultations, diagnoses, reported symptoms, drug prescriptions, referrals to specialist services, and laboratory test results.
Selection of case patients and control subjects
All cases of dementia recorded in CPRD were indexed at the first mention of dementia as a diagnosis or symptom (see Web Table 1 (available at https://dbpia.nl.go.kr/aje) for complete Read code list) or the first prescription of a dementia drug (i.e., memantine, donepezil, rivastigmine, galantamine, or tacrine) if it was followed by a dementia diagnosis code within 12 months.
Case patients were included in the current study if their index date occurred between April 2006 and July 2015 and the patient was aged between 65 and 99 years on that date. Case patients were excluded if the date of diagnosis was unknown, they had less than 6 years of “up-to-standard” data history before the index date, or had any record of motor neuron disease, human immunodeficiency virus infection, acquired immune deficiency syndrome, multiple sclerosis, Down syndrome, or alcohol abuse.
For each case, up to 7 control subjects without dementia at the index date were randomly selected and matched on sex, year of birth (within 3 years), years of available up-to-standard data history, and index of multiple deprivation quintile. The index of multiple deprivation is a weighted sum of indicators of housing, employment, income, education, living environment, and crime for each neighborhood (30). We used incidence density sampling to select control subjects, hence case patients could also be selected as control subjects up to the date of meeting case criteria.
Exposure assessment
We defined a drug-exposure period (DEP) for each case/control group as the period starting after 1 year of up-to-standard data recorded, and at most 20 years before the index date, and ending 4 years before the index date (Web Figure 1) (31). This 4-year lag reduces the risk of protopathic bias, because the use of benzodiazepines in this period may be a marker of undiagnosed dementia (32).
For all patients, we obtained details of all drugs prescribed before the index date. Our primary exposures were the number of defined daily doses (DDDs) prescribed for benzodiazepines (World Health Organization Anatomical Therapeutic Chemical (ATC) category N05BA, N05CD, or N03AE) and benzodiazepine-related drugs (i.e., Z-drugs; ATC N05CF) during the DEP. The DDD is the assumed average maintenance dose per day for a drug based on its main indication in adults; we used the DDD values assigned by the World Health Organization’s Collaborating Center for Drug Statistics Methodology.
We defined “new users” of benzodiazepines as those prescribed benzodiazepines during the DEP but with no benzodiazepine prescriptions in the 12 months before the DEP, and “prevalent” benzodiazepine users as those prescribed benzodiazepines within both the DEP and the 12 months prior (Web Figure 1). New and prevalent users of Z-drugs were defined similarly.
Covariates
Potential confounders were identified as any known or suspected risk factors for dementia (3, 33) or predictors of benzodiazepine initiation (34, 35). Each covariate was ascertained first using only the patient record up to the start of the DEP and second using the patient record up to the end of the DEP.
The following covariates were measured as binary variables reflecting any history of a diagnosis: diabetes; diabetes complications; hyperlipidemia/dyslipidemia; hypertension; stroke/transient ischemic attack; congestive heart disease; heart failure; peripheral arterial disease; atrial fibrillation; angina; myocardial infarction; coronary artery operations; deep vein thrombosis; depression; urinary incontinence; Parkinson’s disease; severe mental illness; drug abuse; epilepsy; anxiety; anxiety symptoms; insomnia, fatigue, or other sleep problems; migraine; headache; back/neck pain; and neuropathic pain. Depression severity was measured as the maximum record in their history (mild, moderate, or severe), and depression duration, defined as the years since first record of a depression diagnosis or symptom.
The following covariates were measured as recorded in the general practitioner’s records in both the 12 months before the start and the end of the DEP: any fall, any fracture, number of consultations, and any prescription for a selective serotonin reuptake inhibitor (ATC N06AB), tricyclic antidepressant (ATC N06AA), or an antipsychotic (ATC N05A). Smoking status (none, former, current), body mass index (weight (kg)/height (m2); <20, 20–24.9, 25–29.9, ≥30), and harmful alcohol use (>49 units/week for men and >35 units/week for women) were measured according to the latest record.
Statistical analyses
We used conditional logistic regression to estimate the association between categorized DDDs (0, >0–29, 30–364, 365–1,459, or ≥1,460 DDDs) of benzodiazepines and Z-drugs and dementia incidence. Odds ratios and 95% confidence intervals were estimated without adjustment and then separately adjusted for birth year, practice region (Scotland, Northern Ireland, Wales, and 10 health regions of England), and the aforementioned covariates.
To test the impact of covariate ascertainment timing, we estimated 2 sets of models, first including covariates measured at the start of the DEP, and second including covariates measured at the end of the DEP. We then estimated associations among new users and prevalent users compared with nonusers in each case.
The impact of each covariate was measured by the change in the log of the odds ratio induced by adding that covariate to a model only including the exposure stratified into new and prevalent use (36). The impact of each covariate was compared when it was measured at the start or end of the DEP. Confidence intervals were calculated by nonparametric bootstrapping.
Finally, to test whether associations between new use and dementia incidence varied with the time between medication initiation and dementia incidence, we stratified odds ratios for any new prescription and total DDDs during the DEP by time of initiation in 3 periods: 15–20, 10–15, or 5–10 years before dementia (among those with at least 16, 11, and 6 years of up-to-standard data history, respectively). For these analyses, we adjusted for covariates at the later of the interval start date and the DEP start date.
Throughout, multiple imputation via chained equations were used to impute missing values of body mass index, harmful alcohol use, and smoking (37) (see Web Appendix for details of imputation models). We used Stata, version 14.2 (StataCorp LP, College Station, Texas) for all statistical analyses. Statistical significance was determined using 2-tailed tests, with a prespecified threshold of P < 0.01.
RESULTS
Of 66,136 cases of dementia recorded in CPRD between 2006 and 2015, 40,770 met inclusion criteria and were matched to 283,933 control subjects (Web Figure 2). The median (interquartile range) DEP was 7.1 (4.0–11.3) years; median age at index date was 83 (78–87) years, and 63% were women (Table 1).
Sociodemographics and Data History of Dementia Case Patients and Control Subjects in the United Kingdom, April 2006–July 2015
| Characteristic . | Dementia Case Patients (n = 40,770) . | Control Subjects (n = 283,933) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Womena | 25,745 | 63.1 | 179,152 | 63.1 |
| Age at index date, yearsa,b | 82.6 (6.8) | 82.6 (6.8) | ||
| Practice level index of multiple deprivation quintilea | ||||
| 1 (least deprived) | 7,867 | 19.3 | 54,766 | 19.3 |
| 2 | 7,928 | 19.4 | 55,220 | 19.4 |
| 3 | 8,756 | 21.5 | 61,032 | 21.5 |
| 4 | 8,389 | 20.6 | 58,407 | 20.6 |
| 5 (most deprived) | 7,830 | 19.2 | 54,508 | 19.2 |
| Country | ||||
| England | 30,615 | 75.1 | 223,468 | 78.7 |
| Northern Ireland | 1,508 | 3.7 | 8,720 | 3.1 |
| Scotland | 5,024 | 12.3 | 25,793 | 9.1 |
| Wales | 3,623 | 8.9 | 25,952 | 9.1 |
| Drug-exposure period lengtha,c, years | 7.1 (4.0–11.3) | 7.1 (4.0–11.3) | ||
| Characteristic . | Dementia Case Patients (n = 40,770) . | Control Subjects (n = 283,933) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Womena | 25,745 | 63.1 | 179,152 | 63.1 |
| Age at index date, yearsa,b | 82.6 (6.8) | 82.6 (6.8) | ||
| Practice level index of multiple deprivation quintilea | ||||
| 1 (least deprived) | 7,867 | 19.3 | 54,766 | 19.3 |
| 2 | 7,928 | 19.4 | 55,220 | 19.4 |
| 3 | 8,756 | 21.5 | 61,032 | 21.5 |
| 4 | 8,389 | 20.6 | 58,407 | 20.6 |
| 5 (most deprived) | 7,830 | 19.2 | 54,508 | 19.2 |
| Country | ||||
| England | 30,615 | 75.1 | 223,468 | 78.7 |
| Northern Ireland | 1,508 | 3.7 | 8,720 | 3.1 |
| Scotland | 5,024 | 12.3 | 25,793 | 9.1 |
| Wales | 3,623 | 8.9 | 25,952 | 9.1 |
| Drug-exposure period lengtha,c, years | 7.1 (4.0–11.3) | 7.1 (4.0–11.3) | ||
a Matching variables.
b Values are expressed as mean (standard deviation).
c Values are expressed as median (interquartile range).
Sociodemographics and Data History of Dementia Case Patients and Control Subjects in the United Kingdom, April 2006–July 2015
| Characteristic . | Dementia Case Patients (n = 40,770) . | Control Subjects (n = 283,933) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Womena | 25,745 | 63.1 | 179,152 | 63.1 |
| Age at index date, yearsa,b | 82.6 (6.8) | 82.6 (6.8) | ||
| Practice level index of multiple deprivation quintilea | ||||
| 1 (least deprived) | 7,867 | 19.3 | 54,766 | 19.3 |
| 2 | 7,928 | 19.4 | 55,220 | 19.4 |
| 3 | 8,756 | 21.5 | 61,032 | 21.5 |
| 4 | 8,389 | 20.6 | 58,407 | 20.6 |
| 5 (most deprived) | 7,830 | 19.2 | 54,508 | 19.2 |
| Country | ||||
| England | 30,615 | 75.1 | 223,468 | 78.7 |
| Northern Ireland | 1,508 | 3.7 | 8,720 | 3.1 |
| Scotland | 5,024 | 12.3 | 25,793 | 9.1 |
| Wales | 3,623 | 8.9 | 25,952 | 9.1 |
| Drug-exposure period lengtha,c, years | 7.1 (4.0–11.3) | 7.1 (4.0–11.3) | ||
| Characteristic . | Dementia Case Patients (n = 40,770) . | Control Subjects (n = 283,933) . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| Womena | 25,745 | 63.1 | 179,152 | 63.1 |
| Age at index date, yearsa,b | 82.6 (6.8) | 82.6 (6.8) | ||
| Practice level index of multiple deprivation quintilea | ||||
| 1 (least deprived) | 7,867 | 19.3 | 54,766 | 19.3 |
| 2 | 7,928 | 19.4 | 55,220 | 19.4 |
| 3 | 8,756 | 21.5 | 61,032 | 21.5 |
| 4 | 8,389 | 20.6 | 58,407 | 20.6 |
| 5 (most deprived) | 7,830 | 19.2 | 54,508 | 19.2 |
| Country | ||||
| England | 30,615 | 75.1 | 223,468 | 78.7 |
| Northern Ireland | 1,508 | 3.7 | 8,720 | 3.1 |
| Scotland | 5,024 | 12.3 | 25,793 | 9.1 |
| Wales | 3,623 | 8.9 | 25,952 | 9.1 |
| Drug-exposure period lengtha,c, years | 7.1 (4.0–11.3) | 7.1 (4.0–11.3) | ||
a Matching variables.
b Values are expressed as mean (standard deviation).
c Values are expressed as median (interquartile range).
By definition, the proportion of patients with a history of each clinical condition increased over the DEP (Web Table 2). For example, up to the start of their DEP, 25,870 patients (8%) had a diagnosis of anxiety and 21,347 (7%) had insomnia. By the end of the DEP this had increased to 41,788 (13%) and 52,578 (16%), respectively. Case patients were more likely than control subjects to have a history of cardiovascular disease and depression, and to visit their general practitioner more frequently.
Among the case patients, 8,010 (20%) were ever prescribed benzodiazepines and 3,130 (8%) were prescribed Z-drugs during their DEP, compared with 52,017 (18%) and 19,163 (7%) of the control subjects, respectively. The 5 most common prescriptions were for temazepam (32% of all benzodiazepine or Z-drug prescriptions), zopiclone (19%), diazepam (18%), nitrazepam (14%), and lorazepam (5%). See Web Table 3 for details of prescribing patterns.
Association between benzodiazepine prescriptions and dementia incidence
The unadjusted odds ratio for dementia and any prescription of a benzodiazepine was 1.09 (95% CI: 1.06, 1.12), but there was little suggestion of a dose-response relationship with the number of DDDs (Table 2). Adjusting for covariates measured at the start of the DEP led to an inverse association between benzodiazepines and dementia (for ≥4 years of DDDs, OR = 0.88, 95% CI: 0.82, 0.95). When adjusting for covariates measured at the end of the DEP, the inverse association appeared stronger (for any use, OR = 0.81, 95% CI: 0.75, 0.87).
Association Between Benzodiazepine Prescriptions and Dementia, by Defined Daily Doses, New or Prevalent Use, and When Covariates Were Measured, in a Nested Case-Control Study in the United Kingdom, December 1988–July 2015
| No. of Benzodiazepine DDDs During DEP . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Measured at Start of DEP . | Measured at End of DEP . | |||
|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | aORa . | 95% CI . | |||
| All users | ||||||||
| Any benzodiazepine prescription | 8,010 | 52,017 | 1.09b | 1.06, 1.12 | 0.99 | 0.96, 1.02 | 0.89b | 0.86, 0.92 |
| DDDs during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 3,949 | 25,390 | 1.10b | 1.07, 1.14 | 1.02 | 0.99, 1.06 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,998 | 12,516 | 1.13b | 1.08, 1.19 | 1.01 | 0.96, 1.06 | 0.88b | 0.84, 0.93 |
| 365–1,459 | 1,143 | 7,775 | 1.04 | 0.98, 1.11 | 0.92 | 0.86, 0.98 | 0.84b | 0.78, 0.89 |
| ≥1,460 | 920 | 6,336 | 1.03 | 0.96, 1.11 | 0.88b | 0.82, 0.95 | 0.81b | 0.75, 0.87 |
| Users stratified by new and prevalent use | ||||||||
| Any benzodiazepine prescription during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Any prescription by new users | 5,058 | 32,245 | 1.11b | 1.08, 1.15 | 1.03 | 1.00, 1.07 | 0.91b | 0.88, 0.95 |
| Any prescription by prevalent users | 2,952 | 19,772 | 1.06b | 1.02, 1.10 | 0.91b | 0.87, 0.95 | 0.85b | 0.81, 0.89 |
| DDDs during DEP | ||||||||
| None | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Within new users | ||||||||
| 0.1–29 | 3,568 | 23,103 | 1.10b | 1.06, 1.14 | 1.02 | 0.99, 1.07 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,135 | 6,987 | 1.15b | 1.08, 1.23 | 1.05 | 0.98, 1.12 | 0.88b | 0.82, 0.94 |
| 365–1,459 | 269 | 1,567 | 1.22b | 1.07, 1.39 | 1.10 | 0.96, 1.25 | 0.94 | 0.82, 1.07 |
| ≥1,460 | 86 | 588 | 1.04 | 0.83, 1.30 | 0.96 | 0.76, 1.20 | 0.84 | 0.67, 1.05 |
| Within prevalent users | ||||||||
| 0.1–29 | 381 | 2,287 | 1.18b | 1.06, 1.32 | 1.00 | 0.89, 1.12 | 0.93 | 0.83, 1.04 |
| 30–364 | 863 | 5,529 | 1.10b | 1.03, 1.19 | 0.97 | 0.90, 1.04 | 0.89b | 0.83, 0.96 |
| 365–1,459 | 874 | 6,208 | 1.00 | 0.93, 1.07 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
| ≥1,460 | 834 | 5,748 | 1.03 | 0.96, 1.11 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
| No. of Benzodiazepine DDDs During DEP . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Measured at Start of DEP . | Measured at End of DEP . | |||
|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | aORa . | 95% CI . | |||
| All users | ||||||||
| Any benzodiazepine prescription | 8,010 | 52,017 | 1.09b | 1.06, 1.12 | 0.99 | 0.96, 1.02 | 0.89b | 0.86, 0.92 |
| DDDs during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 3,949 | 25,390 | 1.10b | 1.07, 1.14 | 1.02 | 0.99, 1.06 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,998 | 12,516 | 1.13b | 1.08, 1.19 | 1.01 | 0.96, 1.06 | 0.88b | 0.84, 0.93 |
| 365–1,459 | 1,143 | 7,775 | 1.04 | 0.98, 1.11 | 0.92 | 0.86, 0.98 | 0.84b | 0.78, 0.89 |
| ≥1,460 | 920 | 6,336 | 1.03 | 0.96, 1.11 | 0.88b | 0.82, 0.95 | 0.81b | 0.75, 0.87 |
| Users stratified by new and prevalent use | ||||||||
| Any benzodiazepine prescription during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Any prescription by new users | 5,058 | 32,245 | 1.11b | 1.08, 1.15 | 1.03 | 1.00, 1.07 | 0.91b | 0.88, 0.95 |
| Any prescription by prevalent users | 2,952 | 19,772 | 1.06b | 1.02, 1.10 | 0.91b | 0.87, 0.95 | 0.85b | 0.81, 0.89 |
| DDDs during DEP | ||||||||
| None | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Within new users | ||||||||
| 0.1–29 | 3,568 | 23,103 | 1.10b | 1.06, 1.14 | 1.02 | 0.99, 1.07 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,135 | 6,987 | 1.15b | 1.08, 1.23 | 1.05 | 0.98, 1.12 | 0.88b | 0.82, 0.94 |
| 365–1,459 | 269 | 1,567 | 1.22b | 1.07, 1.39 | 1.10 | 0.96, 1.25 | 0.94 | 0.82, 1.07 |
| ≥1,460 | 86 | 588 | 1.04 | 0.83, 1.30 | 0.96 | 0.76, 1.20 | 0.84 | 0.67, 1.05 |
| Within prevalent users | ||||||||
| 0.1–29 | 381 | 2,287 | 1.18b | 1.06, 1.32 | 1.00 | 0.89, 1.12 | 0.93 | 0.83, 1.04 |
| 30–364 | 863 | 5,529 | 1.10b | 1.03, 1.19 | 0.97 | 0.90, 1.04 | 0.89b | 0.83, 0.96 |
| 365–1,459 | 874 | 6,208 | 1.00 | 0.93, 1.07 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
| ≥1,460 | 834 | 5,748 | 1.03 | 0.96, 1.11 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DDD, defined daily dose; DEP, drug exposure period; OR, odds ratio.
a Adjusted for all variables in Table 1 and Web Table 2.
bP < 0.01.
Association Between Benzodiazepine Prescriptions and Dementia, by Defined Daily Doses, New or Prevalent Use, and When Covariates Were Measured, in a Nested Case-Control Study in the United Kingdom, December 1988–July 2015
| No. of Benzodiazepine DDDs During DEP . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Measured at Start of DEP . | Measured at End of DEP . | |||
|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | aORa . | 95% CI . | |||
| All users | ||||||||
| Any benzodiazepine prescription | 8,010 | 52,017 | 1.09b | 1.06, 1.12 | 0.99 | 0.96, 1.02 | 0.89b | 0.86, 0.92 |
| DDDs during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 3,949 | 25,390 | 1.10b | 1.07, 1.14 | 1.02 | 0.99, 1.06 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,998 | 12,516 | 1.13b | 1.08, 1.19 | 1.01 | 0.96, 1.06 | 0.88b | 0.84, 0.93 |
| 365–1,459 | 1,143 | 7,775 | 1.04 | 0.98, 1.11 | 0.92 | 0.86, 0.98 | 0.84b | 0.78, 0.89 |
| ≥1,460 | 920 | 6,336 | 1.03 | 0.96, 1.11 | 0.88b | 0.82, 0.95 | 0.81b | 0.75, 0.87 |
| Users stratified by new and prevalent use | ||||||||
| Any benzodiazepine prescription during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Any prescription by new users | 5,058 | 32,245 | 1.11b | 1.08, 1.15 | 1.03 | 1.00, 1.07 | 0.91b | 0.88, 0.95 |
| Any prescription by prevalent users | 2,952 | 19,772 | 1.06b | 1.02, 1.10 | 0.91b | 0.87, 0.95 | 0.85b | 0.81, 0.89 |
| DDDs during DEP | ||||||||
| None | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Within new users | ||||||||
| 0.1–29 | 3,568 | 23,103 | 1.10b | 1.06, 1.14 | 1.02 | 0.99, 1.07 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,135 | 6,987 | 1.15b | 1.08, 1.23 | 1.05 | 0.98, 1.12 | 0.88b | 0.82, 0.94 |
| 365–1,459 | 269 | 1,567 | 1.22b | 1.07, 1.39 | 1.10 | 0.96, 1.25 | 0.94 | 0.82, 1.07 |
| ≥1,460 | 86 | 588 | 1.04 | 0.83, 1.30 | 0.96 | 0.76, 1.20 | 0.84 | 0.67, 1.05 |
| Within prevalent users | ||||||||
| 0.1–29 | 381 | 2,287 | 1.18b | 1.06, 1.32 | 1.00 | 0.89, 1.12 | 0.93 | 0.83, 1.04 |
| 30–364 | 863 | 5,529 | 1.10b | 1.03, 1.19 | 0.97 | 0.90, 1.04 | 0.89b | 0.83, 0.96 |
| 365–1,459 | 874 | 6,208 | 1.00 | 0.93, 1.07 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
| ≥1,460 | 834 | 5,748 | 1.03 | 0.96, 1.11 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
| No. of Benzodiazepine DDDs During DEP . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Measured at Start of DEP . | Measured at End of DEP . | |||
|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | aORa . | 95% CI . | |||
| All users | ||||||||
| Any benzodiazepine prescription | 8,010 | 52,017 | 1.09b | 1.06, 1.12 | 0.99 | 0.96, 1.02 | 0.89b | 0.86, 0.92 |
| DDDs during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 3,949 | 25,390 | 1.10b | 1.07, 1.14 | 1.02 | 0.99, 1.06 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,998 | 12,516 | 1.13b | 1.08, 1.19 | 1.01 | 0.96, 1.06 | 0.88b | 0.84, 0.93 |
| 365–1,459 | 1,143 | 7,775 | 1.04 | 0.98, 1.11 | 0.92 | 0.86, 0.98 | 0.84b | 0.78, 0.89 |
| ≥1,460 | 920 | 6,336 | 1.03 | 0.96, 1.11 | 0.88b | 0.82, 0.95 | 0.81b | 0.75, 0.87 |
| Users stratified by new and prevalent use | ||||||||
| Any benzodiazepine prescription during DEP | ||||||||
| 0 | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Any prescription by new users | 5,058 | 32,245 | 1.11b | 1.08, 1.15 | 1.03 | 1.00, 1.07 | 0.91b | 0.88, 0.95 |
| Any prescription by prevalent users | 2,952 | 19,772 | 1.06b | 1.02, 1.10 | 0.91b | 0.87, 0.95 | 0.85b | 0.81, 0.89 |
| DDDs during DEP | ||||||||
| None | 32,760 | 231,916 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Within new users | ||||||||
| 0.1–29 | 3,568 | 23,103 | 1.10b | 1.06, 1.14 | 1.02 | 0.99, 1.07 | 0.92b | 0.89, 0.96 |
| 30–364 | 1,135 | 6,987 | 1.15b | 1.08, 1.23 | 1.05 | 0.98, 1.12 | 0.88b | 0.82, 0.94 |
| 365–1,459 | 269 | 1,567 | 1.22b | 1.07, 1.39 | 1.10 | 0.96, 1.25 | 0.94 | 0.82, 1.07 |
| ≥1,460 | 86 | 588 | 1.04 | 0.83, 1.30 | 0.96 | 0.76, 1.20 | 0.84 | 0.67, 1.05 |
| Within prevalent users | ||||||||
| 0.1–29 | 381 | 2,287 | 1.18b | 1.06, 1.32 | 1.00 | 0.89, 1.12 | 0.93 | 0.83, 1.04 |
| 30–364 | 863 | 5,529 | 1.10b | 1.03, 1.19 | 0.97 | 0.90, 1.04 | 0.89b | 0.83, 0.96 |
| 365–1,459 | 874 | 6,208 | 1.00 | 0.93, 1.07 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
| ≥1,460 | 834 | 5,748 | 1.03 | 0.96, 1.11 | 0.87b | 0.81, 0.94 | 0.81b | 0.75, 0.87 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DDD, defined daily dose; DEP, drug exposure period; OR, odds ratio.
a Adjusted for all variables in Table 1 and Web Table 2.
bP < 0.01.
New versus prevalent users of benzodiazepines
Of those prescribed benzodiazepines, 37,303 patients (62%) were new users during the DEP, whereas 22,724 (38%) were prevalent users (Table 2). New users had shorter average exposures to benzodiazepines during the DEP than prevalent users, who represented most cases of chronic use. Among new users, there was little evidence of an association between benzodiazepines and dementia incidence when adjusted for covariates measured at the start of the DEP (hence, adjusted for factors recorded before medication initiation; OR = 1.03, 95% CI: 1.00, 1.07), but the negative association was still apparent among prevalent users (OR = 0.91, 95% CI: 0.87, 0.95), for whom the start of the DEP was after medication initiation. When adjusted for covariates measured at the end of the DEP, a negative association was seen for both new use (OR = 0.91, 95% CI: 0.88, 0.95) and prevalent use (OR = 0.85, 95% CI: 0.81, 0.89) of benzodiazepines.
Impact of each covariate
The number of physician consultations, anxiety, insomnia, depression, and antidepressant prescriptions each substantially modified the estimated association between benzodiazepine use and dementia incidence when added to the conditional logistic regression models, whereas other factors did not (Web Figure 3 and Web Table 4). Covariates modified the association more when measured at the end of the DEP; patterns were similar for prevalent and incident use.
Proximity between exposure and outcome
New use of benzodiazepines was not significantly associated with an increased risk of dementia regardless of whether the first prescription was 5–10, 10–15, or 15–20 years before dementia (Table 3). Although estimates are imprecise, associations did appear to increase with closer proximity between exposure initiation and outcome.
Association Between New Benzodiazepine Prescriptions and Dementia, According to When the New Prescription Was Issued, in a Nested Case-Control Study in the United Kingdom, December 1988–July 2015
| No. of DDDs . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Adjusted for Covariates Measured at Start of DEP . | ||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | |||
| New use initiated 15–20 years priorb | ||||||
| Benzodiazepine prescription | ||||||
| No | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| Yes | 560 | 2,916 | 1.06 | 0.97, 1.17 | 0.98 | 0.89, 1.08 |
| DDDs during DEP | ||||||
| 0 | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 283 | 1,646 | 0.96 | 0.84, 1.09 | 0.90 | 0.79, 1.02 |
| 30–364 | 201 | 863 | 1.27c | 1.09, 1.49 | 1.16 | 0.99, 1.36 |
| 365–1,459 | 43 | 232 | 1.02 | 0.74, 1.42 | 0.91 | 0.65, 1.27 |
| ≥1,460 | 33 | 175 | 1.02 | 0.70, 1.48 | 0.97 | 0.66, 1.41 |
| New use initiated 10–15 years priord | ||||||
| Benzodiazepine prescription | ||||||
| No | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| Yes | 1,316 | 6,741 | 1.12c | 1.05, 1.19 | 1.01 | 0.95, 1.08 |
| DDDs during DEP | ||||||
| 0 | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 849 | 4,304 | 1.14c | 1.05, 1.22 | 1.03 | 0.95, 1.11 |
| 30–364 | 322 | 1,756 | 1.05 | 0.93, 1.18 | 0.93 | 0.82, 1.05 |
| 365–1,459 | 107 | 464 | 1.32 | 1.06, 1.63 | 1.17 | 0.95, 1.45 |
| ≥1,460 | 38 | 217 | 0.99 | 0.70, 1.40 | 0.92 | 0.65, 1.30 |
| New use initiated 5–10 years priore | ||||||
| Benzodiazepine prescription | ||||||
| No | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| Yes | 2,564 | 13,636 | 1.14c | 1.09, 1.19 | 1.03 | 0.99, 1.08 |
| DDDs during DEP | ||||||
| 0 | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 1,904 | 10,289 | 1.12c | 1.07, 1.18 | 1.03 | 0.98, 1.08 |
| 30–364 | 528 | 2,707 | 1.18c | 1.08, 1.30 | 1.04 | 0.94, 1.14 |
| 365–1,459 | 117 | 568 | 1.23 | 1.01, 1.50 | 1.08 | 0.88, 1.32 |
| ≥1,460 | 15 | 72 | 1.26 | 0.72, 2.20 | 1.16 | 0.66, 2.02 |
| No. of DDDs . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Adjusted for Covariates Measured at Start of DEP . | ||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | |||
| New use initiated 15–20 years priorb | ||||||
| Benzodiazepine prescription | ||||||
| No | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| Yes | 560 | 2,916 | 1.06 | 0.97, 1.17 | 0.98 | 0.89, 1.08 |
| DDDs during DEP | ||||||
| 0 | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 283 | 1,646 | 0.96 | 0.84, 1.09 | 0.90 | 0.79, 1.02 |
| 30–364 | 201 | 863 | 1.27c | 1.09, 1.49 | 1.16 | 0.99, 1.36 |
| 365–1,459 | 43 | 232 | 1.02 | 0.74, 1.42 | 0.91 | 0.65, 1.27 |
| ≥1,460 | 33 | 175 | 1.02 | 0.70, 1.48 | 0.97 | 0.66, 1.41 |
| New use initiated 10–15 years priord | ||||||
| Benzodiazepine prescription | ||||||
| No | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| Yes | 1,316 | 6,741 | 1.12c | 1.05, 1.19 | 1.01 | 0.95, 1.08 |
| DDDs during DEP | ||||||
| 0 | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 849 | 4,304 | 1.14c | 1.05, 1.22 | 1.03 | 0.95, 1.11 |
| 30–364 | 322 | 1,756 | 1.05 | 0.93, 1.18 | 0.93 | 0.82, 1.05 |
| 365–1,459 | 107 | 464 | 1.32 | 1.06, 1.63 | 1.17 | 0.95, 1.45 |
| ≥1,460 | 38 | 217 | 0.99 | 0.70, 1.40 | 0.92 | 0.65, 1.30 |
| New use initiated 5–10 years priore | ||||||
| Benzodiazepine prescription | ||||||
| No | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| Yes | 2,564 | 13,636 | 1.14c | 1.09, 1.19 | 1.03 | 0.99, 1.08 |
| DDDs during DEP | ||||||
| 0 | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 1,904 | 10,289 | 1.12c | 1.07, 1.18 | 1.03 | 0.98, 1.08 |
| 30–364 | 528 | 2,707 | 1.18c | 1.08, 1.30 | 1.04 | 0.94, 1.14 |
| 365–1,459 | 117 | 568 | 1.23 | 1.01, 1.50 | 1.08 | 0.88, 1.32 |
| ≥1,460 | 15 | 72 | 1.26 | 0.72, 2.20 | 1.16 | 0.66, 2.02 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DDD, defined daily dose; DEP, drug exposure period; OR, odds ratio.
a Adjusted for all variables in Table 1 and Web Table 2.
b Including patients with ≥16 years of up-to-standard data history before the index date.
cP < 0.01.
d Including patients with ≥11 years of up-to-standard data history before the index date. Start of period defined by the later of the start of the DEP and 15 years before the index date.
e Including patients with ≥6 years of up-to-standard data history before the index date. Start of period defined by the later of the start of the DEP and 10 years before the index date.
Association Between New Benzodiazepine Prescriptions and Dementia, According to When the New Prescription Was Issued, in a Nested Case-Control Study in the United Kingdom, December 1988–July 2015
| No. of DDDs . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Adjusted for Covariates Measured at Start of DEP . | ||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | |||
| New use initiated 15–20 years priorb | ||||||
| Benzodiazepine prescription | ||||||
| No | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| Yes | 560 | 2,916 | 1.06 | 0.97, 1.17 | 0.98 | 0.89, 1.08 |
| DDDs during DEP | ||||||
| 0 | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 283 | 1,646 | 0.96 | 0.84, 1.09 | 0.90 | 0.79, 1.02 |
| 30–364 | 201 | 863 | 1.27c | 1.09, 1.49 | 1.16 | 0.99, 1.36 |
| 365–1,459 | 43 | 232 | 1.02 | 0.74, 1.42 | 0.91 | 0.65, 1.27 |
| ≥1,460 | 33 | 175 | 1.02 | 0.70, 1.48 | 0.97 | 0.66, 1.41 |
| New use initiated 10–15 years priord | ||||||
| Benzodiazepine prescription | ||||||
| No | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| Yes | 1,316 | 6,741 | 1.12c | 1.05, 1.19 | 1.01 | 0.95, 1.08 |
| DDDs during DEP | ||||||
| 0 | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 849 | 4,304 | 1.14c | 1.05, 1.22 | 1.03 | 0.95, 1.11 |
| 30–364 | 322 | 1,756 | 1.05 | 0.93, 1.18 | 0.93 | 0.82, 1.05 |
| 365–1,459 | 107 | 464 | 1.32 | 1.06, 1.63 | 1.17 | 0.95, 1.45 |
| ≥1,460 | 38 | 217 | 0.99 | 0.70, 1.40 | 0.92 | 0.65, 1.30 |
| New use initiated 5–10 years priore | ||||||
| Benzodiazepine prescription | ||||||
| No | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| Yes | 2,564 | 13,636 | 1.14c | 1.09, 1.19 | 1.03 | 0.99, 1.08 |
| DDDs during DEP | ||||||
| 0 | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 1,904 | 10,289 | 1.12c | 1.07, 1.18 | 1.03 | 0.98, 1.08 |
| 30–364 | 528 | 2,707 | 1.18c | 1.08, 1.30 | 1.04 | 0.94, 1.14 |
| 365–1,459 | 117 | 568 | 1.23 | 1.01, 1.50 | 1.08 | 0.88, 1.32 |
| ≥1,460 | 15 | 72 | 1.26 | 0.72, 2.20 | 1.16 | 0.66, 2.02 |
| No. of DDDs . | No. of Case Patients . | No. of Control Subjects . | Unadjusted . | Adjusted for Covariates Measured at Start of DEP . | ||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | aORa . | 95% CI . | |||
| New use initiated 15–20 years priorb | ||||||
| Benzodiazepine prescription | ||||||
| No | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| Yes | 560 | 2,916 | 1.06 | 0.97, 1.17 | 0.98 | 0.89, 1.08 |
| DDDs during DEP | ||||||
| 0 | 7,747 | 43,261 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 283 | 1,646 | 0.96 | 0.84, 1.09 | 0.90 | 0.79, 1.02 |
| 30–364 | 201 | 863 | 1.27c | 1.09, 1.49 | 1.16 | 0.99, 1.36 |
| 365–1,459 | 43 | 232 | 1.02 | 0.74, 1.42 | 0.91 | 0.65, 1.27 |
| ≥1,460 | 33 | 175 | 1.02 | 0.70, 1.48 | 0.97 | 0.66, 1.41 |
| New use initiated 10–15 years priord | ||||||
| Benzodiazepine prescription | ||||||
| No | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| Yes | 1,316 | 6,741 | 1.12c | 1.05, 1.19 | 1.01 | 0.95, 1.08 |
| DDDs during DEP | ||||||
| 0 | 18,097 | 105,328 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 849 | 4,304 | 1.14c | 1.05, 1.22 | 1.03 | 0.95, 1.11 |
| 30–364 | 322 | 1,756 | 1.05 | 0.93, 1.18 | 0.93 | 0.82, 1.05 |
| 365–1,459 | 107 | 464 | 1.32 | 1.06, 1.63 | 1.17 | 0.95, 1.45 |
| ≥1,460 | 38 | 217 | 0.99 | 0.70, 1.40 | 0.92 | 0.65, 1.30 |
| New use initiated 5–10 years priore | ||||||
| Benzodiazepine prescription | ||||||
| No | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| Yes | 2,564 | 13,636 | 1.14c | 1.09, 1.19 | 1.03 | 0.99, 1.08 |
| DDDs during DEP | ||||||
| 0 | 31,471 | 191,614 | 1.00 | Referent | 1.00 | Referent |
| 0.1–29 | 1,904 | 10,289 | 1.12c | 1.07, 1.18 | 1.03 | 0.98, 1.08 |
| 30–364 | 528 | 2,707 | 1.18c | 1.08, 1.30 | 1.04 | 0.94, 1.14 |
| 365–1,459 | 117 | 568 | 1.23 | 1.01, 1.50 | 1.08 | 0.88, 1.32 |
| ≥1,460 | 15 | 72 | 1.26 | 0.72, 2.20 | 1.16 | 0.66, 2.02 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; DDD, defined daily dose; DEP, drug exposure period; OR, odds ratio.
a Adjusted for all variables in Table 1 and Web Table 2.
b Including patients with ≥16 years of up-to-standard data history before the index date.
cP < 0.01.
d Including patients with ≥11 years of up-to-standard data history before the index date. Start of period defined by the later of the start of the DEP and 15 years before the index date.
e Including patients with ≥6 years of up-to-standard data history before the index date. Start of period defined by the later of the start of the DEP and 10 years before the index date.
Z-drug prescriptions and dementia
Of those prescribed Z-drugs, 18,704 (84%) patients were new users during the DEP, while 3,589 (16%) patients had received prescriptions during the DEP and additionally in the previous 12 months. There was a positive association between Z-drugs and dementia incidence without adjusting for covariates. No association was observed when adjusting for covariates measured at the start of the DEP, and evidence of a negative association was observed when adjusting for covariates measured after the DEP (Web Tables 5 and 6). The pattern of the impact of individual covariates was almost identical for Z-drugs and benzodiazepines, with depression, antidepressant use, physician consultations, anxiety, and insomnia having the greatest impact on estimated associations (Web Figure 4 and Web Table 7). As with benzodiazepines, this impact was up to 3 or 4 times greater when covariates were measured at the end of the DEP compared with the start of the DEP and was consistent for prevalent and new users of Z-drugs.
DISCUSSION
Associations between benzodiazepine and Z-drug prescriptions and dementia incidence depend on the timing of covariate ascertainment and whether prevalent or only new use is considered. When covariates were only measured before exposure, associations were typically null or slightly positive. When covariates were included in the models that may have occurred before or after initiation of drug exposure, associations were typically negative. Taken together, our results suggest no causal link between benzodiazepines or Z-drug use and later dementia incidence, that any positive association is an artefact of either inadequate control of confounding factors or protopathic bias, and any negative association is the result of adjusting for colliders in regression models.
In every case, adjustment for depression, anxiety, antidepressant use, insomnia, fatigue, and number of recent physician visits had the most impact on our estimates. No other covariate substantially affected the relationships in any analysis. As well as being possible indications for benzodiazepines, depression, anxiety, and sleep disturbance are known symptoms of dementia and are suspected risk factors (3). Therefore, there are several equally plausible explanations for the observed relationships between these variables in our study. Figure 1 illustrates confounding, reverse causation, colliding, and mediating relationships. Panels A and B of Figure 1 show the importance of controlling for neuropsychiatric symptoms; panels C and D illustrate the danger in doing this, because the record of neuropsychiatric symptoms may act as a collider or a mediator. Note that in each case, neuropsychiatric symptoms might equally be recorded before or after the measured exposure and so these scenarios cannot be definitively distinguished theoretically or empirically.
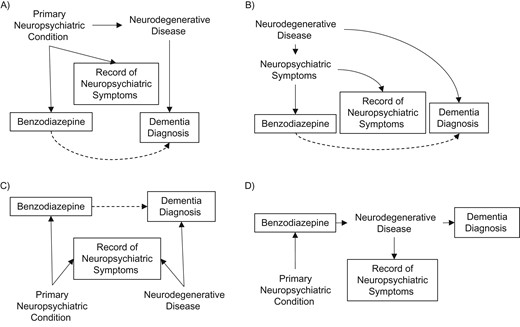
Directed acyclic graphs in a nested case-control study in the United Kingdom, December 1988–July 2015, illustrating theoretically plausible relationships between psychiatric conditions, benzodiazepine prescription (exposure), neurodegenerative disease, and the record of psychiatric symptoms (measured covariate that might be caused by a primary neuropsychiatric condition or a latent neurodegenerative disease) and dementia diagnosis (outcome). Solid outlines indicate observed variables. Dashed lines indicate false associations induced by omitted variable bias: A) confounding by indication; B) reverse causation; and C) adjusting for a collider. D) In the case of a genuine relationship between benzodiazepines and dementia, “record of neuropsychiatric symptoms” after treatment initiation may reflect a mediator of the relationship.
Nevertheless, by varying the timing of covariate ascertainment compared with the timing to treatment initiation, we can place reasonable bounds on causal associations by considering whether each analysis is more likely to under- or overestimate it. For new users, the start of the DEP might be some time before treatment initiation, and so controlling for covariates measured up to this time risks residual confounding and overestimating associations. Covariates measured at the end of the DEP may have occurred after treatment and so including these risks underestimation. For prevalent users, any measured covariate may have occurred after treatment initiation, hence underestimation is more likely, whereas univariable analyses and those with short lag times are likely to lead to overestimation.
The key strengths of our study include the detailed evaluation of the impact of varying study design parameters and use of an exposure period up to 20 years before diagnosis of dementia for a significant number of cases. Diagnosis of dementia in CPRD has been validated with a positive predictive value of 95% (2). The available data allowed us to carefully consider the roles of a wide range of potential covariates measured at different points. Measurement of exposure was based on prescription rather than use, but these are likely to be similar, particularly for chronic users.
Substantively, our study updates and builds on the findings of Imfeld et al. (18), who reported no significant association with benzodiazepine or Z-drug use and risk of dementia, also based on a case-control study nested within CPRD, although using a period for case ascertainment that began before financial incentives for the accurate recording of dementia diagnoses in primary care in the United Kingdom. Gray et al. (17) reported a small association among low users that was not observed when the lag period was extended beyond 2 years, again suggesting no causal link. Authors for a Swiss study also reported no association between benzodiazepine prescriptions and new dementia medication prescriptions, despite only allowing a 2-year lag period (38).
Study design choices might explain previously reported positive associations between benzodiazepine use and dementia. In 2 studies that did not apply any lag between exposure and outcome, causal effect likely was overestimated (14, 16). In a study based on a Canadian insurance claims database, a significant association was reported with a dose-response relationship (15). Although they controlled for anxiety and sleep disturbance, the authors did not have any record of these indications for most users, suggesting that the control of confounding factors was inadequate.
Inclusion of prevalent users in pharmacoepidemiologic studies is challenging. However, authors of previous studies examining benzodiazepine use and dementia incidence reported only slightly smaller associations with prevalent use compared with new use (14, 39).
In summary, we found no evidence that benzodiazepine or Z-drug use is associated with risk of dementia. However, because benzodiazepines have known adverse effects, including falls and sedation, and lead to tolerance (6), prescribers should continue to follow guidelines on avoiding or limiting their use.
Our study reinforces the challenges in estimating associations between long-term cumulative exposures and adverse events with long latent or prodromal periods, particularly where indications for the exposure are also prodromal symptoms of the outcome. Nevertheless, these remain important questions that observational studies provide almost the only opportunity to answer, and so these challenges must be addressed. Investigators should carefully consider the causal framework for potential covariates, when measured at different time points and among prevalent and new users, and should be mindful that prodromal periods for neurodegenerative diseases could be extremely long. Given the inherent difficulty of measuring confounders and of separating confounding from mediating or colliding effects in these cases, the aim for observational studies should not necessarily be to provide single unbiased effect estimates, but such studies can provide robust upper or lower bounds on effect sizes, depending on study design, that should be considered alongside other forms of evidence using, for example, a triangulation framework to narrow the range of plausibly true causal effects (40).
ACKNOWLEDGMENTS
Author affiliations: School of Health Sciences, University of East Anglia, Norwich, United Kingdom (Kathryn Richardson, Carlota M. Grossi, George M. Savva); Norwich Medical School, University of East Anglia, Norwich, United Kingdom (Katharina Mattishent, Yoon K. Loke, Nicholas Steel, Chris Fox); Division of Population Health Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland (Kathleen Bennett); School of Life and Health Sciences, Aston University, Birmingham, United Kingdom (Ian Maidment); School of Medicine, Indiana University, Indianapolis, Indiana (Malaz Boustani); Institute of Health and Society/Institute for Ageing, Newcastle University, Newcastle, United Kingdom (Fiona E. Matthews, Louise Robinson); School of Medicine, Medical Sciences & Nutrition, University of Aberdeen, Aberdeen, United Kingdom (Phyo K. Myint); Department of Pharmacy Practice, College of Pharmacy, Purdue University, Lafayette, Indiana (Noll L. Campbell); and Cambridge Institute of Public Health, University of Cambridge, Cambridge, United Kingdom (Carol Brayne).
This research was supported by funding from the UK Alzheimer’s Society (grant AS-PG-2013-017).
We thank Tarita Murray-Thomas (Medicines and Healthcare Products Regulatory Agency) for extracting the Clinical Practice Research Datalink data, Keshav Bajaj (Aston University) for assisting with medication coding, and Barry Plumpton, Ann McLauchlan, Barbara di Vita, and Gloria Swan for providing much appreciated assistance in interpretation and oversight as Alzheimer’s Society Research Network volunteers.
This work was presented as a poster at the annual meeting of the British Pharmacological Society, London, United Kingdom, December 13–15, 2016.
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The interpretation and conclusions contained in this report are those of the authors alone.
Conflict of interest: none declared.
Abbreviations
- ATC
Anatomical Therapeutic Chemical
- CI
confidence interval
- CPRD
Clinical Practice Research Datalink
- DDD
defined daily dose
- DEP
drug exposure period
- OR
odds ratio




