-
PDF
- Split View
-
Views
-
Cite
Cite
Siaw Li Chan, Xin Yi, Emily Wysocki, Rachael Bridgman, Jocelyn Gutierrez, Krzysztof Mikrut, Edward Ki-Yun Leung, Kiang-Teck J Yeo, Jonathan L Miller, Development of a Nonradioactive Platelet Serotonin Uptake and Release Assay by Micro-Liquid Chromatography Tandem Mass Spectrometry Using Minimal Blood Volume, American Journal of Clinical Pathology, Volume 152, Issue 6, December 2019, Pages 718–724, https://doi.org/10.1093/ajcp/aqz094
Close - Share Icon Share
Abstract
Analysis of platelet functional responses to stimuli is presently quite limited with respect to measurement of dense granule secretion. We sought to develop a nonradioactive assay of stimulated serotonin release using liquid chromatography tandem mass spectrometry (LC-MS/MS).
Citrated whole blood (200 μL) was incubated with deuterated serotonin (d45-HT). Following uptake by platelets, blood was diluted 10-fold and aliquots were incubated with platelet stimuli. Following stimulation, blood was further diluted, centrifuged, and supernatant was assayed for released d45-HT by micro-LC–MS/MS.
This study demonstrated a broad linear range of 50 to 2,000 pg/mL d45-HT, with a total precision of less than 15.0% coefficient of variation at all quality control levels and a limit of quantitation of 50 pg/mL.
Quantification of d45-HT by micro-LC–MS/MS assay offers a highly sensitive, nonradioactive methodology for quantitating platelet serotonin uptake and dense granule secretion, requiring only small volumes of patient blood.
Platelets play a critical role in the maintenance of vascular integrity.1-4 Identifying an actual abnormality of platelet function that may be contributing to increased bleeding in the individual patient, however, is not an easy task,5 particularly when only very small quantities of blood may be available. Such abnormalities include a wide range of both inherited and acquired platelet disorders.4,6,7
Light transmission aggregometry (LTA) is widely considered to be the gold standard methodology for the assessment of platelet function.8 LTA measures changes in light transmittance that reflect platelet activation-induced aggregation when an agonist is added to a stirred platelet suspension. LTA provides useful information pertaining to platelet shape change, lag phase for aggregation, maximal rate and extent of aggregation, as well as reversal of aggregation. Inferences as to dense granule secretion may sometimes be made based on these traces, but more definitive assessment requires the use of aggregometers equipped with a luminescence channel capable of directly monitoring dense granule release of ATP.
LTA requires laborious preparation of platelet-rich plasma8,9 and typically requires a significant volume of patient blood in order to employ a standardized panel of platelet agonists. In the case of pediatric patients, the volume of the blood draw itself may limit the availability of such testing, particularly in the neonatal period. Moreover, because LTA is inherently dependent upon the frequency of physical platelet collisions in a stirred suspension, the reliability of this methodology progressively decreases with increasing degrees of thrombocytopenia, becoming largely unusable at platelet counts under 100,000/μL.10,11
Accordingly, there is an unmet need to obtain clinically relevant information pertaining to platelet function from small amounts of patient blood. Functional flow cytometry for the analysis of platelet response to a variety of stimuli has been employed for decades in research settings and increasingly has been introduced into some clinical laboratories. This approach typically minimizes time-consuming platelet preparation, instead simply utilizing dilutions of citrated whole blood. The most commonly measured platelet responses are activation of the platelet glycoprotein IIb/IIIa (GPIIb/IIIa) fibrinogen receptor and the release of α-granules (reflected by the extracellular translocation of P-selectin from the α-granule membranes). Such approaches, however, leave unresolved the potentially vital clinical question of whether or not there may be an abnormality of dense granule release from the platelets.12 The present study seeks to fill this gap, through development of a highly sensitive micro-liquid chromatography tandem mass spectrometry (LC-MS/MS) method that can quantitatively measure deuterated serotonin as a marker of platelet dense granule release using only a minimal amount of blood.
Materials and Methods
Materials
High-performance liquid chromatography (HPLC)-grade H2O, HPLC-grade acetonitrile, and HPLC-grade methanol were obtained from Fisher Scientific. Methyl-5-hydroxytryptamine oxalate salt (m-5-HT), L-ascorbic acid, 70% perchloric acid, thrombin receptor activating peptide (TRAP), and the thromboxane A2 analogue U46619 were purchased from Sigma-Aldrich. The GPVI collagen receptor activator, convulxin, was purchased from Santa Cruz Biotechnology. Deuterated serotonin-α,α,β,β-d4 creatine sulfate complex (d45-HT) was purchased from C/D/N Isotopes. m-5-HT and d45-HT have reported chemical and isotopic purity of greater than 98% and were used without further purification. Solid-phase extraction cartridges were purchased from Phenomenex.
Preparation of Modified Tyrode’s Buffer
Modified Tyrode’s buffer (MTB) was prepared as 1 L of H2O containing 10 mmol/L Hepes, 137 mmol/L NaCl, 2.8 mmol/L KCL, 1 mmol/L MgCl2, 12 mmol/L NaHCO3, 0.4 mmol/L Na2HPO4.7H2O, 0.35% w/v bovine serum albumin, 5.5 mmol/L glucose, pH adjusted to 7.4.
Preparation of Calibrators, Internal Standard, and Quality Control Materials
Stock solutions of d45-HT (1 mg/mL) and internal standard (IS) m-5-HT (1 mg/mL) were prepared by dissolving the lyophilized compounds in HPLC-grade H2O. Working solutions of varying d45-HT concentrations for both calibrators and quality control (QC) materials were prepared similarly, but separately, by diluting the stock solution with 100% methanol (MeOH). Seven calibrator concentrations (50, 75, 100, 250, 500, 1,000, and 2,000 pg/mL) and three concentrations of QC materials (50, 500, and 1,500 pg/mL) were prepared using appropriate dilutions of the working and stock solutions.
Platelet Stimulation
Blood was obtained from eight healthy adult volunteers in accordance with procedures approved and ethical standards established by the institutional review board of the University of Chicago Medical Center. Six of these donors were not currently taking any medication. The remaining two donors disclosed that they were currently taking selective serotonin reuptake inhibitors (SSRIs). Blood (1.8 mL) was drawn into a pediatric-size light blue-top tube containing 0.2 mL of 3.2% sodium citrate and gently mixed. From this tube 190 μL was taken and incubated with 10 μL of 4 μg/mL d45-HT for 15 minutes at 37°C. Following d45-HT uptake, the citrated blood was diluted 10-fold with MTB buffer. Separate aliquots (100 μL each) of this further-diluted blood were incubated with platelet stimuli for 10 minutes at 37°C without stirring, in an effort to minimize the effect of platelet count upon dense granule secretion. Following preliminary experiments with a variety of potential stimuli, TRAP, U46619, and convulxin were found to produce the most consistent d45-HT release signals under the nonstirring conditions employed and were accordingly selected as stimuli for these studies. Following stimulation, the blood was diluted an additional 10-fold with MTB buffer, 400 µL of 100 µg/mL prostaglandin E1 added, and the sample was then centrifuged for 10 minutes at 570g to obtain a supernatant of releasate. Total d45-HT uptake was determined by lysis of unstimulated platelets similarly diluted with MTB buffer.
Sample Preparation for LC-MS/MS
Aliquots of each calibrator and QC (350 µL) were transferred into 1.5-mL microcentrifuge tubes followed by addition of 25 µL IS working solution. For sample extraction, 10 µL of 50 mg/mL ascorbic acid was added to all samples followed by 5 µL of 70% perchloric acid. Samples were subjected to protein cleanup using Strata-X polymeric reversed-phase cartridges. Samples were finally eluted with 500 µL of MeOH containing 2% formic acid. Eluate from the extract was evaporated under nitrogen gas at 37°C, and the residue was resuspended in 100 µL of MeOH/H2O (5/95) (v/v) containing 0.1% formic acid. Samples were vigorously vortex mixed before centrifugation at 300g for 5 minutes. After the final centrifugation of samples in microcentrifuge tubes, samples were transferred to amber vials with tapered glass inserts and capped for instrument analysis.
Micro-LC–MS/MS Instrumentation
A QTRAP 6500 triple-quadrupole mass spectrometer (SCIEX) equipped with an IonDrive Turbo V source was operated in positive electrospray ionization mode. An Eksigent Ekspert micro-LC 200 system (SCIEX) with a CTC PAL autosampler provided chromatographic separation using a reversed-phase C18 analytical column (YMC Triart; 0.5 mm, 50 mm, and 3 µm particle size). The mobile phases consisted of water with 0.1% formic acid (phase A) and MeOH with 0.1% formic acid (phase B). The micro-LC system was programmed to deliver a flow rate of 25 µL/min starting with 2% mobile phase B for 0.5 minutes, linear gradient to 95% mobile phase B between 0.5 and 1.5 minutes, held steady at 95% mobile phase B for 1.8 minutes, followed by re-equilibration to initial condition. The full LC run was 4 minutes. The autosampler injection syringe and valve were washed eight times after each injection with acetonitrile and water. The QTRAP 6500 instrument settings were optimized for d45-HT signal. The electrospray ionization source was operated with ion spray voltage at 4,000 V at a temperature of 200°C; declustering potential was set at 25 V and entrance potential was 10 V. A high-purity nitrogen generator (Parker Balston) provided nitrogen gas with the following settings: curtain gas, 60 psi; source gas, 60 psi; and gas1/gas 2, 100 psi. Two mass transitions were monitored for d45-HT (quantifier, 181.1/164; qualifier, 181/118), and one transition was used to monitor IS (191/115). Other instrument settings optimized for each transition are shown in Table 1. All MS data were acquired and processed with Analyst 1.6.2 software. Calibration curves were constructed by plotting the peak area ratio between d45-HT and IS vs the concentration of d45-HT in calibrators, using the MultiQuant 3.0 software (SCIEX).
| Compound . | Q1, m/z . | Q3, m/z . | Collision Energy, V . | Collision Cell Exit Potential, V . | Dwell Time, ms . | Declustering Potential, V . |
|---|---|---|---|---|---|---|
| d45-HT | 181.10 | 164.00 | 14 | 10 | 75 | 25 |
| d45-HT | 181.00 | 118.00 | 40 | 10 | 75 | 25 |
| m-5-HT | 191.10 | 115.00 | 39 | 10 | 25 | 31 |
| Compound . | Q1, m/z . | Q3, m/z . | Collision Energy, V . | Collision Cell Exit Potential, V . | Dwell Time, ms . | Declustering Potential, V . |
|---|---|---|---|---|---|---|
| d45-HT | 181.10 | 164.00 | 14 | 10 | 75 | 25 |
| d45-HT | 181.00 | 118.00 | 40 | 10 | 75 | 25 |
| m-5-HT | 191.10 | 115.00 | 39 | 10 | 25 | 31 |
d45-HT, deuterated serotonin; m-5-HT, methyl-5-hydroxytryptamine oxalate salt; Q1, m/z, precursor ion, mass/charge ratio; Q3, m/z, product ion, mass/charge ratio.
| Compound . | Q1, m/z . | Q3, m/z . | Collision Energy, V . | Collision Cell Exit Potential, V . | Dwell Time, ms . | Declustering Potential, V . |
|---|---|---|---|---|---|---|
| d45-HT | 181.10 | 164.00 | 14 | 10 | 75 | 25 |
| d45-HT | 181.00 | 118.00 | 40 | 10 | 75 | 25 |
| m-5-HT | 191.10 | 115.00 | 39 | 10 | 25 | 31 |
| Compound . | Q1, m/z . | Q3, m/z . | Collision Energy, V . | Collision Cell Exit Potential, V . | Dwell Time, ms . | Declustering Potential, V . |
|---|---|---|---|---|---|---|
| d45-HT | 181.10 | 164.00 | 14 | 10 | 75 | 25 |
| d45-HT | 181.00 | 118.00 | 40 | 10 | 75 | 25 |
| m-5-HT | 191.10 | 115.00 | 39 | 10 | 25 | 31 |
d45-HT, deuterated serotonin; m-5-HT, methyl-5-hydroxytryptamine oxalate salt; Q1, m/z, precursor ion, mass/charge ratio; Q3, m/z, product ion, mass/charge ratio.
Analytical Performance and Validation
Precision, Analytical Measuring Range, and Limit of Quantification Studies
Precision studies of this method were performed using in-house prepared QC materials. Assay repeatability was determined by consecutively measuring three levels of QC materials (50, 500, and 1,500 pg/mL) by performing 20 injections (five replicates of each QC sample were prepared and four injections were made from each replicate). Carryover was assessed during the within-day repeatability test. Reproducibility was determined by daily testing of the QC materials in duplicates over 20 days. The limit of quantification (LOQ) was assessed by running a series of diluted QC materials of various d45-HT concentrations over a period of 10 days and the coefficient of variation (CV) at 20% was used as the cutoff to define LOQ.
Matrix Effect and Recovery Studies
Evaluation of ionization suppression or enhancement was performed by postextraction spike matrix comparison.13 Three sets of samples were created: set A was prepared using standard dissolved in MeOH; set B was prepared by spiking extracted MTB buffer with d45-HT (postextraction spiked samples); and set C was made by spiking MTB buffer with the d45-HT before extraction (preextraction spiked samples). Matrix effect (ME) is expressed as a ratio of the mean peak area of an analyte in postextraction spiked sample (set B) to the mean peak area of the same analyte in standard solution (set A), multiplied by 100 (% ME = B/A × 100). Recovery of extraction (RE) is defined as a ratio of the mean peak of preextraction spiked sample (set C) to the mean peak of the same analyte in postextraction spiked sample (set B), multiplied by 100 (% RE = C/B × 100).
Interference Studies
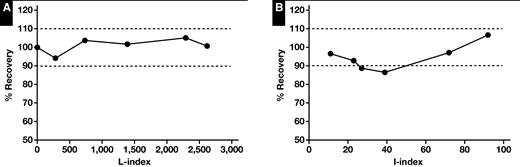
Interference studies were conducted by evaluating the effects of intralipid and bilirubin on the micro-LC–MS/MS assay performance. We analyzed the impact of lipemic samples by spiking donor whole blood with six increasing concentration of intralipid (L-index, 2-2,620). Similarly, icteric samples (I-index, 11-92) were assayed for exogenous serotonin. All samples were assayed in duplicate and compared to baseline values. Serum indices were measured on a Cobas 8000 system (Roche) by spectrophotometry.
Results
Analytical Performance and Validation
All analytical performance and validation were conducted in accordance with the College of American Pathologists and Clinical and Laboratory Standards Institute guidelines for laboratory-developed tests. Figure 1 is an example of a chromatogram overlay of both d45-HT quantifier and qualifier, as well as the IS extracted from MTB buffer of a calibrator containing 500 pg/mL of d45-HT. d45-HT concentrations were determined using the quantifier mass transition of mass-to-charge ratio (m/z) 181.1 to 164.0. The assay repeatability and reproducibility of this method showed CVs less than 10% for all levels of QCs Table 2. The analyte measuring range was shown to be linear at a range of 50 to 2,000 pg/mL Figure 2. LOQ (used to assess the smallest concentration the assay can reliability measure) was determined to be 50 pg/mL, corresponding to a CV of less than 20% Figure 3, with a signal to noise ratio of greater than 20. No carryover between high and low concentrations of samples was observed in this methodology Figure 4.
| . | Intraassay Precision . | Interassay Precision . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control Material . | Mean, pg/mL . | SD . | CV, % . | No. . | Mean, pg/mL . | SD . | CV, % . | No. . |
| QC 1 | 48 | 2 | 5 | 20 | 49 | 3 | 7 | 20 |
| QC 2 | 485 | 36 | 8 | 20 | 490 | 21 | 4 | 20 |
| QC 3 | 1,492 | 57 | 4 | 20 | 1,457 | 78 | 5 | 20 |
| . | Intraassay Precision . | Interassay Precision . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control Material . | Mean, pg/mL . | SD . | CV, % . | No. . | Mean, pg/mL . | SD . | CV, % . | No. . |
| QC 1 | 48 | 2 | 5 | 20 | 49 | 3 | 7 | 20 |
| QC 2 | 485 | 36 | 8 | 20 | 490 | 21 | 4 | 20 |
| QC 3 | 1,492 | 57 | 4 | 20 | 1,457 | 78 | 5 | 20 |
CV, coefficient of variation; LC-MS/MS, liquid chromatography tandem mass spectrometry; QC, quality control.
| . | Intraassay Precision . | Interassay Precision . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control Material . | Mean, pg/mL . | SD . | CV, % . | No. . | Mean, pg/mL . | SD . | CV, % . | No. . |
| QC 1 | 48 | 2 | 5 | 20 | 49 | 3 | 7 | 20 |
| QC 2 | 485 | 36 | 8 | 20 | 490 | 21 | 4 | 20 |
| QC 3 | 1,492 | 57 | 4 | 20 | 1,457 | 78 | 5 | 20 |
| . | Intraassay Precision . | Interassay Precision . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control Material . | Mean, pg/mL . | SD . | CV, % . | No. . | Mean, pg/mL . | SD . | CV, % . | No. . |
| QC 1 | 48 | 2 | 5 | 20 | 49 | 3 | 7 | 20 |
| QC 2 | 485 | 36 | 8 | 20 | 490 | 21 | 4 | 20 |
| QC 3 | 1,492 | 57 | 4 | 20 | 1,457 | 78 | 5 | 20 |
CV, coefficient of variation; LC-MS/MS, liquid chromatography tandem mass spectrometry; QC, quality control.
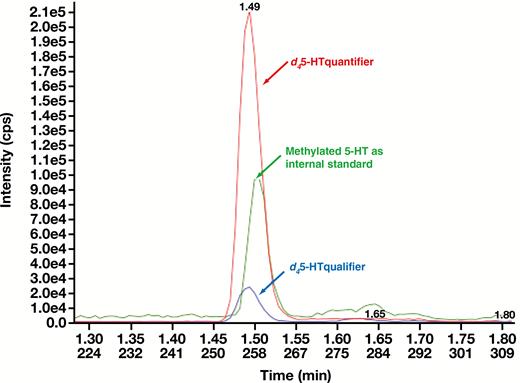
Multiple reaction monitoring chromatogram overlay (quantifier, m/z 181.1-164.0; qualifier, m/z 181-118; IS, m/z 191-115) of a calibrator level 5 containing 500 pg/mL of serotonin. cps, counts per second; d45-HT, deuterated serotonin.
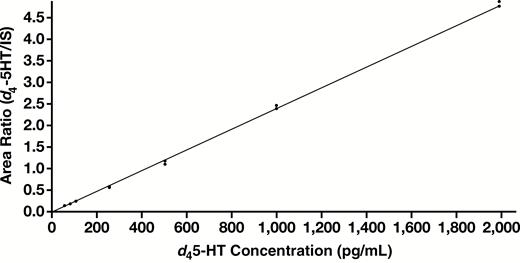
Calibration curve for d45-HT. d45-HT micro-liquid chromatography tandem mass spectrometry assay was linear from 50 pg/mL to 2,000 pg/mL with R = 0.999 (y = 0.00238x + 0.00651). d45-HT, deuterated serotonin.
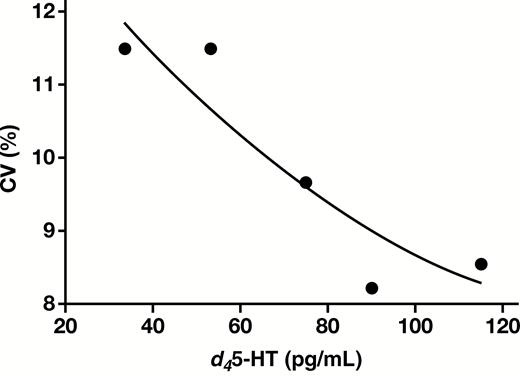
The limit of quantification of this assay was 50 pg/mL with a corresponding CV of less than 20%. CV, coefficient of variation; d45-HT, deuterated serotonin.
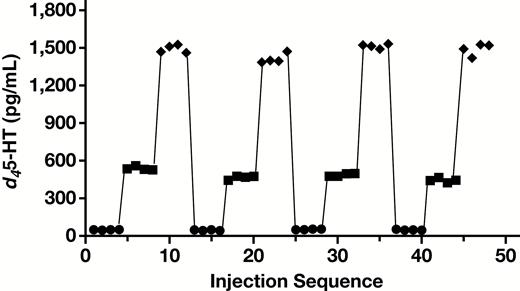
Carryover study using quality control material level 1 (50 pg/mL), level 2 (500 pg/mL), and level 3 (1,500 pg/mL). d45-HT, deuterated serotonin.
Due to whole blood being the sample of choice, we analyzed the impact of mild to severely lipemic and icteric samples on d45-HT measurements Figure 5. No significant interference was observed with L-index up to 2,620 and I-index up to 92.
Sample extraction recovery was approximately 75% in this assay. No significant ion suppression of d45-HT was observed in samples diluted with MTB buffer Table 3.
| Assessment . | Analyte Concentration, % . | ||
|---|---|---|---|
| . | 50 pg/mL . | 500 pg/mL . | 1,500 pg/mL . |
| Matrix effect | 86 | 81 | 72 |
| Extraction recovery | 75 | 76 | 77 |
| Assessment . | Analyte Concentration, % . | ||
|---|---|---|---|
| . | 50 pg/mL . | 500 pg/mL . | 1,500 pg/mL . |
| Matrix effect | 86 | 81 | 72 |
| Extraction recovery | 75 | 76 | 77 |
d45-HT, deuterated serotonin; LC-MS/MS, liquid chromatography tandem mass spectrometry.
| Assessment . | Analyte Concentration, % . | ||
|---|---|---|---|
| . | 50 pg/mL . | 500 pg/mL . | 1,500 pg/mL . |
| Matrix effect | 86 | 81 | 72 |
| Extraction recovery | 75 | 76 | 77 |
| Assessment . | Analyte Concentration, % . | ||
|---|---|---|---|
| . | 50 pg/mL . | 500 pg/mL . | 1,500 pg/mL . |
| Matrix effect | 86 | 81 | 72 |
| Extraction recovery | 75 | 76 | 77 |
d45-HT, deuterated serotonin; LC-MS/MS, liquid chromatography tandem mass spectrometry.
Healthy Donor Studies
A guiding principle in the method development was to minimize mechanical manipulation of the platelets, in order to prevent potential inadvertent platelet stimulation. Accordingly, sequential dilutions were employed to the extent possible, rather than centrifugation. In preliminary experiments, a variety of d45-HT concentrations for incubation with diluted whole blood were explored. As can be seen in Figure 6, at a concentration of 200 ng/mL, d45-HT was essentially taken up fully by the platelets of six normal donors not taking SSRI medication, whose platelet count ranged from 150,000 to 360,000 platelets/μL. In contrast, in the two donors currently taking SSRI medication, d45-HT uptake was dramatically reduced down to 58% and 46% of the added d45-HT.
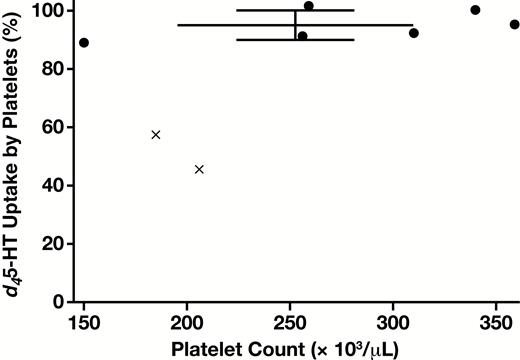
Platelet uptake of d45-HT (concentration of d45-HT 200 ng/mL at the time of incubation with platelets and 2,000 pg/mL after final blood dilution prior to analysis). Error bars indicate mean ± SD for uptake by six normal donors (with individual data points shown by filled circles). Note that nearly complete d45-HT uptake is observed across the broad range of donor platelet counts. Reduced uptake in the case of two additional donors taking selective serotonin reuptake inhibitor medication are indicated by × symbols. d45-HT, deuterated serotonin.
Despite a reduction in the absolute amount of d45-HT taken up in the SSRI donors, the secretory response by these platelets appeared quite comparable to the other six donors. Specifically, when challenged by TRAP or by the thromboxane A2 analogue U46619, the d45-HT released as a percentage of total d45-HT uptake was indistinguishable between the SSRI and non-SSRI donors Figure 7. There was a trend towards the low end of secretion for the SSRI donors in response to the GPVI collagen receptor activator convulxin, although not of statistical significance. d45-HT release was characteristically lower in response to U46619 than to TRAP or to convulxin, although readily distinguishable from background, and with a notably tight range from 13% to 18% release.
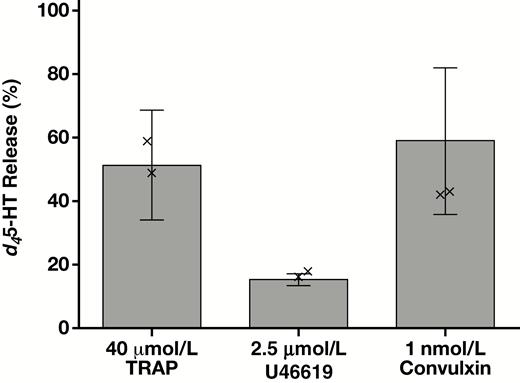
Platelet d45-HT release following incubation without stirring for 15 minutes at 37°C with the indicated stimuli. d45-HT secretion is shown as percent release with respect to total platelet uptake for each of eight donors. The two donors who were taking selective serotonin reuptake inhibitor medication, while taking up (Figure 6) and releasing decreased amounts of d45-HT on an absolute basis, nevertheless showed indistinguishable release of d45-HT on a percentage basis (represented by × symbols) with respect to the other six donors, and hence were included in the statistical calculations (mean ± SD). d45-HT, deuterated serotonin, TRAP, thrombin receptor activating peptide.
Discussion
The present proof-of-principle study introduces a novel, nonradioactive method for determination of platelet dense granule release that is both analytically robust and sensitive. This approach borrows conceptually from the classic radioactive serotonin release assay, instead employing a nonradioactive serotonin isotope d45-HT, and assaying both its uptake into platelets and its stimulated release by high-sensitivity micro-LC–MS/MS. This study was developed with an emphasis on minimizing platelet handling until the final separation steps required to distinguish cellular from extracellular content of the isotopic d45-HT. Accordingly, a series of dilutions of whole blood were employed, rather than isolating and further manipulating platelet-rich plasma. The LC-MS/MS methodology provided sufficient sensitivity for selection of an incubation concentration of 200 ng/mL d45-HT, low enough to assure virtually complete uptake over a wide range of platelet counts, while still producing a sufficiently robust analytical signal in the releasates.
The present proof-of-principle study was limited to blood samples containing platelet counts within the normal range. It remains to be determined how low a platelet count will prove capable of being assayed for dense granule secretion by such an approach. With this issue in mind, an effort was made in the present study for the method to be as independent of platelet count as possible. In particular, stirring was omitted to minimize any impact on the response to platelet stimuli from platelet collisions. Under nonstirring conditions, reproducible dense granule secretion was obtained in response to 40 μmol/L TRAP, 1.0 nmol/L convulxin, and 2.5 μmol/L U46619. In conjunction with the potential extension of this approach to actual clinical diagnosis of platelet disorders and the establishment of robust, locally developed reference intervals for stimulus-induced dense granule secretion, it may be worth reconsidering the possibility of stirring, particularly in the caseof agents such as collagen, ADP, or ristocetin. Some disorders, such as Glanzmann thrombasthenia, typically impair platelet aggregation with little or no diminution of dense granule secretion,14 and would not be anticipated to yield distinctly abnormal results in uptake and release of d45-HT. Of critical importance, however, in guiding the choice of agonist panels to be employed for clinical diagnosis will be inclusion in laboratory test development of patients with known disorders of dense granule secretion.
The micro-LC–MS/MS analysis of platelet uptake and release of d45-HT may also find utility as an alternative to the radioactive serotonin release assay15 presently used for assessment of heparin-induced thrombocytopenia. Most, if not all, of the methods currently employed in the radioactive serotonin release assay should be readily transferable to the micro-LC–MS/MS approach, with avoidance of radioactivity.
The quite small volume of blood required by the micro-LC–MS/MS approach might potentially support extension of platelet dense granule release analysis to newborns, from whom only limited volumes of blood may be safely obtained. Although not yet established with actual thrombocytopenic blood specimens, it is certainly conceivable that this novel methodology might eventually help in addressing the perplexing clinical question of how functionally responsive are the residual circulating platelets in thrombocytopenic patients. In addition to requiring only a small volume of patient blood, this methodology avoids the need to wash platelets or even to prepare platelet-rich plasma, instead employing diluted whole blood. Lastly, monitoring platelet dense granule release by LC-MS/MS assay of d45-HT is not adversely affected by lipemic or icteric blood samples, in contrast to the limitations imposed by these interfering substances when optical methods for platelet function evaluation are employed, such as LTA or lumiaggregation.8 In conclusion, this novel method of quantitating serotonin uptake and release is analytically quite robust and may permit assessment of this critical aspect of platelet function in patients for whom study of dense granule secretion was not previously possible.
Acknowledgment
We thank SCIEX for providing equipment support for this project.
This work was supported by the Department of Pathology, University of Chicago.
References
Author notes
Dr Yi is currently with the Department of Pathology and Genomic Medicine, Houston Methodist Hospital and Research Institute, Houston, TX
Dr Leung is currently with the Department of Pathology and Laboratory Medicine, Children’s Hospital Los Angeles, Keck School of Medicine of the University of Southern California.




