-
PDF
- Split View
-
Views
-
Cite
Cite
Vincent Rusanganwa, Jean Bosco Gahutu, Magnus Evander, Anna-Karin Hurtig, Clinical Referral Laboratory Personnel’s Perception of Challenges and Strategies for Sustaining the Laboratory Quality Management System: A Qualitative Study in Rwanda, American Journal of Clinical Pathology, Volume 152, Issue 6, December 2019, Pages 725–734, https://doi.org/10.1093/ajcp/aqz092
Close - Share Icon Share
Abstract
To explore challenges explaining the decrease in quality performance and suggest strategies to improve and sustain laboratory quality services.
Twenty key informants’ interviews from laboratory personnel were conducted in five laboratories. Four had previously shown a decrease in quality performance. Interviews were transcribed verbatim and analyzed using inductive thematic analysis.
Two themes emerged: (1) insufficient coordination and follow-up system towards accreditation, where lack of coordination, follow-up, and audits explained the decrease in performance; (2) inadequate resource optimization, where insufficient knowledge in Laboratory Quality Management System (LQMS), ownership by laboratory workforce, and insufficient stakeholders’ communication contributed to low-quality performance.
The coordination, follow-up, and assessments of LQMS, in conjunction with training of laboratory workforce, would establish an institutional culture of continuous quality improvement (CQI) towards accreditation and sustainment of quality health care. To achieve CQI culture, routine gap checking and planning for improvement using a system approach is required.
Despite the current progress of health sciences in the improvement of health care provision, the availability and accessibility of quality health care remains a challenge across the world, especially in low-income countries.1-4 To ensure the availability and quality of health care, a strong, responsive health system is paramount, not only encompassing different components but ensuring effective complementarity and commitment to the delivery of quality services. The quality of health care is an outcome of quality institutional culture, which is supported by different core values such as a sense of ownership and spirit of belonging. These values and philosophies are conveyed by effective leadership through policies and strategies and are owned by a health workforce that efficiently manages health system resources towards quality service delivery.5-7
Additionally, in clinical settings, different services and systems are engaged in patient health care provision, namely clinical, diagnostic, and supporting cross-cutting services. These services and systems are interconnected and interdependent to serve patients and to deliver continuous quality health care. Laboratories are crucial because clinical services depend on them for decisions regarding patient management.8,9 The availability of laboratory tests, as well as accurate and reliable results, contributes importantly to the quality of patient treatment.10
In 2008 to 2009, conscious of the gaps existing in laboratory systems and the vital role of clinical laboratories in health care quality improvement, countries within the World Health Organization (WHO) African region, and their involved development partners, adopted a framework for the improvement of laboratory systems for reliable services with a view to supporting health care quality improvement.11 Based on this consensus, the Strengthening Laboratory Management Towards Accreditation (SLMTA) approach, with the Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) as a checklist, was established.11-14
Following this adoption, 47 countries were implementing the SLMTA program in 617 laboratories by 2014, and two additional countries joined the program later to make a total of 49 countries.15,16 However, since 2009, when the program was launched,9 only 81 laboratories have been accredited.17 The ultimate goal of the SLMTA program is to support public laboratories in the implementation of the laboratory quality management system (LQMS) towards international accreditation, ensuring quality health care.18,19 The effectiveness of the program has been shown by improvements in LQMSs in endline audits 16; however, much work remains to be done for the majority of clinical laboratories in Africa to boost and sustain LQMS standards for accreditation and quality health services.10,17
The intervention of the SLMTA program included baseline and endline laboratory audits in several countries, and the implementation seems to have improved the quality in many low-income countries.16,20 A study published in 2014 showed that 302 laboratories that completed the SLMTA program in 2013 had an average score of 64% on the checklist at the exit audit, which was an increase from 39% at baseline.16 However, now almost a decade since the start of the program, an evaluation would be useful because it would provide data regarding the path that laboratories take towards accreditation in different countries. Such data would be informative, as it would identify the barriers limiting achievement of accreditation and sustaining LQMS, which contribute to the optimum goal, quality health care.
In the Rwanda development agenda, established in its long-term vision for 2020,21 the health sector received particular attention to tackle the enormous health problems after the 1994 genocide. These problems consisted of prevention, promotion, cure, and rehabilitation in the context of a destroyed health system, including but not limited to human resources, infrastructure, and equipment for health facilities. The aim was to ensure availability and accessibility of attainable quality health care.22,23 Many reforms have been successfully implemented and better health outcomes have been achieved.24,25 The health services that delivered these better health outcomes were aiming for quality standards. It was in that regard, that Rwanda embraced the accreditation process as a strategy for the achievement of this objective. Three referral hospitals were enrolled in the process of international accreditation, with a baseline in 2006.22 In the same objective, five clinical referral laboratories, including those affiliated with referral hospitals and the National Reference Laboratory (NRL), were enrolled in the SLMTA program in 2010 for their accreditation process. As the process continued, to date, all 48 public hospitals are enrolled in the accreditation process, including their laboratories. Specifically, laboratories are using the SLMTA/SLIPTA system. Among these, four referral hospitals, with their laboratories, and the NRL are in the process of international accreditation, with one hospital having already achieved international accreditation. The 44 remaining hospitals and their laboratories are undergoing the national accreditation process, among which there are five named satellite laboratories due to their geographical location, being peripheral and near national borders. These satellite laboratories have the additional role of transborder epidemiologic surveillance and are paid particular attention based on that mandate. The five referral laboratories enrolled in the SLMTA program showed good progress towards accreditation from 2010 to 2012, three of which scored four stars and the other two scored three stars, with the objective of achieving the maximum of five stars in the WHO grading scale26 needed to apply for international accreditation. However, in contrast to the good performance found in 2012, a study conducted in 2017 highlighted a strong decrease in the quality management system in four of the five laboratories, with the exception of one laboratory that reached five stars27 and recently applied for international accreditation. The laboratory system organization is displayed in Figure 1.
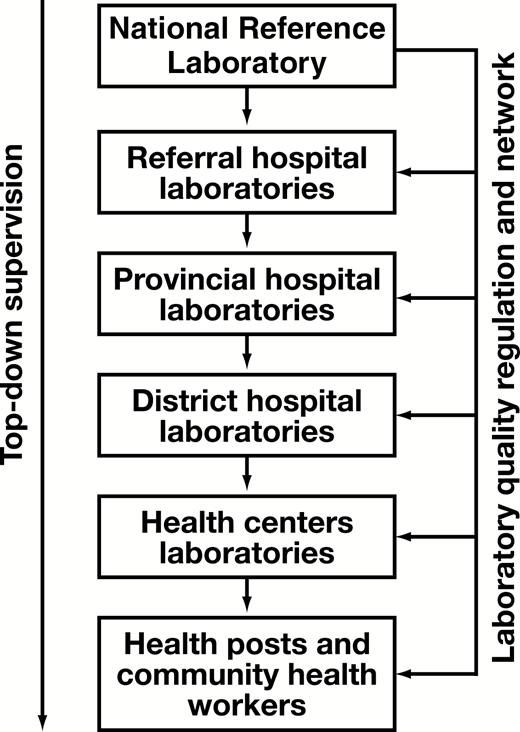
Rwanda clinical laboratory system organization. The provincial level is still functioning at the district level.
Based on the findings of the 2017 study,27 the current study aimed to explore the challenges that influenced this decrease in the quality management system and to suggest strategies for the improvement and sustainment of quality laboratory services. The present study used qualitative methods offering a better understanding of the reasons and context underlying the decrease in performance. This study provided a new perspective on the accreditation process in Rwanda, and serves as the basis for hypotheses at the regional level regarding the matters of the laboratory accreditation process and the quality of services.
Materials and Methods
Setting
Rwanda is a Central-East African country of 26,338 km2 and a current population size of 12,089,721.28 The bottom-up health care referral system is organized from community, health centers, district hospitals, to national referral hospitals. The laboratory services are included according to the service package at each health care level. The supervision of laboratory services, similar to other clinical services, is cascaded from top to bottom. The NRL regulates laboratory services and ensures quality of laboratory work nationally (Figure 1). Our study was conducted in five referral laboratories that were included in the first cohort of the SLMTA program in 2010. All five are government institutions, and one of them, which decreased in quality performance, has been recently contracted to private management, although still owned by the government.
Study Design
We conducted interviews with key informants from all five laboratories. The one that showed a good performance was included based on its role in overall laboratory coordination and regulation. The method was selected due to its appropriate approach in understanding complex phenomena from the participants’ perspectives.
Data Collection and Informants
The profiles of the key informants were purposely selected by the research team and communicated to the heads of the different laboratories. The selected profiles were managerial positions at different levels and operational staff with at least 6 years of experience, as well as SLMTA/SLIPTA experts. Following their identification, 20 key informants were contacted by telephone for a face-to-face meeting with the first author to explain the study and schedule the interview. Some of the selected key informants combined more than one selected profile. The key informants had different backgrounds and professional experiences, but all had laboratory work in common. The minimum age of the participants was 30 years and the maximum was 57 years, with an average age of 41.8 years. Among the 20 participants, 17 were male and three were female. The profiles were our entry point. The professional experience varied from 6 to 35 years, with an average of 15.3 years. The academic background was diverse and composed of two PhD holders, five at the master’s degree level, 11 with a bachelor’s degree, and two with an advanced diploma.
An open-ended interview guide was prepared, inspired by the findings of our first study,27 the WHO health system framework,29 and the preunderstanding by the first author (V.R.) of the system context. The interview guide was mainly articulated around laboratory achievements since their SLMTA enrollment, challenges and opportunities to deliver quality laboratory services, and clients’ satisfaction, as well as recommendations. Verbal consent was obtained from all key informants, after which the interview process was started. The interviews were conducted in English at the interviewee’s workplace between February and April 2018 and were digitally recorded by the first author. The length of the interview ranged from 38 to 53 minutes. Additionally, during the 3-day assessment of each laboratory in the first study,27 notes were systematically taken, during which challenges and opportunities explaining the strengths and weaknesses were provided by teams of laboratory staff in their specific services. Furthermore, following analysis of the first study, a debriefing regarding the results of each individual laboratory was organized, which included staff and managers, and these exchanges completed and enriched our notes. The key informant interviews were transcribed verbatim by a hired, qualified and experienced person in verbatim transcription after signing a confidentiality agreement to protect the participants’ identities. The transcripts were cross-checked by the first author in comparison with the digitally recorded data.
Data Analysis
For data analysis, inductive thematic analysis was used as described by Braun and Clarke.30 We read the transcripts several times to get a feeling for the participants’ perspectives. The contents of the text, reporting participants’ perspectives on laboratory quality performance, challenges, barriers, or opportunities, were highlighted with different colors. The highlighted text across all transcripts was extracted to form units by maintaining the meaning and participants’ perspectives. Codes were manually assigned to the meaning units by first author and discussed with A.K.H. and M.E. Subsequently, the codes were grouped based on their similarities, reviewed in light of text content, and assigned to subcategories. The subcategories were reviewed by revisiting the transcripts, which were labelled for the grouping to form categories that were discussed among V.R., A.K.H., and M.E. Finally, converging categories were combined and negotiated between the aforementioned authors in light of the WHO health system building blocks to generate two themes Table 1.
Themes Describing the Participants’ Perspectives on Accreditation Challenges
| Category . | Theme . |
|---|---|
| Missing coordination and regular external audits crucial for accreditation and sustaining LQMS | Insufficient coordination and follow-up system towards accreditation |
| Lack of supervision, follow-up, and mentorship in referral laboratories | |
| Insufficient ownership, accountability, and national domestication | |
| SLMTA/SLIPTA, appropriate instrument to improve laboratory service | |
| Insufficient training in QMS and accreditation | Inadequate resource optimization |
| Turnover and insufficient ownership by staff | |
| Reagents stock-out and maintenance issues affecting service delivery and client satisfaction | |
| Communication improved but lack of meetings between laboratory and clinical services |
| Category . | Theme . |
|---|---|
| Missing coordination and regular external audits crucial for accreditation and sustaining LQMS | Insufficient coordination and follow-up system towards accreditation |
| Lack of supervision, follow-up, and mentorship in referral laboratories | |
| Insufficient ownership, accountability, and national domestication | |
| SLMTA/SLIPTA, appropriate instrument to improve laboratory service | |
| Insufficient training in QMS and accreditation | Inadequate resource optimization |
| Turnover and insufficient ownership by staff | |
| Reagents stock-out and maintenance issues affecting service delivery and client satisfaction | |
| Communication improved but lack of meetings between laboratory and clinical services |
LQMS, laboratory quality management system; SLIPTA, stepwise laboratory improvement process towards accreditation; SLMTA, strengthening laboratory management towards accreditation.
Themes Describing the Participants’ Perspectives on Accreditation Challenges
| Category . | Theme . |
|---|---|
| Missing coordination and regular external audits crucial for accreditation and sustaining LQMS | Insufficient coordination and follow-up system towards accreditation |
| Lack of supervision, follow-up, and mentorship in referral laboratories | |
| Insufficient ownership, accountability, and national domestication | |
| SLMTA/SLIPTA, appropriate instrument to improve laboratory service | |
| Insufficient training in QMS and accreditation | Inadequate resource optimization |
| Turnover and insufficient ownership by staff | |
| Reagents stock-out and maintenance issues affecting service delivery and client satisfaction | |
| Communication improved but lack of meetings between laboratory and clinical services |
| Category . | Theme . |
|---|---|
| Missing coordination and regular external audits crucial for accreditation and sustaining LQMS | Insufficient coordination and follow-up system towards accreditation |
| Lack of supervision, follow-up, and mentorship in referral laboratories | |
| Insufficient ownership, accountability, and national domestication | |
| SLMTA/SLIPTA, appropriate instrument to improve laboratory service | |
| Insufficient training in QMS and accreditation | Inadequate resource optimization |
| Turnover and insufficient ownership by staff | |
| Reagents stock-out and maintenance issues affecting service delivery and client satisfaction | |
| Communication improved but lack of meetings between laboratory and clinical services |
LQMS, laboratory quality management system; SLIPTA, stepwise laboratory improvement process towards accreditation; SLMTA, strengthening laboratory management towards accreditation.
Trustworthiness
Certain measures were adopted to ensure the trustworthiness of the study. To ensure the credibility of the study, a literature review on the topic was conducted prior to the formulation of the interview guide, data collection, or analysis. Participants were from diverse backgrounds and institutions, with different experiences and ages. The information captured from the process of our previous study27 enriched our understanding of the context. The whole process of data analysis was discussed and agreed among three authors (V.R., M.E., and A.K.H.) step by step. Two authors (V.R. and J.B.G.) have a broader understanding of the study setting context, because they are from the same system. The dependability was ensured by the fact that the interviews with all participants focused on the same area. Our first study provided enough insight in advance to frame interviews. Because the first author (V.R.) knew the context, developed the interview guide, conducted all interviews, and analyzed data, it allowed consistency of the entire process towards the objective. Finally, the rich description of the study context enhanced the transferability.
Ethical Consideration
The research proposal was reviewed, and clearance Nos. 0059/RNEC/2017 and 111/RNEC/2018 were obtained from the Rwanda National Ethics Committee. Research authorization was obtained from the Ministries of Health and Education prior to its implementation. Additional permission was requested from the leadership of the institutions to which the evaluated laboratories belonged. The research project was presented and discussed at study sites prior to data collection. Moreover, the objectives of the study were explained to the key informants individually, who voluntarily accepted participation.
Results
From analysis of the collected data, two themes emerged: (1) insufficient coordination and follow-up system towards accreditation, which was related to the organization and strategies for the continuous improvement of laboratory quality; and (2) inadequate resource optimization, which was related to the participants’ perspectives on the laboratory workforce, availability of reagents, and equipment maintenance to ensure continuity of services and information systems.
Insufficient Coordination and Follow-up System Towards Accreditation
Most of the key informants highlighted the roles of leadership, coordination of the accreditation process across laboratories, LQMS training in the SLMTA program, and regular external audits as key actions that led to the success during the 2010 to 2012 period. It was a regular process of checks, planning, and implementation of improvement projects. Because the concept of accreditation was not yet firmly established in the laboratory culture, the lack of regular audits, especially external audits, were stipulated by informants as one of the challenges that influenced the decrease in laboratory quality performance. According to the participants, the process described above, supported by coordinated follow-up and an accountability system, should be kept to help sustain the quality management system towards accreditation and quality health care.
“…2012 was the last audit that was conducted, and most of the time when the people know that someone will come to ask what they have done they get prepared. So, from 2012, there were no audits conducted; there was not even a plan to say that within five years there will be someone to come to assess what you have done. So, when people are not assessed they come back in their routine. So that, I think that is the major challenge that we are having…” Staff, lab 3.
Most of the participants highlighted that certain district laboratories, especially satellite ones, are performing better in the quality management system than referral laboratories. These laboratories, together with the NRL, have been supported by a World Bank project since 2011, and have been assessed on quarterly basis by the NRL and annually by the East Africa Public Health Laboratory Network in the framework of the World Bank Project.
“…So, satellite laboratories had to be ready to undergo that assessment every year. I think that was the strength of satellites laboratories to sustain their quality management systems at 4 stars for most of them, because they knew that they would be assessed……So that regular assessment is part of something that can improve the quality management systems, while referral labs were not assessed regularly every year…” In charge of quality, lab 1.
By contrast, in referral laboratories, there was no supervision or mentorship. The accreditation process in laboratories was at its initial phase. Most of the participants said that to ensure the continuity towards the objective, which is accreditation, supervision, mentorship, and coordination were necessary. However, coordination was lacking in these referral laboratories, which led each laboratory to evolve its own routine diagnostic activities with less attention paid to the quality management system and no form of accountability or follow-up.
“… five years without follow-up, you are also going to forget, you are also going to sleep. So, that is among the challenges; and as I told you, the solution is to resume the follow-up. Because before, people were at four stars, three stars, but as you see through the assessment that you have conducted, people have gone back one star, meaning that the follow up is the key to sustainability.” Quality manager, lab 5.
Participants stated that there was a lack of a national system approach in the laboratory accreditation process. After 2012, each referral laboratory had to rely on itself; therefore, some of these laboratories left this stepwise approach, while others maintained it but with less effort. The national accreditation system would establish policies and other guiding documents, such as a strategic plan, so that laboratories could adapt them to their own context. Such a system would organize a reporting system and regular audits, even ranking institutions according to their quality performance. The system would gather laboratories together to share the audit findings and new strategies, such as a peer evaluation mechanism. In the current situation, LQMS depends on the good will and capabilities of individual laboratories and their leaders.
“…it depends on the will of individuals and not on an organized system…but I wish the Ministry of Health would launch that approach to rank laboratories. Then they say this laboratory is five stars, this one is three stars. So, when you hear that you are one star, and your colleague has five stars, you will change your mind and you will have to improve…” Head of laboratory, lab 2.
The key informants expressed that having a national accreditation system would increase accountability, national and institutional ownership, and coordination mechanisms, facilitating national domestication of laboratory accreditation. Establishment of a national accreditation body was suggested that would be responsible for the accreditation process, not only for laboratories, but also for other clinical services.
“…We need to create a national accreditation body, a big one, for laboratories, but also to include other fields if you want…” In charge of quality, lab 1.
Commitment of leadership at the hospital and laboratory levels was also stated as a key element in the accreditation process, which was very active during 2010 to 2012. However, after this period, the involvement of these leaders decreased, while it should instead continue in order to reach and maintain accreditation. The main focus of the leaders would be to ensure supervision, which requires accountability and alignment of all institutional stakeholders towards quality improvement and resource provision.
“…I remember when we started, each person was involved, starting from the cleaner to the upper management, the directors of hospitals. I don’t know why, maybe it is because we had an external person who came to help and assess everything, you felt that everybody was involved. It is different today, that is why you will find that some laboratories have dropped in star rating, it is because the upper management of referral hospitals are no longer much involved in the program...” Staff, lab 1.
A few participants explained the lack of laboratory follow-up and decrease in performance by the fact that these laboratories were enrolled in the accreditation process with accrediting organizations that had different checklists and, thus, did not use the SLIPTA checklist. They explained that referral laboratories are not regularly assessed, because they cannot be bothered with SLIPTA while following other accreditation checklists. However, the majority stressed the utility of SLMTA/SLIPTA as a program that builds the capacity and support for quality improvement by a stepwise approach towards accreditation. The two approaches are complementary, because SLMTA/SLIPTA helps the process of quality improvement towards accreditation, while the accrediting organization comes to assess the standards in order to provide accreditation if the standards are met. Additionally, the accrediting organizations in Rwanda assess the entire hospital, while SLIPTA was appreciated by the participants as a specific, deeper, and focus tool for laboratory quality improvement.
“…the WHO has its own checklist, when you use the SLIPTA checklist for WHO, sometimes you can find gaps because it is very specific to the laboratories, while the COHSASA (The Council for Health Service Accreditation of Southern Africa) one is very wide and not specific to the laboratory. It concerns all aspects of clinical services and laboratories globally.” Staff, lab 2.
Inadequate Resource Optimization
Not only coordination and follow-up, which are the main roles of leadership, but also different issues related to health system resources were highlighted by the participants in the present study. The main ones were laboratory workforce, commodities, and information systems.
Commonly, all participants testified that the accreditation was a new concept for laboratory personnel. It was stipulated that accreditation and quality management system were not taught in preservice training, which constituted a weakness to be addressed. Laboratory staff were exposed to these concepts once in practice. Additionally, considering routine laboratory activities, some staff believed that the related accreditation activities were extra work; such a mindset was identified as a lack of ownership and a major challenge towards accreditation.
“…For lab technicians, they need some courses called laboratory management, but really they don’t focus on accreditation. Most of the staff know accreditation in their practices, not at the school. This is one. Number two, it is mindset. Even though they have understood the definition of accreditation, most staff don’t want to hear about the accreditation, they think accreditation is extra work. Whereas the accreditation is included in their daily activities, you will see some people say we have a lot of work.” Head of laboratory, lab 2.
All participants commented positively on the good impact of SLMTA training on personnel mindset in favor of LQMS and accreditation. However, only a few staff from the studied laboratories had the opportunity to be trained, with the exception of the NRL, which was described as having almost all trained staff. In the remaining laboratories, with an average of 45 staff members in each laboratory, only approximately four staff from each laboratory had been trained, and some of those had left these institutions. The turnover of the SLMTA-trained staff, as well as some laboratory leaders, was highlighted in the interviews as negatively impacting the accreditation process, because new leaders did not understand the accreditation.
“…Here we have a problem as you said, a problem of staff turnover, especially the head of department. This year we can have a head of department, the next we have another head of department; this turnover disturbs our management. Because the head of department does not yet know the system, doesn’t yet know how to do things, then comes another one, it is like starting again. But we also have the problem of staff mindset that says that accreditation is nothing, it is for laboratory management, for quality managers, it is not for us. That is a big problem.” Laboratory manager, lab 2.
Some key informants reported a high workload as one of the challenges that hindered the process of accreditation. Most of the staff’s time was occupied with diagnostics and delivering results, with less attention dedicated to accreditation activities. They wished to have a dedicated quality manager to monitor and deal with LQMS and the accreditation process, but some laboratories were missing this position, or it was combined with other duties.
“…This is the big issue we have. For example, as you see in our checklist, you must have a safety officer, but now we don’t have a safety officer; we have proposed for the new structure to put a safety officer and a quality manager in place; but according to the information I have, they didn’t add them to the structure...” Laboratory manager, lab 4.
“…I have been working in this lab for twenty years. The main problem we have, I think, is the workload. We have many patients, many activities in the hospital. I think another issue is the insufficient number of laboratory technicians in the laboratory…” Staff, lab 2
All participants reported stock-out of reagents as a major concern in all studied laboratories, together with the issue of equipment maintenance. However, most equipment has a back-up system. The stock-out was mainly due to the long process of the procurement system and outside supplies, while the problem of equipment maintenance was due to the lack of local biomedical engineers. Such issues caused service interruption and affect clients. Harmonization of equipment was suggested to reduce this problem.
“…As I have said, the biggest problem we have is stock-out. This is the biggest problem we have. The second problem is the quality of reagents, some reagents are not good. But the first problem we have in this laboratory is stock-out. We used to have a problem of machines due to the lack of maintenance, but now that we have started using the leasing system, the problem has reduced because the responsibility is with the supplier. Our responsibility is to buy reagents. But the big problem is stock-out…” Staff, lab 5.
Most of the studied laboratories have an online platform, where test requests and results can be posted. Only one laboratory did not have such online tools; instead the results were picked up hourly by personnel and taken to different clinical services. Most key informants reported that the online platform facilitated the communication between the laboratory and clinicians with respect to reporting results. In all laboratories, critical values were directly communicated first by telephone and then sent through the normal channel. This was facilitated by a common user group system, where all staff of one institution could call each other on their mobile telephones with a flat prepaid amount of money paid by the institution. The use of e-mails in other types of communication was reported in all laboratories.
“We do have a system. Even the clinicians request tests through that software. Even us, we put the results in that system, but in case of a critical value, a critical result, it is our mandate to communicate it immediately using a cell phone, and you document it in the appropriate books…” Laboratory manager, lab 4.
Within the teaching laboratories, daily morning staff meetings were organized, during which cases and work plans were discussed. On top of other administrative channels of communication, these meetings were the platforms where related service challenges could be discussed; however, most of the key informants declared that online communication was not sufficient for the quality management system. Formal meetings between laboratory staff and clinicians were recommended in order to discuss quality gaps that need addressing. Such meetings were nonexistent in the majority of hospitals, except in one laboratory, which sent laboratory technicians to different clinical services to discuss quality issues.
“…We try but it is not regular. We use written announcements, sometimes we use e-mail, sometimes now we use WhatsApp, but it is not regular. According to the accreditation, we must have a meeting with them to explain what is new, what happened…” Laboratory manager, lab 5.
Despite the existing challenging situation of a decrease in performance, some participants believed that the gaps can be addressed with available resources. What mattered was the organization of existing human resources for audits, supervision, and mentorship.
“…To me, nothing is missing, because we have Human Resources, we have trained personnel, we have facilitators, we have mentors, we have master trainers, well-qualified. We have infrastructures in our laboratories, we have equipment, we have the political willing to achieve accreditation, but what we are missing to achieve that is the follow-up.” Staff, lab 3.
“…The management needs to put more emphasis on pushing the laboratory managers to make them feel that quality is their duty all of the time.” Staff, lab 1.
Discussion
According to the participants’ perspectives, the present study showed the main challenges that explain the decrease in quality performance in clinical referral laboratories in Rwanda and suggests strategies for improvement. These challenges were mainly insufficient coordination of the accreditation process across laboratories, lack of follow-up and regular laboratory assessments, as well as insufficient quality management system knowledge and no sense of ownership for certain laboratory personnel. The stock-out of reagents and equipment maintenance were also reported as challenges, as well as the lack of formal discussions between the clinical and laboratory services regarding laboratory quality issues.
The present study highlights that the laboratory accreditation and the LQMS are not yet well integrated in the mindset of the majority of personnel in the laboratory system in Rwanda. Following initiation of the SLMTA program, national stakeholders should take over and perform regular laboratory audits, supervision, mentorships, and training, to create and reinforce the culture of continuous quality improvement towards accreditation. The findings show that in four of five laboratories that had decreased in their quality performance, there was a lack of regular audits, supervision, and mentorship after 2012. Such structured follow-up should ensure the continuity of the quality management system, leading to continuous quality improvement and accreditation. The lack of this culture explained the decrease in quality performance. In accordance with other studies, our findings suggest the necessity of creating and maintaining an institutional culture of continuous quality improvement for sustainable quality health care.31-34
Our findings reveal the lack of a systems approach for continuous laboratory quality improvement. The quality performance reached in 201226 only lasted for a short period,27 based on SLMTA intervention and individual commitment, instead of being a system-based performance. An effective strategy system approach would ensure the continuity of quality performance even after phasing out the SLMTA intervention. Other studies in different contexts have highlighted the importance of such a systems approach as an unending circle of identifying gaps, data driven knowledge, planning, and implementation of projects to ensure continuous quality improvement.2-4,6,32 The participants underlined the lack of coordination and insufficient involvement of leaders, because the role of leadership and coordination is paramount in establishing and maintaining an effective system to deliver the expected quality performance.3 However, when there are no regular audits and no sharing of problems to feed internal reviews, leaders may ignore the quality performance status of laboratories. This is a reason why establishment of quality system approach would be a better solution.
Different challenges related to health system resources were stressed as contributors to the low laboratory quality performance. Insufficient LQMS knowledge and ownership by most of the laboratory workforce, as well as the turnover of the personnel, were reported as the main challenges. In relation to turnover of the workforce, to some extent it could be a problem if many people leave; however, a certain amount of movement is unavoidable. An effective health system can control such turnover by retention strategies and the recruitment of new skilled people. Even though the issues of reagents stock-out and equipment maintenance were highlighted as an existing problem that affected the quality of laboratory services, the current situation is likely to be no worse than in 2012, when the quality performance was better, because some improvement measures were implemented. Additionally, back-up laboratory equipment exists in most cases of machine breakdown to avoid interruption of service. It is obvious that the existing problems of reagents stock-out and broken equipment could affect the quality of services, but that alone cannot explain the observed decrease in quality of performance. While technology has improved communication with respect to test requests and results reporting, formal regular meetings between clinical and laboratory services were reported as a challenge, because these stakeholder meetings are important for discussing and solving quality problems. These resource-related challenges have also been reported in two studies in similar contexts.35,36
The present study shows that the registered performance in 2012 was a reaction to the SLMTA intervention, which brought external expertise to train laboratory staff and mentor them through quality improvement projects. It was also a regional move during that time, across many countries and laboratories. This general enterprise likely influenced the improvement; however, our findings indicate that this achieved performance was not capitalized in a country-wide laboratory system for continuous improvement. Such lack of continuity suggests that the registered performance was not a result of an overall institutional quality improvement plan, which explains why there was no follow-up, resulting in a decrease in laboratory performance.34
The SLMTA was appreciated as a program that brought awareness and LQMS skills. The SLIPTA checklist was reported as an appropriate tool for laboratory quality assessment due to its completeness and specificity for laboratory quality improvement. The fact that an entire hospital is enrolled in accreditation with another accrediting organization should not be a reason for not using a stepwise approach, because the SLMTA/SLIPTA prepare laboratories for accreditation while contributing to quality improvement.18
The regional adoption of investment in laboratory services aims to contribute to quality health care. The case in Rwanda cannot represent all countries enrolled in the SLMTA because the context and practices may be different; however, it can serve as an insight into how far they are towards the initial objective, especially when less than 13% of globally enrolled laboratories have been accredited after almost a decade. In accordance with current emphasis on the quality of health care through universal health coverage to achieve the third Sustainable Development Goal,37 strengthening and sustaining the quality of laboratory services is paramount. Regional and international organizations in African countries should investigate how to tackle the problem of continuous quality improvement through a systems approach for its sustainability. The reversal in quality performance of four out of five laboratories in Rwanda and the low rate of accredited laboratories among enrolled laboratories in the SLMTA program, raise the question of sustainability. Without straining national efforts, the regional and global level of mentorship and follow-up should be maintained, to accompany a new SLMTA initiative in enrolled countries. For sustainability, the demonstrated trend of laboratory quality improvement should be followed-up to reach and maintain laboratories’ accreditation.
In Rwanda, the quality of health care and accreditation of health facilities, including laboratories, is a national investment. All 49 public hospitals, including laboratories, are enrolled in the accreditation process. The findings of the present study highlight the main challenges that hinder quality laboratory services in Rwanda and advocate for more coordination of the laboratory accreditation process and follow-up. A systems approach for continuous quality improvement that would regularly assess all laboratory systems and plan accordingly would effectively and efficiently accompany the process. The findings from the studied laboratories serve as a warning to prevent the same experience in the remaining health facilities enrolled in the accreditation process, which could be a waste of investment.
Conclusion
The present study explored the challenges that explain the decrease in the quality performance of clinical referral laboratories in Rwanda. During the SLMTA intervention, laboratories showed a good performance in 2012, while their evaluation in 2017 showed a decreased quality performance. Our findings highlight that the lack of effective coordination of the accreditation process and follow-up, the unestablished culture of continuous quality improvement, and the lack of a system approach, explain the decrease in laboratory quality performance. Such a system approach should identify barriers that hinder the laboratory quality system, which should be regularly addressed. Establishment of a system approach by searching for gaps in the quality system to inform planning and implementation of improvement projects would ensure continuous quality improvement in these laboratories. Such an approach would serve to establish quality culture and would benefit the entire health care system.
This work was supported by the Swedish International Development Cooperation Agency (grant numbers 51160027-04 and 51160059-10).
References
Yao K, Maruta T, Luman ET, et al. The SLMTA programme: transforming the laboratory landscape in developing countries. Afr J Lab Med. 2014;3. doi:10.4102/ajlm.v3i2.194




