-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Sale, Marianna Noale, Simona Cintoli, Gloria Tognoni, Chiara Braschi, Nicoletta Berardi, Stefania Maggi, Lamberto Maffei, the Train the Brain Consortium , Long-term beneficial impact of the randomised trial ‘Train the Brain’, a motor/cognitive intervention in mild cognitive impairment people: effects at the 14-month follow-up, Age and Ageing, Volume 52, Issue 5, May 2023, afad067, https://doi.org/10.1093/ageing/afad067
Close - Share Icon Share
Abstract
No treatment options are currently available to counteract cognitive deficits and/or delay progression towards dementia in older people with mild cognitive impairment (MCI). The ‘Train the Brain’ programme is a combined motor and cognitive intervention previously shown to markedly improve cognitive functions in MCI individuals compared to non-trained MCI controls, as assessed at the end of the 7-month intervention. Here, we extended the previous analyses to include the long-term effects of the intervention and performed a data disaggregation by gender, education and age of the enrolled participants. We report that the beneficial impact on cognitive functions was preserved at the 14-month follow-up, with greater effects in low-educated compared to high-educated individuals, and in women than in men.
Key Points
The beneficial impact of the Train the Brain intervention on cognitive functions was preserved at the 14-month follow-up.
The effects were greater in low-educated compared to high-educated individuals.
The beneficial effects were more evident in women than in men.
Introduction
Ageing is considered as the major risk factor for decline in cognitive domains such as memory, attention and executive functions [1]. While a pathological ageing process can lead to severe age-related forms of dementia, the underlying neurobehavioural impairments develop across a temporal continuum, which goes from early stages of silent perturbations, through an intermediate state called mild cognitive impairment (MCI) [2]. At this stage, the subjective cognitive decline becomes objectivable by neuropsychological tests in the absence of impairments in daily activity performance [3].
Meta-analysis studies suggest an annual conversion rate from MCI to dementia in a range from about 9.5% [4] to 20% [5], making this stage particularly suitable for strategies aimed at slowing down the evolution towards overt dementia. In this context, modifiable lifestyle factors play a prominent role, with increased levels of physical exercise and cognitive activity being considered particularly efficacious either as protective factors against dementia, or as key elements of non-pharmacological interventions for patients with MCI or early dementia (see [6] for a recent review).
We recently performed a randomised, clinical trial called as ‘Train the Brain’, investigating the impact of a combined motor and cognitive intervention in older people with a diagnosis of MCI. Previously published results showed that this treatment markedly improved cognitive functions in trained subjects compared to non-trained controls, as assessed immediately at the end of the 7-month intervention programme [7].
Here, we extended the previous analysis to include the long-term effects at the 14-month follow-up (i.e. 7 months past the end of the intervention) and to perform a detailed data disaggregation by subject gender, education level and age.
Material and methods
An extensive description of the methods can be found in [7] and in the Appendix.
Participants
Participants, recruited from the general population and enrolled in the Neurology Unit (Azienda Ospedaliera Universitaria Pisa, AOUP) of Santa Chiara Hospital, Pisa (Italy), were older people (age > 65, <89), with a confirmed diagnosis of MCI (amnestic and non-amnestic types). All participants matched the inclusion criteria described in the original paper [7] and in the Appendix.
Subjects were randomly assigned to either the multidomain training group (MCI-training), or to the no-training condition (MCI-no-training), in a 1:1 ratio, considering a computer-generated simple randomisation sequence. Randomisation was performed after the baseline assessment by a statistician not otherwise involved in the study and with no contact with the study participants. Clinicians and psychologists involved in the training were different from the outcome evaluators.
All participants were required to sign an informed consent prior to the tests administration. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Regional Ethical Committee for Clinical Experimentation. ClinicalTrials.gov Identifier is NCT01725178 (8 November 2012).
Cognitive assessment
For the MCI diagnosis, the diagnostic criteria proposed by the European Consortium on Alzheimer’s Disease Working Group on MCI were applied [8]. A comprehensive battery of neuropsychological tests was chosen to assess the performance in several cognitive domains. The battery included the following tests: Mini-Mental State Examination (MMSE), Clock Drawing Test (CDT), Clinical Dementia Rating Scale (CDR) and Geriatric Depression Scale.
Patients diagnosed as MCI underwent an evaluation which included Alzheimer’s Disease Assessment Scale-cognitive (ADAS-Cog) to measure the severity of the most important symptoms of AD. The clinical MCI evaluation provided the baseline (T0) and the two cognitive assessments at the follow-ups (T7 and T14).
Combined physical-cognitive training
Enrolled participants were assigned to mixed-gender classes of maximum 10 individuals each, to promote social interactions, and given two sessions of supervised cognitive training of 60 min each per day, three times a week and one of supervised aerobic physical activity of 60 min each per day, three times a week, for 7 months. A detailed description of the cognitive training programme is given in the Appendix.
Statistical analysis
Baseline characteristics of study participants at follow-ups (7 and 14 months from the baseline) were compared with those lost to follow-up, considering the χ2 or Fisher’s exact test for categorical variables and Generalised Linear Models after testing for homoscedasticity (Levene’s test; in case of heteroscedasticity, the Welch’s ANOVA was considered) or the nonparametric Wilcoxon rank sum test for quantitative ones.
Shapiro–Wilk test and D’Agostino-Pearson test were used to test the normal distribution of quantitative variables. Mixed-effects models were considered to study the changes in ADAS-Cog scores related to group (MCI-training; MCI-no training), time and group × time interaction. Adjustment for baseline ADAS-Cog score, gender, age, years of education and covariates differently distributed across groups were applied. A covariance model accounting for unequally spaced measurements and Tukey adjustment for multiple comparisons were considered. Intent-to-treat analyses were made using multiple imputation of missing outcomes [9]; 50 imputed datasets were combined considering Proc MIAnalyze. Further analyses evaluated also the interaction of group × time with years of school (≤5 versus >5 years), sex and age (<75 versus ≥ 75 years; 75 is the median value of the age distribution of participants) to evaluate the training-specific effect for education groups, sex and age groups, respectively. The macro ‘type3_MI_mixed’ was used to obtain a single, weighted Type III statistic.
Statistical significance was assumed for a P-value < 0.05. The analyses were performed using SAS statistical package release 9.4 (SAS Institute Inc., Cary, NC).
Results
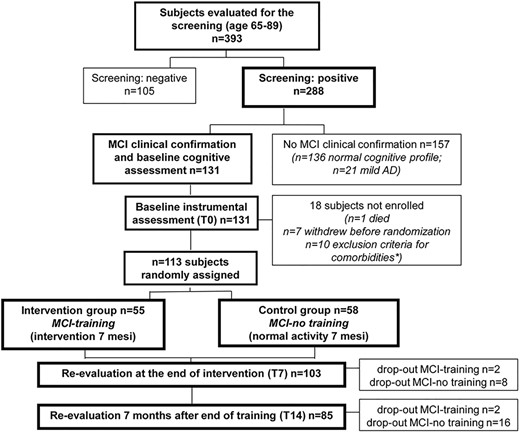
One hundred thirteen persons were enrolled and randomised to the intervention: n = 55 in the MCI-training group and n = 58 in the MCI-no-training group (Figure 1). The training programme lasted for 7 months and was completed by 53 MCI-training subjects and 50 MCI-no training subjects, with a significantly higher drop-out rate in the MCI-no-training group [7]. Cognitive evaluations were performed 7 months after baseline (i.e. soon after the end of the training period) on 103 participants (T7, 53 MCI-training and 50 MCI-no training subjects) and 14 months after baseline on 85 participants (T14, 51 MCI-training and 34 MCI-no-training). On comparing the participants included in these analyses with those lost at follow-ups, no significant differences were found, with the only exception of gender: men were more frequently lost at the T14 follow-up (see Supplementary Table S1).
No significant differences were present between the two groups for gender, age, education, MMSE and CDT at both T7 (gender P = 0.495, age P = 0.4744, education P = 0.7633, MMSE P = 0.3783 and CDT P = 0.8541) and at T14 (gender P = 0.7217, age P = 0.3832, education P = 0.9047, MMSE P = 0.3888 and CDT P = 0.7663). Baseline characteristics of the enrolled subjects are presented in Supplementary Tables S1 and S2.
Estimated means and 95% confidence intervals (CIs) for ADAS-Cog for each group at different time points, from mixed-model repeated measures analyses, are presented in Supplementary Table S3 (between-groups differences) and Supplementary Table S4 (within-group differences). A significant beneficial effect of the training on ADAS-Cog scores was evident at T7, as already reported [7], and was confirmed at the T14 follow-up (Figure 2): the mean estimated change in ADAS-Cog with respect to the baseline value in the MCI-training group was −1.57 (95% CI (−2.50, −0.64)), while the corresponding value in the MCI-no-training group was +0.72 (95% CI (−0.23, 1.68)). The mean difference between the two groups in relation to change in ADAS-Cog at T14 was −2.08 (95% CI (−3.25, −0.91)), highly statistically significant (P < 0.001), with an effect size (ES) in the medium range (ES = −0.52; 95% CI (−0.55, −0.49)).
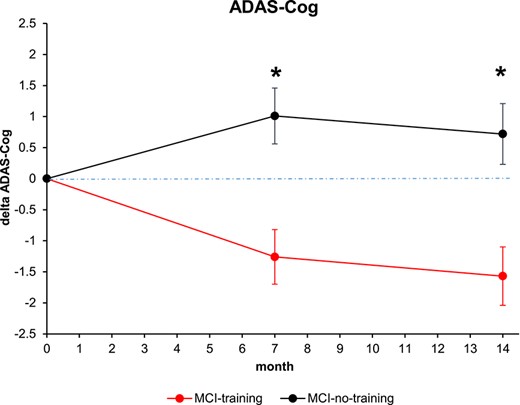
Changes in cognitive score at the ADAS-Cog score. Estimated means and SE from mixed-model repeated measures analyses, adjusted for baseline ADAS-Cog, age, sex, education, MMSE and CDT. Asterisk means P < 0.05.
Thirty-eight participants (33.6%) reported ≤5 years of education, while 75 reported >5 years of education. The low-educated group at the screening performed significantly worse at CDT (6.9 (SE = 2.0) versus 8.2 (SE = 1.9), P = 0.0004), while no significant differences were found in relation to MMSE and CDR. Mixed-effect models to study changes with time in ADAS-Cog scores revealed that the interaction group × time with dichotomized education had borderline significance (P = 0.0764); the results of the training were significant both in high and low education training groups at both time points, though more evident among participants with lower education (Figure 3a) than among those with higher education (Figure 3b). In participants with >5 years of schooling, the differences in post-training ADAS-Cog between the training and control groups were: −1.6 (95% CI (−2.9, −0.3), P = 0.0181) at T7 and −1.6 (95% CI (−3.1, −0.2); P = 0.0242) at T14. In participants with lower education, the differences in post-training ADAS-Cog was almost twice that of high education groups: the mean difference between low education training and control groups was −3.1 (95% CI (−5.1, −1.2), P = 0.0020) at T7 and −3.2 (95% CI (−5.3, −1.2), P = 0.0028) at T14 (Supplementary Table S3). Thus, training was beneficial both for high and low education MCI subjects, and in both groups, the training effects were maintained at the T14 follow-up.
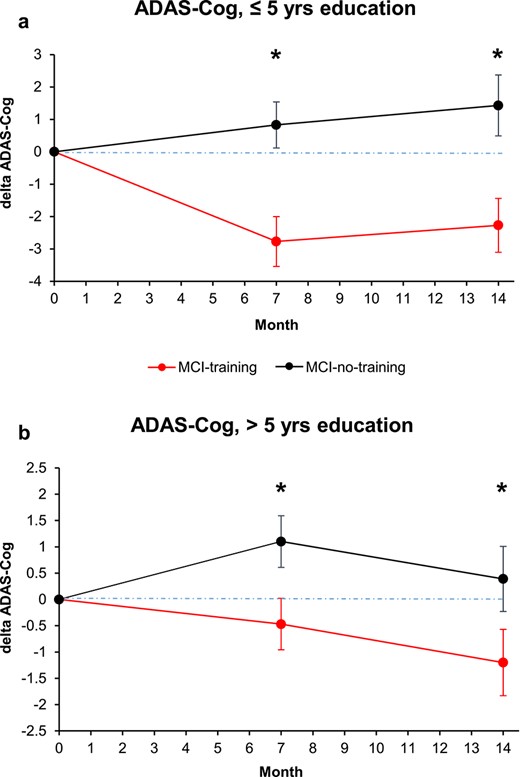
Changes in cognitive score at the ADAS-Cog score, analysed by years of education dichotomized (a, less educated; b, more educated subjects). Estimated means and SE from mixed-model repeated measures analyses, adjusted for baseline ADAS-Cog, age, sex, MMSE and CDT. Asterisk means P < 0.05.
Women (n = 55, 48.7%) obtained significantly lower scores at CDT with respect to men in the screening phase (7.3 (SE = 0.3) versus 8.2 (SE = 0.2); P = 0.0354), while no significant differences were detected for MMSE and CDR. The interaction group × time with gender was not statistically significant (P = 0.2710). However, the results of the training on ADAS-Cog scores seem to be more evident among women (Supplementary Table S3; Figure 4a and b). At T7, both men and women showed a significant effect of training: the mean difference between training and no training females was −2.3 (95% CI (−3.9, −0.8); P = 0.0035) and that for males was −1.9 (95% CI (−3.4, −0.3); P = 0.0187). At T14, the mean difference between training and no training females was −3.1 (95% CI (−4.7, −1.5); P = 0.0003); the mean difference between training and no training males was −1.2 (95% CI (−2.9, 0.5), P = 0.1655), reduced with respect to what found at T7 and not reaching statistical significance, likely for the reduction in sample size.
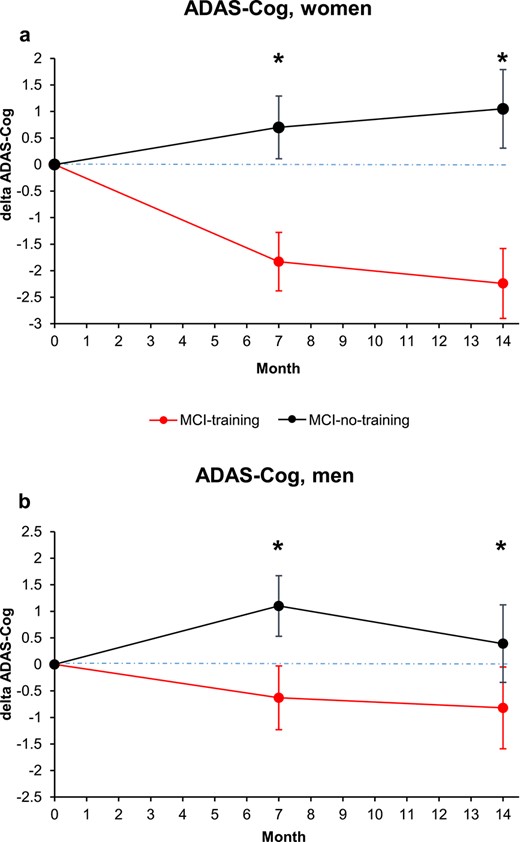
Changes in cognitive score at the ADAS-Cog score, analysed by subject gender (a, women; b, men). Estimated means and SE from mixed-model repeated measures analyses, adjusted for baseline ADAS-Cog, age, education, MMSE and CDT. Asterisk means P < 0.05.
Participants aged ≥75 years were 54 (47.8%) and they performed slightly better, at the screening, at the MMSE with respect to participants aged <75 years (26.0 (SE 0.3) versus 25.2 (SE 0.3); P = 0.0391), while for CDR and CDT, differences were not significant. Interaction group*time*age group dichotomized was not significant (P = 0.6614) (Supplementary Table S3; Figure 5a and b). The effect of training on ADAS-Cog scores was significant both among participants aged <75 years (mean difference between training groups at T7, −2.3 (95% CI (−3.8, −0.8), P = 0.0033; at T14, −2.4 (95% CI (−4.0, −0.8), P = 0.0035), and among participants aged ≥75 years (mean difference between training groups at T7, −2.1 (95% CI (−3.7, −0.4), P = 0.0133); at T14, −2.0 (95% CI (−3.8, −0.1); P = 0.0376).
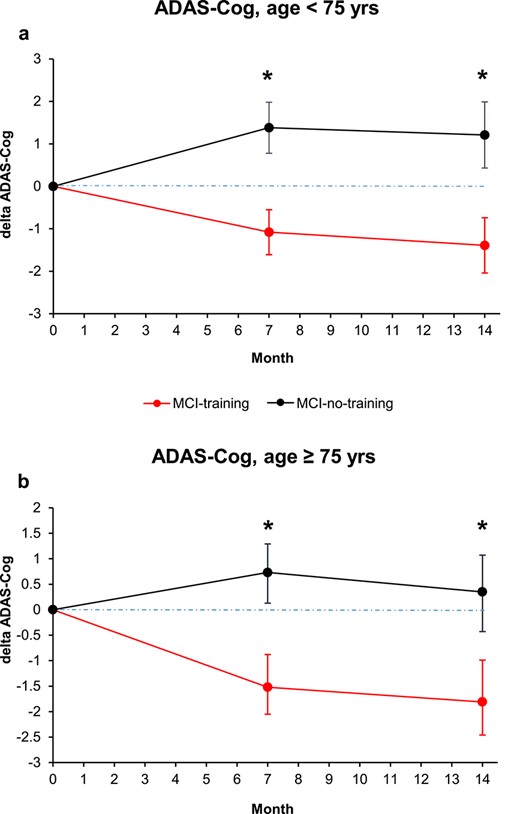
Changes in cognitive score at the ADAS-Cog score, analysed by years of subject age dichotomized (a, <75 years; b, >75 years). Estimated means and SE from mixed-model repeated measures analyses, adjusted for baseline ADAS-Cog, sex, education, MMSE and CDT. Asterisk means P < 0.05.
Discussion
In the present paper, we expanded our previous work on the acute effects of a combined cognitive-motor training, named the ‘Train the Brain’, in a population of older persons with MCI diagnosis.
The first aim of the study was to accomplish a follow-up analysis of cognitive data performed 7 months past the end of the intervention, i.e. 14 months since the baseline assessment. The present data show that the acute beneficial effects elicited by the intervention were maintained over time, with a medium ES and a mean ADAS-Cog difference between the intervention and the control groups of −2.08. The ADAS-Cog changes were in the 1–3-point range, either as improvements in trained people or as decline in non-trained subjects. In pharmacological studies focused on more severe forms of cognitive deterioration (e.g. Alzheimer’s disease patients), a ‘4-point change at 6 months’ criterion is assumed as conventional for the assessment of the clinical meaningfulness [10]. However, considering that (i) the ‘Train the Brain’ intervention is a totally non-pharmacological approach; (ii) the targeted population had a MCI and that (iii) the follow-up in the present study was at 14 months, and the clinical meaningfulness of the results is likely better portrayed by a less stringent criterion. Indeed, even with pharmacological approaches, other studies reported an average improvement of much <4 points at 6 months and 1 year [11], suggesting that a given n-point decline on ADAS-Cog needs to be interpreted in the context of the overall response. The prevailing consensus is that, for ADAS-Cog at 1 year, a change of 2 may be appropriate for meaningful changes [12]. In agreement with this view, we previously reported that the cognitive improvements at the T7 follow-up were also evident at the level of single cognitive domains and resulted in a reduction of neuropsychiatric symptoms and a marked improvement in the quality of life [13].
Strikingly, the global cognitive performance in the current study displayed an improvement with respect to baseline in the MCI-training group, while it undertook deterioration in the MCI-no-training group. This underscores the clinical relevance of the ‘Train the Brain’ intervention and of similar programs in terms of their impact on a possible delay of dementia onset. To date, very few studies have validated the long-term effects of the tested programs on older MCI persons, with most cases reporting the impact of a protracted cognitive-motor stimulation analysed within the time limits of the period in which the intervention was still active, i.e. without post-intervention follow-ups (e.g. [14]).
The second aim of our study was to perform a disaggregation of the data in terms of education, gender and age. We report that, at the baseline, the lower-educated group displayed a worse cognitive performance in the CDT with respect to the higher-educated group. This finding can be appraised within the context of the so-called cognitive reserve hypothesis by which different degrees of lifetime mental stimulation contribute to the inter-individual variability in late-life cognitive abilities and protection against the effects of ageing [15–18]. Moreover, our finding is in agreement with recent results from the FINGER study, a multidomain intervention, including cognitive training combined with physical exercise components [19]. In this study, the authors focused the attention on another key source of mental engagement likely involved in the construction of cognitive reserve, i.e. occupational complexity [20, 21], reporting that higher levels of occupational complexity were associated with better baseline cognition [22]. While the cognitive benefit of the FINGER intervention did not vary significantly among participants with different levels of occupational complexity, we found that the beneficial effects of our ‘Train the Brain’ intervention were more pronounced among individuals with lower education than among those with higher education, although being statistically significant in both groups, both at T7 and at T14. Albeit distinct, the two variables involved in the cognitive reserve are likely not independent, as late levels of occupational complexity might be at least partially accounted for by early levels of education (see [23, 24]. As a note of caution, it should also be noted that the FINGER population is representative of individuals at risk for dementia, but in the absence of a cognitive impairment, as is instead the case of the cohort included in our ‘Train the Brain’ study.
While men have a greater risk for MCI [25], women display greater longitudinal rates of progression towards dementia than men (e.g. [26, 27]. This qualifies MCI women as a particularly vulnerable population. However, data on sociodemographic variables, such as gender, that may influence training gains remain rare. We show that the beneficial effects of the training were more marked among women, particularly at T14, in agreement with previous evidence for stronger cognitive training improvements of female compared to male MCI patients (e.g. [28]). Moreover, the higher loss to follow-up in men compared to women might indicate that they dropped the training programme due to a worsening of their cognitive status. In fact, at T7, the females who underwent the training have a significant improvement in ADAS-Cog, while males did not. Still at T7, untrained females did not experience a significant worsening, while untrained males worsened significantly in ADAS-Cog.
In parallel to gender, a variable affecting the risk for dementia in MCI individuals is age. The prevalence of MCI has been reported to vary from 6.7% at 60–64 years, to 14.8% between ages 75 and 79 and to 25.8% for ages 80–84, with the rate of progression from MCI to dementia being between 9 and 20% per year depending on the specific characteristics of the population [5]. Importantly, we found a similar impact of the intervention on both age groups of <75 years of age and of >75 years, a result that strengthens the general application of the ‘Train the Brain’ intervention.
The mechanisms underlying the beneficial effects of the intervention remain unclear. Exercise and exposure to environmental enrichment in animal models are known to boost hippocampal neurogenesis, to reduce amyloid-β levels, to increase neurotrophins and to improve cognitive abilities in normal and pathological ageing (see [29]. In humans, physical exercise in older people reverses hippocampal volume loss, cognitive decline and cardiovascular physical performance [30]. Exercise, alone or in combination with cognitive stimulation, can also affect the cerebral blood flow [7, 31], a sensitive parameter that, in individuals with MCI, is affected prior to the transition to Alzheimer’s disease [32]. Finally, a growing body of research suggests that ageing is associated with enhanced inflammation in the brain and that chronic neurodegenerative diseases are associated with an abnormal inflammatory response [33–37]. Although a tight link among inflammation, ageing and dementia is clearly emerging, knowledge about the possibility to target neuroinflammation with suitable intervention strategies is still lacking.
This study has some limitations. First, the measurement of cognitive impairment was based on selected cognitive tests and could obviously be prone to errors. Second, the number of participants was limited even if a related strength was that they were directly recruited from the community. Third, the two main components of the intervention, namely motor and cognitive stimulation, were not independently assessed for their specific contribution to the global effect. Fourth, the results concerning MRI assessment of cerebral volumetry and perfusion at T14 are still in progress. Future studies should overcome these limitations.
Conclusions
This study provides strong support to the increasing body of evidence that indicates that the ‘Train the Brain’ intervention exerts beneficial effects in older people at risk for cognitive decline and dementia [7, 13, 38, 39]. The maintenance of the cognitive effects several months post-intervention is particularly encouraging, as it underscores the role of combined cognitive-motor interventions in reducing or delaying dementia conversion in MCI subjects.
Further research with larger sample sizes for the subgroups and longer follow-up evaluations is warranted.
Acknowledgements
Institutional Review Board Statement-Study design and protocol, including subject privacy and sensitive data treatment, have been approved by the Regional Ethical Committee for Clinical Experimentation. ClinicalTrials.gov Identifier is NCT01725178 (8 November 2012). All methods included in the protocol were carried out in accordance with the guidelines laid down in the Declaration of Helsinki of 1975 (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/), revised in 2013. We thank Renzo Di Renzo, Elena Novelli and Elena Orsucci for their precious technical assistance to the execution of the study.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This intervention was made possible by the generous funding of ‘Fondazione Pisa’ to the project ‘Train the Brain’ (Bando Ricerca Scientifica in Neuroscienze 2007 of Fondazione Cassa di Risparmio di Pisa).
Data Availability Statement
Data comply with human subjects protection and as such are not publicly available.
References
Author notes
Alessandro Sale and Marianna Noale Equal contribution to this work.
Acknowledgement of collaborative authorship: The Train the Brain Consortium members are: L. Maffei, E. Picano, M. G. Andreassi, A. Angelucci, F. Baldacci, L. Baroncelli, T. Begenisic, P. F. Bellinvia, N. Berardi, L. Biagi, J. Bonaccorsi, E. Bonanni, U. Bonuccelli , A. Borghini, C. Braschi, M. Broccardi, R. M. Bruno, M. Caleo, C. Carlesi, L. Carnicelli, G. Cartoni, L. Cecchetti, M. C. Cenni, R. Ceravolo, L. Chico, S. Cintoli, G. Cioni, M. Coscia, M. Costa , G. D’Angelo, P. D’Ascanio, M. De Nes, S. Del Turco, E. Di Coscio, M. Di Galante , N. di Lascio, F. Faita, I. Falorni, U. Faraguna, A. Fenu, L. Fortunato, R. Franco, L. Gargani, R. Gargiulo, L. Ghiadoni, F. S. Giorgi, R. Iannarella, C. Iofrida, C. Kusmic, F. Limongi, M. Maestri, M. Maffei, S. Maggi, M. Mainardi, L. Mammana, A. Marabotti, Mariotti V. , E. Melissari, A. Mercuri, S. Micera, S. Molinaro, R. Narducci, T. Navarra, M. Noale, C. Pagni, S. Palumbo, R. Pasquariello, S. Pellegrini, P. Pietrini, T. Pizzorusso, A. Poli, L. Pratali, A. Retico, E. Ricciardi, G. Rota, A. Sale, S. Sbrana, G. Scabia, M. Scali, D. Scelfo , R. Sicari, G. Siciliano, F. Stea, S. Taddei, G. Tognoni, A. Tonacci , M. Tosetti, S. Turchi & L. Volpi, Institute of Neuroscience of the CNR, Via G. Moruzzi 1, 56100 Pisa, Italy. Institute of Clinical Physiology of the CNR, Via G. Moruzzi 1, 56124 Pisa, Italy. Department of Clinical and Experimental Medicine-Neurology Unit, University of Pisa & AOU Pisa, Italy. IRCCS Stella Maris, Viale del Tirreno 341, Calambrone, Italy. Department of Surgical, Medical, Molecular Pathology and of Critical Care, University of Pisa, Via Savi 10, 56126, Pisa, Italy. Department of Clinical and Experimental Medicine, Pisa University, Via Savi 10, 56126 Pisa, Italy. Department of Translational research and New technologies in Medicine and Surgery, Pisa University, Via Savi 10, 56126 Pisa, Italy. Bertarelli Foundation Chair in Translational Neuroengineering, Center for Neuroprosthetics and Institute of Bioengineering, Ecole Polytechnique Federale de Lausanne, CH-1015 Lausanne, Switzerland. Scuola Superiore Sant’Anna, P.za Martiri della Libertà 33, 56127 Pisa, Italy. National Institute of Nuclear Physics (INFN), Pisa Section, Largo B. Pontecorvo, 3 56127, Pisa, Italy.




Comments