-
PDF
- Split View
-
Views
-
Cite
Cite
Amanda Brass, Andrew P Shoubridge, Nicolas Larby, Levi Elms, Sarah K Sims, Erin Flynn, Caroline Miller, Maria Crotty, Lito E Papanicolas, Steve L Wesselingh, Lidia Morawska, Scott C Bell, Steven L Taylor, Geraint B Rogers, Targeted reduction of airborne viral transmission risk in long-term residential aged care, Age and Ageing, Volume 51, Issue 12, December 2022, afac316, https://doi.org/10.1093/ageing/afac316
Close - Share Icon Share
Abstract
COVID-19 has demonstrated the devastating consequences of the rapid spread of an airborne virus in residential aged care. We report the use of CO2-based ventilation assessment to empirically identify potential ‘super-spreader’ zones within an aged care facility, and determine the efficacy of rapidly implemented, inexpensive, risk reduction measures.
Key Points
CO2 monitoring of indoor spaces identifies areas of high respiratory viral transmission risk.
Staff spaces (but not communal areas) were identified as being high-risk areas.
Simple ventilation interventions reduced infection transmission risk.
Introduction
Outbreaks of COVID-19 in long-term residential aged care facilities (RACF) have proven devastating. The combination of high transmission rates and resident vulnerability has led to rates of mortality that are higher than any other setting, including acute care hospital facilities [1]. In response, aged care providers and public health authorities have enacted measures to reduce the risk of SARS-CoV-2 exposure to prevent transmission within facilities. These measures include social distancing, reduced visitation (site lockdowns), the wearing of personal protective equipment (PPE) by staff and visitors, and isolation of those with active infection. The impact of these measures on the well-being and quality-of-life of residents, more than half of whom have reduced cognitive ability or dementia [2], has been considerable [3]. The need to identify less disruptive measures to protect RACF populations from airborne viral transmission (including respiratory viruses other than SARS-CoV-2) is urgent.
There are multiple strategies to reduce rates of indoor viral transmission, including those that prevent ingress of infectious agents (e.g. lockdowns and occupancy limits to restrict visitors and resident movements), those that reduce resident vulnerability (e.g. vaccination programmes and PPE use) and those that prevent the accumulation of viable viral particles within the circulating air of an RACF environment [4, 5]. This latter group of measures is more effective as it does not require any behaviour changes to staff or visitors. It also offers long-term advantages by avoiding the need to place additional burden on facility residents and staff, or the need to limit access to facilities by family members. Several strategies to reduce the build-up of viable viral particles can be employed, including the use of high-efficiency particulate absorbing air-filtration, or ultraviolet C disinfection devices [3, 4]. However, most effective approach is arguably to remove viral particles through increased ventilation [6].
Outdoor venues are associated with a low relative risk for airborne viral transmission because of the rapid clearance of virus-containing airborne particles through natural air movement. Indoor spaces, where air exchange is considerably reduced, are estimated to have a SARS-CoV-2 transmission risk that is more than 18 times higher [7]. Increasing rates of air exchange within the built environment would reduce this transmission risk substantially. The principal reason that this strategy has not been employed widely is the considerable costs associated with heating or cooling external air [8]. Instead, many facilities, including RACFs, typically employ high rates of air recirculation, inadvertently resulting in recirculation of the virus as well [6].
Increasing ventilation within facilities that house vulnerable populations has been advocated widely during the COVID-19 pandemic [9]. However, the implementation of ventilation measures has been limited. The ability to target increases in air exchange to those zones within facilities that carry the highest transmission risk would greatly increase the financial feasibility of improving ventilation. Here we report the use of a rapid, low-cost strategy to empirically identify potential ‘super-spreader’ zones within an RACF, and the efficacy of the targeted ventilation-based risk reduction measures that were deployed in response.
Methods
The assessed facility was a two-storey, 8,400 m2, 110-bedroom RACF in South Australia. A total of 25 communal rooms were accessible to both staff and residents, including dining rooms, lounge rooms, activity rooms, toilets and a hair salon. In addition, 24 areas were accessible only to staff, including meeting rooms, offices, nurse stations, lunchrooms, kitchens and laundry facilities.
Sixty-two wall-mounted, remotely monitored CO2 sensors (Aranet4 Pro Sensors, CO2 Radical, Australia) were used to monitor CO2 levels (logged at 60-s intervals) over a 4-week period in February 2022. Sensors were deployed across all 25 communal rooms, 17 of the 24 staff-only areas, as well as 20 connecting sites such as corridors and lifts. Nurse stations, single occupancy offices and some staff change rooms were not assessed. Sensors were typically placed on walls ~2 m off the ground and not directly adjacent to windows or doors. While atmospheric CO2 levels remain relatively constant, indoor CO2 levels increase as a result of human respiration. Elevated CO2 levels have been used widely as a marker of indoor air quality and, relative to occupancy, a measure of air exchange and ventilation efficiency. Indeed, such measures correlate strongly with infectious disease outcomes, such as absenteeism because of acute respiratory infection [10].
Zones with a high transmission risk were identified based on CO2 levels exceeding 1,000 parts per million (ppm) for a period of 15 min or more. These CO2 peaks were based on levels recommended by The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) of 600 ppm above outdoor concentrations (~420 ppm) [11].
During this period, all staff and visitors to the RACF were required to wear N95 face masks (or equivalent), goggles or face shield, and be vaccinated against COVID-19. The zones employed a range of heating, ventilation and air conditioning (HVAC) systems, including evaporative cooling with 100% fresh ducted air, refrigerated reverse cycle ducted air, refrigerated reverse cycle split system and exhaust air.
Where high-risk zones were identified, the ventilation rate was assessed by CO2 tracer gas decay curve, as previously described [12], with air changes per hour (ACH) calculated by non-linear regression. Interventions to address ventilation in high-risk zones were performed according to building facility recommendations. Assessment of additional running costs was calculated by an AC current and voltage data logger over a 1-week period.
Results
All 62 wall-mounted sensors recorded CO2 levels over the survey period with negligible impact to staff and residents. Of the 45 monitors in mixed zones (accessed by both staff and residents), none showed indications of poor ventilation. However, of the 17 staff-only zones, three (17.6%) were identified as having a high airborne infection transmission risk. These included one staff lunchroom and two staff meeting rooms (Figure 1A). Interventions to minimise airborne infection transmission risk were identified based on the zone usage, the HVAC modality employed and the ability to achieve improved ventilation rapidly and at low relative cost.
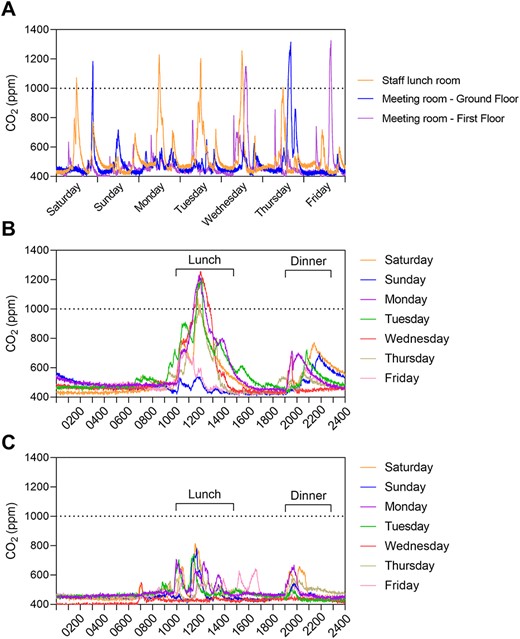
CO2 levels in a long-term RACF. (A) Showing high CO2 peaks in three high-risk airborne infection transmission zones. (B) CO2 levels over a 24-h period in the staff lunchroom over the course of a week prior to the installation of the extraction fan, showing high prolonged peaks between 1,000–1,400 and 1,900–2,200, corresponding with staff lunch and dinner breaks. (C) Lunchroom CO2 levels after the installation of the extraction fan, showing reduced peaks.
High-risk zone 1: the staff lunchroom had a floor area of 19 m2 with a refrigerated reverse cycle split system HVAC. Despite room density restrictions of one person per 4 m2, high, regular and prolonged CO2 peaks were identified in the lunchroom corresponding to meal breaks (Figure 1B). Of note, staff were not masked while using this room and CO2 levels did not return to baseline levels for several hours after these peak periods. To quantify room ventilation, a subsequent CO2 decay curve was generated over a 6-h period, during which the staff lunchroom was not occupied. The resulting ACH was 0.65 (Supplementary Figure S1), which is 10-fold lower than the Australian Standards for ventilation in a healthcare setting [13]. Installation of an extraction fan ($3,400 AUD) at 100 L/s was identified as an add-on ventilation option. Retesting ventilation by CO2 decay curve after extraction fan installation yielded an improved ACH of 5.30 (Supplementary Figure S1), and no peaks during mealtimes (Figure 1C). Ongoing extraction fan running costs were estimated at $40.20 AUD per year.
High-risk zones 2 and 3: two staff meeting rooms also had CO2 peaks indicative of high transmission risk. The first room had a floor area of 50 m2 and a refrigerated reverse cycle ducted air system controlled by a remote. CO2 peaks in this room were less frequent than the staff lunchroom, with a total of two peaks >1,000 ppm during the 4-week survey period. Facility management noted that the HVAC system was rarely running during meetings. An intervention strategy was therefore minimal, with the HVAC system programming adjusted to automatically turn on while the room is in use. The second room had a floor area of 20 m2 and a refrigerated reverse cycle split system HVAC. Like the first meeting room, two peaks >1,000 ppm were recorded during the survey period. However, unlike the first meeting room, existing HVAC could not be easily improved. Interim protocols were therefore implemented, continuing a mandated mask use and room density limit for staff.
Discussion
Globally, the COVID-19 pandemic has again underlined the vulnerability of those living in long-term residential aged care to airborne transmission of viral infections. In addition to COVID-19, seasonal respiratory viruses, including seasonal influenza and respiratory syncytial virus, represent a major cause of morbidity and mortality for residents [14]. Indeed, following a period of reduced exposure, seasonal influenza peaks are predicted to pose ongoing and significant health threats [15]. Targeting effective risk reduction measures to areas that are likely to contribute disproportionately viral spread could provide considerable protection. We identified staff-access rooms (particularly the tearoom) as high risk and deployed simple ventilation strategies to reduce this risk. Notably, the staff tearoom has been identified as high transmission risk zones in other healthcare settings [16].
In our analysis, we used passive and simulated CO2 levels to identify high transmission risk zones. Such approaches are simple, inexpensive and readily deployable, allowing for high airborne transmission risk zones within facilities to be identified rapidly and mitigation strategies to be enacted. CO2 sensors that allow accurate and longitudinal monitoring typically range from $200 to $500 AUD. Sensors can be deployed in large numbers to assess ventilation of areas within a site in parallel, as here. Alternatively, smaller numbers of units can be used to assess areas sequentially to minimise equipment costs. While CO2 levels do not account for other factors affecting airborne transmission risk, such as PPE and amount of interaction between individuals [17], CO2 levels have been used to model indoor infection risk [18]. For example, a CO2 concentration of 1,000 ppm in a room with 10 people corresponds to an influenza reproductive number (R0) of 5 [18]. In our analysis, we applied CO2 peaks based on levels recommended by ASHRAE [11], which are based on consideration of a number of factors, including comfort. Alignment of target levels more closely with empirically assessed viral transmission rates would enable further protocol refinements. In conclusion, the approach we describe demonstrates rapid, targeted, improvements in air quality management, based on empirical facility surveys, which has the potential to be applied across RACFs as well as other high-risk settings including detention facilities, hospitals or schools.
Acknowledgements
We would like to thank the RACF staff and residents for their assistance in undertaking this study.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study was funded through the Australian Medical Research Future Fund (GNT2005904). S.L.T. is supported by an NHMRC Emerging Leader Fellowship (GNT2008625). G.B.R. is supported by an NHMRC Senior Research Fellowship (GNT1155179) and a Matthew Flinders Professorial Fellowship. C.M. is supported by an NHMRC Emerging Leader Fellowship (GNT1195421) and undertakes commissioned research for SA Health.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Author notes
Joint senior.




Comments