-
PDF
- Split View
-
Views
-
Cite
Cite
Dengfeng Xu, Yifei Lu, Xian Yang, Da Pan, Yuanyuan Wang, Shiyu Yin, Shaokang Wang, Guiju Sun, Effects of fish oil-derived n-3 polyunsaturated fatty acid on body composition, muscle strength and physical performance in older people: a secondary analysis of a randomised, double-blind, placebo-controlled trial, Age and Ageing, Volume 51, Issue 12, December 2022, afac274, https://doi.org/10.1093/ageing/afac274
Close - Share Icon Share
Abstract
the effects regarding n-3 polyunsaturated fatty acid (n-3 PUFA) supplementation on sarcopenia have been explored by several clinical trials. Nonetheless, the use of n-3 PUFA for improving body composition, muscle strength and physical performance in older people is conflicting.
our aim was to perform a randomised, double-blind, controlled trial to evaluate the effects of 6-month n-3 PUFA supplementation on body composition, muscle strength and physical performance in older Chinese people.
in this double-blind, placebo-controlled trial, 200 eligible subjects were randomly assigned to receive 4 g/day fish oil capsules (1.34 g eicosapentaenoic [EPA] + 1.07 docosahexaenoic [DHA]) or 4 g/day corn oil capsules (EPA + DHA <0.05 g) for 6 months. The primary outcomes were the changes of body composition, muscle strength (hand grip strength) and physical performance (Timed Up and Go time). Secondary outcomes were the changes in serum lipid profiles.
compared with control group, fish oil-derived n-3 PUFA supplementation resulted in significant increases in thigh circumference (interaction time × group effect P < 0.001), total skeletal muscle mass (interaction time × group effect P < 0.001) and appendicular skeletal muscle mass (interaction time × group effect P < 0.001); the differences were still significant even after height correction. Muscle strength and physical performance including hand grip strength (interaction time × group effect P < 0.001) and Timed Up and Go time (interaction time × group effect P < 0.001) were also improved after a 6-month fish oil-derived n-3 PUFA intervention. In terms of serum lipid profiles, fish oil-derived n-3 PUFA supplementation could significantly reduce serum level of triglyceride (interaction time × group effect P = 0.012) and increase high density lipoprotein cholesterol (interaction time × group effect P < 0.001); while no significant improvement was found in serum concentrations of total cholesterol (interaction time × group effect P = 0.413) and low density lipoprotein cholesterol (interaction time × group effect P = 0.089).
our present trial demonstrated that a 6-month fish oil-derived n-3 PUFA supplementation could beneficially affect the body composition, muscle strength, physical performance and serum lipid profiles in older people, which could be into considerations when making strategies aiming to the primary prevention of sarcopenia.
Key Points
n-3 polyunsaturated fatty acid (PUFA) could improve lipid profiles.
n-3 PUFA could confer beneficial effects on body composition, muscle strength and physical performance.
n-3 PUFA could be taken into considerations when making strategies aiming to the primary prevention of sarcopenia.
Introduction
Age-related declines in muscle mass, strength and physical performance are significantly associated with increased risk of most chronic diseases, such as dementia, frailty and sarcopenia [1]. It is estimated that muscle mass and strength begin to decline at a rate of ~0.5–1% and 3–4% per year in older people [2, 3]. It exists a vicious circle starting from the ageing leading to poor physical performance, and finally to sarcopenia which increase the risk of adverse outcomes in older people. Thus, identification of modifiable risk factors for physical performance decline is essential to develop effective strategies for the primary prevention of disability in older people.
The reasons for age-related declines in muscle mass are multifactorial, including chronic inflammation [4], altered hormonal profile [5, 6], inadequate physical activity [7] and other conditions. For instance, prospective study suggested that compared with low C-reactive protein (CRP <1.4 μg/ml), patients with high CRP levels (>6.1 μg/ml) were significantly correlated with a 2- to 3-fold greater risk of losing more than 40% of muscle strength [8]. In addition, it has been proven that the growth and differentiation of skeletal muscles could be regulated via growth hormone-insulin-like growth factor axis (GH-IGF axis) [9]. Animal experiments also provided the evidence for an increased synthesis of skeletal muscle protein following a GH replacement therapy in aged rat [10]. However, we should note that the efficacy of pharmacological therapy in aged-related diseases might be limited due to the special physiological state of being weak and sickly in older people.
In contrast, impressive body of evidence has increasingly focused on the crucial role of exercise and nutritional supplementation in the prevention of the loss of muscle mass and strength (sarcopenia) [11–13]. Particularly resistance training, a group of exercises that require subjects to exercise against an increasing external load, has been indicated to play a protective role in management of muscle strength and physical function in older adults [14]. However, sometimes, it is not always easy for frail older adults with diminishing physiological function to keep a regular physical activity [15, 16]. Consequently, nutrition therapy might be a desirable alternative in muscle health [17].
Nutritional supplement, which is defined as products intended to provide nutrients or non-nutrient chemicals which are claimed to have biologically beneficial effects including minerals, vitamins, amino acids and other substances, could also show positive links to the improvements of muscle mass, strength and function in older adults [18, 19]. N-3 PUFAs are a group of long-chain fatty acids including eicosapentaenoic (EPA) and docosahexaenoic (DHA) and can be found in most fatty fish. Epidemiological data have revealed a positive association between low levels of EPA and low muscle parameters values [20]. On the other hand, rodents experiment indicated that n-3 PUFA supplementation could improve muscle strength and quality in high fat diet-induced C57BL/6 mice [21]. Evidence from clinical trial also supported the beneficial effect of n-3 PUFA supplementation on muscle mass and function [22]. These prior studies indicated the important role of n-3 PUFA supplementation in maintaining physical independence in older people. However, another randomised controlled trial showed that there was no significant effect of a 1.3 g/day n-3 PUFA supplementation on body composition and handgrip strength in older adults at risk or having low lean mass at baseline [23].
Given this background, this randomised controlled trial aims to assess the effects of fish oil-derived n-3 PUFA on body composition, muscle strength and physical performance on older Chinese people. The results would provide evidence for the role of n-3 PUFA supplementation in the primary prevention of sarcopenia.
Methods
Study design and population
In this preplanned comparative analysis, the population comes from the patients who are over 60 years old in a randomised controlled trial our group conducted before [24, 25]. The study was designed as a 6-month, randomised, double-blind, placebo-controlled trial. All outcomes measured in the present trial were collected three times, which were at the baseline (month 0), middle (month 3) and end (month 6) of the trial, respectively. The study protocol was approved by the ethic committee of Zhongda Hospital Affiliated Southeast University, and registered in the China Ethics Committee of Registering Clinical Trials (ChiCTR-TRC-14005084, ChiCTR-IOR-16008435). Written informed consent was obtained from all participants.
The older adults (≥60 years) were recruited via posters and advertisements placed in Guanlin Hospital in Yixing City, China. Prior to the trial, we collected some information about the diet background and physical activity of volunteers through a brief questionnaire. Volunteers were excluded if they have: (i) daily supplementation of n-3 PUFA for the last 6 months; (ii) diagnoses of serious kidney, liver or digestive tract disease; (iii) volunteers with severe joint and muscle disease; (iv) heavy smoking or drinking and (v) poor compliance.
Randomisation and sample size
Randomisation was performed by computer software with Excel 2016 (Microsoft Office, USA).
Intervention
According to the computer-generated random numbers, the enrolled volunteers were randomly and equally allocated to control group and experiment group in a blinded fashion. The subjects in experiment group were required to supplement 4 g/day of capsule of fish oil-derived n-3 PUFA (1.34 g EPA + 1.07 DHA), while subjects in control group were required to consume 4 g/day of corn oil capsule (EPA + DHA <0.05 g). The main fatty acid compositions of two test capsules are presented in supplemental files, Supplementary Table S1. For eligible participants, a 3-day 24-h dietary recall questionnaire and a physical activity record were completed at the beginning and end of the trial, respectively. All test samples were provided by BYHEALTH Co., Ltd. Guangdong, China. The appearance and taste of capsules in two groups were produced to be consistent, so both participants and study personnel were blinded to the treatment assignment. During the whole trial, participants were asked to maintain their dietary habits and physical activity. The intervention samples were provided to the subjects once every 2 weeks, and any adverse effects or concerns from volunteers could be communicated with study personnel.
Outcomes measurement
General anthropometrics
Height, body weight, waistline and hipline were measured with standardised techniques. The thigh circumference was measured directly below the gluteal fold, while subjects were required to stand vertically with their legs as wide as their shoulders. The mean value of left thigh circumference and right thigh circumference was regarded as the final thigh circumference. Systolic and diastolic blood pressures were measured with an automated electronic device (Omron HEM-770A, Kyoto, Japan).
Body composition
Muscle strength and physical performance
Hand grip strength
The CAMRY Hand dynamometer (EH101, Xiangshan, Guangdong) was used to evaluate muscle function following a standardised procedure [27].
Timed Up and Go time
Participants were required to take a rise from a chair, walk 3 m, turn around, walk back to the chair and sit down [28].
Biochemical detection
Fasting blood samples were collected by venipuncture and centrifuged at 1500 × g for 15 min at 4°C, then stored at −80°C until the final analysis. Clinical chemistry parameters including total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), serum total proteins and albumin were measured by enzymatic-colorimetric methods with an automatic biochemical analyzer (Beckman, DxC800, USA).
Statistical analysis
Continuous and categorical variables were presented as mean|$\pm$|SD, and counts (percentages), respectively. The effects of n-3 PUFA on body composition, muscle strength, physical performance and lipid profiles during the intervention were compared by f-test from analysis of variance for repeated measures, among which the group effect was fixed. The principle of intention to treat analysis was followed, and the method of last observation carried forward was used for imputation of missing data. All analyses were conducted in SPSS version 18.0 (SPSS Inc, USA) with a 5% level of significance.
Results
Baseline characteristics
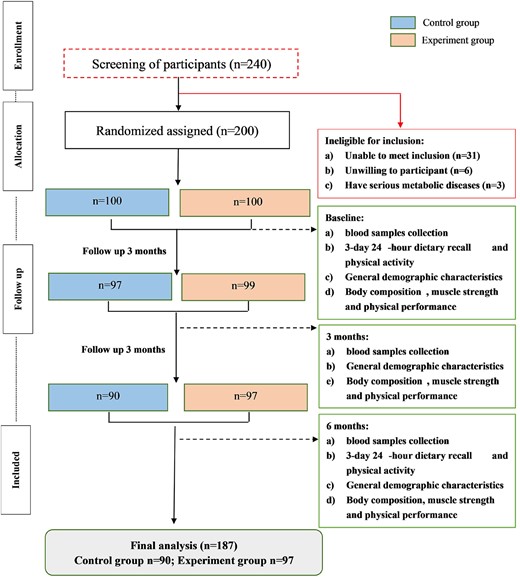
We screened 240 older adults for eligibility, in which 200 older adults were randomly assigned to control group and experiment group (Figure 1). After 3-month intervention, four participants dropped out of which two changed their residence, and two declined to continue the trial because of some side effects unrelated to the trial. After 6-month intervention, there were another nine participants who were excluded from the study, six of whom had poor compliance, and three dropped out for some personal reasons. The rate of dropped out during the whole trial was 6.5%. Therefore, a total of 187 samples completed the entire study (90 in control group and 97 in experiment group). General anthropometric and clinical characteristics between groups at baseline are shown in Table 1.
| Characteristics . | Control group (n = 100) . | Experiment group (n = 100) . |
|---|---|---|
| Age (year) | 67.31 ± 5.03 | 66.63 ± 4.21 |
| Gender (M/F) | 42/58 | 42/58 |
| Weight (kg) | 63.36 ± 8.95 | 64.57 ± 9.00 |
| Height (cm) | 160.31 ± 7.70 | 158.58 ± 7.76 |
| Waistline (cm) | 87.19 ± 7.94 | 87.51 ± 9.77 |
| Hipline (cm) | 96.45 ± 7.61 | 97.62 ± 5.86 |
| Waist-hip ratio | 0.91 ± 0.10 | 0.90 ± 0.09 |
| BMI (kg/m2) | 24.63 ± 2.94 | 25.64 ± 2.72 |
| SBP (mm Hg) | 143.52 ± 16.79 | 143.43 ± 18.36 |
| DBP (mm Hg) | 83.01 ± 7.55 | 82.84 ± 8.23 |
| Muscle mass (kg) | 41.09 ± 7.24 | 41.58 ± 7.32 |
| Body fat mass (kg) | 19.59 ± 6.35 | 20.50 ± 7.00 |
| Body fat percentage (%) | 30.83 ± 8.31 | 31.51 ± 8.06 |
| Basal metabolic rate | 1237.63 ± 173.49 | 1286.27 ± 198.89 |
| Creatinine (umol/L) | 62.76 ± 11.32 | 61.50 ± 12.03 |
| Uric acid (umol/L) | 320.77 ± 74.76 | 323.32 ± 84.07 |
| Characteristics . | Control group (n = 100) . | Experiment group (n = 100) . |
|---|---|---|
| Age (year) | 67.31 ± 5.03 | 66.63 ± 4.21 |
| Gender (M/F) | 42/58 | 42/58 |
| Weight (kg) | 63.36 ± 8.95 | 64.57 ± 9.00 |
| Height (cm) | 160.31 ± 7.70 | 158.58 ± 7.76 |
| Waistline (cm) | 87.19 ± 7.94 | 87.51 ± 9.77 |
| Hipline (cm) | 96.45 ± 7.61 | 97.62 ± 5.86 |
| Waist-hip ratio | 0.91 ± 0.10 | 0.90 ± 0.09 |
| BMI (kg/m2) | 24.63 ± 2.94 | 25.64 ± 2.72 |
| SBP (mm Hg) | 143.52 ± 16.79 | 143.43 ± 18.36 |
| DBP (mm Hg) | 83.01 ± 7.55 | 82.84 ± 8.23 |
| Muscle mass (kg) | 41.09 ± 7.24 | 41.58 ± 7.32 |
| Body fat mass (kg) | 19.59 ± 6.35 | 20.50 ± 7.00 |
| Body fat percentage (%) | 30.83 ± 8.31 | 31.51 ± 8.06 |
| Basal metabolic rate | 1237.63 ± 173.49 | 1286.27 ± 198.89 |
| Creatinine (umol/L) | 62.76 ± 11.32 | 61.50 ± 12.03 |
| Uric acid (umol/L) | 320.77 ± 74.76 | 323.32 ± 84.07 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
| Characteristics . | Control group (n = 100) . | Experiment group (n = 100) . |
|---|---|---|
| Age (year) | 67.31 ± 5.03 | 66.63 ± 4.21 |
| Gender (M/F) | 42/58 | 42/58 |
| Weight (kg) | 63.36 ± 8.95 | 64.57 ± 9.00 |
| Height (cm) | 160.31 ± 7.70 | 158.58 ± 7.76 |
| Waistline (cm) | 87.19 ± 7.94 | 87.51 ± 9.77 |
| Hipline (cm) | 96.45 ± 7.61 | 97.62 ± 5.86 |
| Waist-hip ratio | 0.91 ± 0.10 | 0.90 ± 0.09 |
| BMI (kg/m2) | 24.63 ± 2.94 | 25.64 ± 2.72 |
| SBP (mm Hg) | 143.52 ± 16.79 | 143.43 ± 18.36 |
| DBP (mm Hg) | 83.01 ± 7.55 | 82.84 ± 8.23 |
| Muscle mass (kg) | 41.09 ± 7.24 | 41.58 ± 7.32 |
| Body fat mass (kg) | 19.59 ± 6.35 | 20.50 ± 7.00 |
| Body fat percentage (%) | 30.83 ± 8.31 | 31.51 ± 8.06 |
| Basal metabolic rate | 1237.63 ± 173.49 | 1286.27 ± 198.89 |
| Creatinine (umol/L) | 62.76 ± 11.32 | 61.50 ± 12.03 |
| Uric acid (umol/L) | 320.77 ± 74.76 | 323.32 ± 84.07 |
| Characteristics . | Control group (n = 100) . | Experiment group (n = 100) . |
|---|---|---|
| Age (year) | 67.31 ± 5.03 | 66.63 ± 4.21 |
| Gender (M/F) | 42/58 | 42/58 |
| Weight (kg) | 63.36 ± 8.95 | 64.57 ± 9.00 |
| Height (cm) | 160.31 ± 7.70 | 158.58 ± 7.76 |
| Waistline (cm) | 87.19 ± 7.94 | 87.51 ± 9.77 |
| Hipline (cm) | 96.45 ± 7.61 | 97.62 ± 5.86 |
| Waist-hip ratio | 0.91 ± 0.10 | 0.90 ± 0.09 |
| BMI (kg/m2) | 24.63 ± 2.94 | 25.64 ± 2.72 |
| SBP (mm Hg) | 143.52 ± 16.79 | 143.43 ± 18.36 |
| DBP (mm Hg) | 83.01 ± 7.55 | 82.84 ± 8.23 |
| Muscle mass (kg) | 41.09 ± 7.24 | 41.58 ± 7.32 |
| Body fat mass (kg) | 19.59 ± 6.35 | 20.50 ± 7.00 |
| Body fat percentage (%) | 30.83 ± 8.31 | 31.51 ± 8.06 |
| Basal metabolic rate | 1237.63 ± 173.49 | 1286.27 ± 198.89 |
| Creatinine (umol/L) | 62.76 ± 11.32 | 61.50 ± 12.03 |
| Uric acid (umol/L) | 320.77 ± 74.76 | 323.32 ± 84.07 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Effects of fish oil-derived n-3 PUFA supplementation on general anthropometrics
The results showed that fish oil-derived n-3 PUFA supplementation for 6 months could significantly increase thigh circumference in older Chinese adults (interaction time × group effect P < 0.001, Table 2). However, no significant difference in waistline (interaction time × group effect P = 0.569, Table 2), hipline (interaction time × group effect P = 0.746, Table 2) and waist–hip ratio (interaction time × group effect P = 0.437, Table 2) was found between groups.
Effects of oil-derived n-3 PUFA supplementation on thigh circumference, waistline, hipline and waist–hip ratio
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TC (cm) | 48.67 ± 3.77 | 47.90 ± 5.99 | 47.64 ± 5.91 | 48.88 ± 3.90 | 49.55 ± 3.76 | 49.56 ± 3.66 | 0.508 | 0.042 | <0.001 |
| Waistline (cm) | 87.19 ± 7.94 | 87.62 ± 8.27 | 88.47 ± 9.24 | 87.51 ± 9.77 | 87.76 ± 10.04 | 87.97 ± 10.43 | 0.099 | 0.992 | 0.569 |
| Hipline (cm) | 96.45 ± 7.61 | 96.38 ± 6.78 | 98.64 ± 8.71 | 97.62 ± 5.86 | 97.02 ± 6.33 | 99.57 ± 5.87 | <0.001 | 0.304 | 0.746 |
| Waist-hip ratio (%) | 90.78 ± 9.69 | 91.25 ± 10.38 | 90.08 ± 10.32 | 89.72 ± 9.10 | 90.53 ± 8.75 | 88.29 ± 8.75 | <0.001 | 0.344 | 0.437 |
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TC (cm) | 48.67 ± 3.77 | 47.90 ± 5.99 | 47.64 ± 5.91 | 48.88 ± 3.90 | 49.55 ± 3.76 | 49.56 ± 3.66 | 0.508 | 0.042 | <0.001 |
| Waistline (cm) | 87.19 ± 7.94 | 87.62 ± 8.27 | 88.47 ± 9.24 | 87.51 ± 9.77 | 87.76 ± 10.04 | 87.97 ± 10.43 | 0.099 | 0.992 | 0.569 |
| Hipline (cm) | 96.45 ± 7.61 | 96.38 ± 6.78 | 98.64 ± 8.71 | 97.62 ± 5.86 | 97.02 ± 6.33 | 99.57 ± 5.87 | <0.001 | 0.304 | 0.746 |
| Waist-hip ratio (%) | 90.78 ± 9.69 | 91.25 ± 10.38 | 90.08 ± 10.32 | 89.72 ± 9.10 | 90.53 ± 8.75 | 88.29 ± 8.75 | <0.001 | 0.344 | 0.437 |
Data were shown by mean|$\pm$|SD; f-test from analysis of variance for repeated measures for difference between groups. TC: thigh circumference.
Effects of oil-derived n-3 PUFA supplementation on thigh circumference, waistline, hipline and waist–hip ratio
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TC (cm) | 48.67 ± 3.77 | 47.90 ± 5.99 | 47.64 ± 5.91 | 48.88 ± 3.90 | 49.55 ± 3.76 | 49.56 ± 3.66 | 0.508 | 0.042 | <0.001 |
| Waistline (cm) | 87.19 ± 7.94 | 87.62 ± 8.27 | 88.47 ± 9.24 | 87.51 ± 9.77 | 87.76 ± 10.04 | 87.97 ± 10.43 | 0.099 | 0.992 | 0.569 |
| Hipline (cm) | 96.45 ± 7.61 | 96.38 ± 6.78 | 98.64 ± 8.71 | 97.62 ± 5.86 | 97.02 ± 6.33 | 99.57 ± 5.87 | <0.001 | 0.304 | 0.746 |
| Waist-hip ratio (%) | 90.78 ± 9.69 | 91.25 ± 10.38 | 90.08 ± 10.32 | 89.72 ± 9.10 | 90.53 ± 8.75 | 88.29 ± 8.75 | <0.001 | 0.344 | 0.437 |
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TC (cm) | 48.67 ± 3.77 | 47.90 ± 5.99 | 47.64 ± 5.91 | 48.88 ± 3.90 | 49.55 ± 3.76 | 49.56 ± 3.66 | 0.508 | 0.042 | <0.001 |
| Waistline (cm) | 87.19 ± 7.94 | 87.62 ± 8.27 | 88.47 ± 9.24 | 87.51 ± 9.77 | 87.76 ± 10.04 | 87.97 ± 10.43 | 0.099 | 0.992 | 0.569 |
| Hipline (cm) | 96.45 ± 7.61 | 96.38 ± 6.78 | 98.64 ± 8.71 | 97.62 ± 5.86 | 97.02 ± 6.33 | 99.57 ± 5.87 | <0.001 | 0.304 | 0.746 |
| Waist-hip ratio (%) | 90.78 ± 9.69 | 91.25 ± 10.38 | 90.08 ± 10.32 | 89.72 ± 9.10 | 90.53 ± 8.75 | 88.29 ± 8.75 | <0.001 | 0.344 | 0.437 |
Data were shown by mean|$\pm$|SD; f-test from analysis of variance for repeated measures for difference between groups. TC: thigh circumference.
Effects of fish oil-derived n-3 PUFA supplementation on skeletal muscle mass and body fat
The supplementation of fish oil-derived n-3 PUFA led to a significant increase of skeletal muscle mass including TSMM (interaction time × group effect P < 0.001, Table 3) and ASMM time × group (interaction effect P < 0.001, Table 3) compared with control group. The increases were still significant after height correction (TSMMI, interaction time × group effect P < 0.001; ASMMI, interaction time × group effect P < 0.001 Table 3). There was no significant treatment difference in body fat mass (interaction time × group effect P = 0.245, Table 3), fat free mass (interaction effect time × groupP = 0.522, Table 3) and body fat percentage (interaction time × group effect P = 0.205, Table 3).
Effects of oil-derived n-3 PUFA supplementation on skeletal muscle mass and body fat
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TSMM (kg) | 23.93 ± 4.25 | 23.69 ± 4.30 | 23.64 ± 4.19 | 24.24 ± 4.22 | 24.92 ± 4.34 | 24.96 ± 4.20 | 0.074 | 0.108 | <0.001 |
| TSMMI (kg/m2) | 9.27 ± 1.24 | 9.18 ± 1.24 | 9.17 ± 1.24 | 9.58 ± 1.09 | 9.85 ± 1.14 | 9.87 ± 1.00 | 0.084 | <0.001 | <0.001 |
| ASMM (kg) | 18.05 ± 3.22 | 17.71 ± 3.24 | 17.60 ± 3.09 | 18.37 ± 3.44 | 18.86 ± 3.50 | 19.04 ± 3.36 | 0.373 | 0.035 | <0.001 |
| ASMMI (kg/m2) | 6.99 ± 0.92 | 6.86 ± 0.91 | 6.82 ± 0.89 | 7.26 ± 0.94 | 7.45 ± 0.96 | 7.53 ± 0.86 | 0.321 | <0.001 | <0.001 |
| BFM (kg) | 19.59 ± 6.35 | 19.58 ± 5.80 | 19.65 ± 5.45 | 20.50 ± 6.99 | 19.78 ± 6.44 | 19.66 ± 6.61 | 0.308 | 0.655 | 0.245 |
| FFM (kg) | 44.06 ± 7.69 | 44.57 ± 7.34 | 44.64 ± 7.65 | 44.15 ± 7.73 | 44.99 ± 8.41 | 44.42 ± 8.07 | 0.062 | 0.925 | 0.522 |
| BFP (%) | 30.83 ± 8.31 | 30.96 ± 8.17 | 31.07 ± 7.61 | 31.51 ± 8.06 | 30.56 ± 8.53 | 30.27 ± 8.59 | 0.449 | 0.870 | 0.205 |
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TSMM (kg) | 23.93 ± 4.25 | 23.69 ± 4.30 | 23.64 ± 4.19 | 24.24 ± 4.22 | 24.92 ± 4.34 | 24.96 ± 4.20 | 0.074 | 0.108 | <0.001 |
| TSMMI (kg/m2) | 9.27 ± 1.24 | 9.18 ± 1.24 | 9.17 ± 1.24 | 9.58 ± 1.09 | 9.85 ± 1.14 | 9.87 ± 1.00 | 0.084 | <0.001 | <0.001 |
| ASMM (kg) | 18.05 ± 3.22 | 17.71 ± 3.24 | 17.60 ± 3.09 | 18.37 ± 3.44 | 18.86 ± 3.50 | 19.04 ± 3.36 | 0.373 | 0.035 | <0.001 |
| ASMMI (kg/m2) | 6.99 ± 0.92 | 6.86 ± 0.91 | 6.82 ± 0.89 | 7.26 ± 0.94 | 7.45 ± 0.96 | 7.53 ± 0.86 | 0.321 | <0.001 | <0.001 |
| BFM (kg) | 19.59 ± 6.35 | 19.58 ± 5.80 | 19.65 ± 5.45 | 20.50 ± 6.99 | 19.78 ± 6.44 | 19.66 ± 6.61 | 0.308 | 0.655 | 0.245 |
| FFM (kg) | 44.06 ± 7.69 | 44.57 ± 7.34 | 44.64 ± 7.65 | 44.15 ± 7.73 | 44.99 ± 8.41 | 44.42 ± 8.07 | 0.062 | 0.925 | 0.522 |
| BFP (%) | 30.83 ± 8.31 | 30.96 ± 8.17 | 31.07 ± 7.61 | 31.51 ± 8.06 | 30.56 ± 8.53 | 30.27 ± 8.59 | 0.449 | 0.870 | 0.205 |
Data were shown by mean|$\pm$|SD; f-test from analysis of variance for repeated measures for difference between groups.
TSMM, total skeletal muscle mass; TSMMI, total skeletal muscle mass index; ASMM, appendicular skeletal muscle mass; ASMMI, appendicular skeletal muscle mass index; BFM, body fat mass; FFM, fat free mass; BFP, body fat percentage.
Effects of oil-derived n-3 PUFA supplementation on skeletal muscle mass and body fat
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TSMM (kg) | 23.93 ± 4.25 | 23.69 ± 4.30 | 23.64 ± 4.19 | 24.24 ± 4.22 | 24.92 ± 4.34 | 24.96 ± 4.20 | 0.074 | 0.108 | <0.001 |
| TSMMI (kg/m2) | 9.27 ± 1.24 | 9.18 ± 1.24 | 9.17 ± 1.24 | 9.58 ± 1.09 | 9.85 ± 1.14 | 9.87 ± 1.00 | 0.084 | <0.001 | <0.001 |
| ASMM (kg) | 18.05 ± 3.22 | 17.71 ± 3.24 | 17.60 ± 3.09 | 18.37 ± 3.44 | 18.86 ± 3.50 | 19.04 ± 3.36 | 0.373 | 0.035 | <0.001 |
| ASMMI (kg/m2) | 6.99 ± 0.92 | 6.86 ± 0.91 | 6.82 ± 0.89 | 7.26 ± 0.94 | 7.45 ± 0.96 | 7.53 ± 0.86 | 0.321 | <0.001 | <0.001 |
| BFM (kg) | 19.59 ± 6.35 | 19.58 ± 5.80 | 19.65 ± 5.45 | 20.50 ± 6.99 | 19.78 ± 6.44 | 19.66 ± 6.61 | 0.308 | 0.655 | 0.245 |
| FFM (kg) | 44.06 ± 7.69 | 44.57 ± 7.34 | 44.64 ± 7.65 | 44.15 ± 7.73 | 44.99 ± 8.41 | 44.42 ± 8.07 | 0.062 | 0.925 | 0.522 |
| BFP (%) | 30.83 ± 8.31 | 30.96 ± 8.17 | 31.07 ± 7.61 | 31.51 ± 8.06 | 30.56 ± 8.53 | 30.27 ± 8.59 | 0.449 | 0.870 | 0.205 |
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TSMM (kg) | 23.93 ± 4.25 | 23.69 ± 4.30 | 23.64 ± 4.19 | 24.24 ± 4.22 | 24.92 ± 4.34 | 24.96 ± 4.20 | 0.074 | 0.108 | <0.001 |
| TSMMI (kg/m2) | 9.27 ± 1.24 | 9.18 ± 1.24 | 9.17 ± 1.24 | 9.58 ± 1.09 | 9.85 ± 1.14 | 9.87 ± 1.00 | 0.084 | <0.001 | <0.001 |
| ASMM (kg) | 18.05 ± 3.22 | 17.71 ± 3.24 | 17.60 ± 3.09 | 18.37 ± 3.44 | 18.86 ± 3.50 | 19.04 ± 3.36 | 0.373 | 0.035 | <0.001 |
| ASMMI (kg/m2) | 6.99 ± 0.92 | 6.86 ± 0.91 | 6.82 ± 0.89 | 7.26 ± 0.94 | 7.45 ± 0.96 | 7.53 ± 0.86 | 0.321 | <0.001 | <0.001 |
| BFM (kg) | 19.59 ± 6.35 | 19.58 ± 5.80 | 19.65 ± 5.45 | 20.50 ± 6.99 | 19.78 ± 6.44 | 19.66 ± 6.61 | 0.308 | 0.655 | 0.245 |
| FFM (kg) | 44.06 ± 7.69 | 44.57 ± 7.34 | 44.64 ± 7.65 | 44.15 ± 7.73 | 44.99 ± 8.41 | 44.42 ± 8.07 | 0.062 | 0.925 | 0.522 |
| BFP (%) | 30.83 ± 8.31 | 30.96 ± 8.17 | 31.07 ± 7.61 | 31.51 ± 8.06 | 30.56 ± 8.53 | 30.27 ± 8.59 | 0.449 | 0.870 | 0.205 |
Data were shown by mean|$\pm$|SD; f-test from analysis of variance for repeated measures for difference between groups.
TSMM, total skeletal muscle mass; TSMMI, total skeletal muscle mass index; ASMM, appendicular skeletal muscle mass; ASMMI, appendicular skeletal muscle mass index; BFM, body fat mass; FFM, fat free mass; BFP, body fat percentage.
Effects of fish oil-derived n-3 PUFA supplementation on muscle strength and physical performance
There were significant improvements on muscle strength and physical performance following a 6-month fish oil-derived n-3 PUFA supplementation. Compared with control group, fish oil-derived n-3 PUFA could significantly increase hand grip strength (interaction time × group effect P < 0.001, Table 4) and reduce the Timed Up and Go time (interaction time × group effect P < 0.001, Table 4).
Effects of oil-derived n-3 PUFA supplementation on muscle strength and physical performance
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TUG (s) | 12.08 ± 1.47 | 12.24 ± 1.58 | 12.37 ± 1.66 | 12.17 ± 1.80 | 12.07 ± 1.89 | 11.83 ± 1.85 | 0.155 | 0.389 | <0.001 |
| HGS (kg) | 23.57 ± 5.19 | 23.18 ± 5.21 | 22.71 ± 4.98 | 23.41 ± 5.01 | 24.25 ± 5.08 | 24.56 ± 4.91 | 0.084 | 0.195 | <0.001 |
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TUG (s) | 12.08 ± 1.47 | 12.24 ± 1.58 | 12.37 ± 1.66 | 12.17 ± 1.80 | 12.07 ± 1.89 | 11.83 ± 1.85 | 0.155 | 0.389 | <0.001 |
| HGS (kg) | 23.57 ± 5.19 | 23.18 ± 5.21 | 22.71 ± 4.98 | 23.41 ± 5.01 | 24.25 ± 5.08 | 24.56 ± 4.91 | 0.084 | 0.195 | <0.001 |
Data were shown by mean|$\pm$|SD; f-test from analysis of variance for repeated measures for difference between groups. TUG, Timed Up and Go; HGS, hand grip strength.
Effects of oil-derived n-3 PUFA supplementation on muscle strength and physical performance
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TUG (s) | 12.08 ± 1.47 | 12.24 ± 1.58 | 12.37 ± 1.66 | 12.17 ± 1.80 | 12.07 ± 1.89 | 11.83 ± 1.85 | 0.155 | 0.389 | <0.001 |
| HGS (kg) | 23.57 ± 5.19 | 23.18 ± 5.21 | 22.71 ± 4.98 | 23.41 ± 5.01 | 24.25 ± 5.08 | 24.56 ± 4.91 | 0.084 | 0.195 | <0.001 |
| Parameters . | Control group (n = 100) . | Experiment group (n = 100) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | 3 months . | 6 months . | Baseline . | 3 months . | 6 months . | Time . | Group . | Interaction . | |
| TUG (s) | 12.08 ± 1.47 | 12.24 ± 1.58 | 12.37 ± 1.66 | 12.17 ± 1.80 | 12.07 ± 1.89 | 11.83 ± 1.85 | 0.155 | 0.389 | <0.001 |
| HGS (kg) | 23.57 ± 5.19 | 23.18 ± 5.21 | 22.71 ± 4.98 | 23.41 ± 5.01 | 24.25 ± 5.08 | 24.56 ± 4.91 | 0.084 | 0.195 | <0.001 |
Data were shown by mean|$\pm$|SD; f-test from analysis of variance for repeated measures for difference between groups. TUG, Timed Up and Go; HGS, hand grip strength.
Effects of fish oil-derived n-3 PUFA supplementation on serum lipids profiles and proteins
Fish oil-derived n-3 PUFA conferred a significantly decreased effect on serum TG concentration (interaction time x group effect P = 0.012, Supplemental files, Supplementary Table S2) when compared with control group; besides, the level of serum HDL-C was notable increased after supplementing fish oil-derived n-3 PUFA for 6 months (interaction time x group effect P < 0.001, Supplemental files, Supplementary Table S2). However, no significant difference was found in serum TC (interaction time x group effect P = 0.413, Supplemental files, Supplementary Table S2) and LDL-C (interaction time × group effect P = 0.089, Supplementary Table S2) concentration between groups. In terms of serum proteins, fish oil-derived n-3 PUFA had no effect on either the concentration of serum total protein (interaction time × group effect P = 0.080, Supplemental files, Supplementary Table S2) or the concentration of serum albumin (interaction time × group effect P = 0.820, Supplemental files, Supplementary Table S2).
Adverse effects
Except for the two participants who refused to continue the trial due to some side effects unrelated to the trial after 3-month intervention, no other participants withdrew from the trial because of the test foods consumed.
Discussion
Age-associated declines in muscle mass and strength could lead to adverse outcomes in older people. We performed a randomised, double-blind, placebo-controlled trial to evaluate the effects of a 6-month fish oil-derived n-3 PUFA supplementation on body composition, muscle strength, physical performance and lipid profiles in older Chinese people. The results suggested that compared with control group, fish oil-derived n-3 PUFA supplementation effectively improved skeletal muscle parameters, including increasing the mass and strength of skeletal muscle and decreasing the time of TUG, as well as regulating serum lipid profiles, indicating a strong protective effect against age-associated diseases in older people.
Despite the critical role of resistance training and supplementation of anabolic agent (e.g. testosterone and GH) in the management of skeletal muscle mass and function, nutritional supplements might be a preferred method to prevent the declines in muscle mass, strength and physical performance, and avoid undesirable effects caused by drug treatments for older people [12]. In the present trial, we demonstrated that fish oil-derived n-3 PUFA supplementation significantly increased thigh circumference and skeletal muscle mass in older people; in addition, muscle strength (hand grip strength) and physical performance (Timed Up and Go time) are also improved. Epidemiological studies had revealed a significant association between dietary n-3 PUFA intake and reduced risks of both muscle weakness and lower-extremity functional impairment [29, 30]. Indeed, Smith et al. found that a 6-month fish oil-derived n-3 PUFA intervention could significantly increase thigh muscle volume and hand grip strength in healthy older adults [22], which showed a high consistency with our present trial conducted in older Chinese people. We speculated the mechanism behind these beneficial effects could be partly attributed to the elevated rate of muscle protein synthesis stimulated by n-3 PUFA [31, 32]. Our results also provided the evidence for the recommendations for the treatments of sarcopenia proposed by Geriatrics Branch Chinese Medical Association [33, 34]. However, some previous studies showed contradictory results, whose conclusions did not support the beneficial effects of n-3 PUFA supplementation in improvements of muscle quality. Murphy et al. [35] found no beneficial effect of either leucine-enriched protein supplementation alone or combined with fish oil-derived n-3 PUFA in muscle mass and function in well-nourished older adults. A similar negative result was also concluded from a secondary analysis of the Multidomain Alzheimer Preventive Trial conducted in older people with a low intervention dose of n-3 PUFA supplementation (800 mg DHA + 225 mg EPA) [36]. These inconsistent results could be due to the differences in study design, population selection, intervention duration and dosage of intervention between studies. For example, results from a randomised controlled trial conducted in 126 postmenopausal women indicated that n-3 PUFA supplementation for 6 months led to a significant improvement in walking speed, but no significant changes in hand grip strength and repeated chair rises were observed [37]. Moreover, gender difference was also one of confounding factors which should be noted. Da Boit et al. [38] found that the increased effects of n-3 PUFA supplementation in muscle quality and function were only observed in older women but not in older men. Given the conflicting findings of n-3 PUFA supplementation in muscle improvements, the evidence of using n-3 PUFA supplement to prevent sarcopenia in clinical practice should be further explored.
Affirmative evidence from recent meta-analysis based on randomized controlled trial. supported that n-3 PUFA supplementation can improve overall body muscle mass and strength [39]. However, another meta-analysis conducted by Ma et al. presented conflicting results, in which they found that supplementation with n-3 PUFA did not confer a significant effect on muscle mass in older adults; besides, the authors found that the beneficial effect of n-3 PUFA supplementation on grip strength was only observed in the population of America, but non-significant in Europe [40]. These inconsistent results might be important, for that they drive us to consider the discrepancies of different races to n-3 PUFA supplementation on muscle health. Just as found by Kothapalli et al., compared with Non-Asian populations, Asian are more likely to synthesise long-chain PUFA due to an adaptive genetic polymorphism within the FADS2 gene, which is a key gene responsible for inhibition of protein degradation [41, 42]. Indeed, cross-sectional studies have demonstrated that effects of FADS polymorphism on desaturase indices are ethnic-specific [43, 44]. With this in mind, our present study highlights the need for further region-specific researches.
The exact mechanisms by which n-3 PUFA improves muscle health are not well clarified. However, some pathways related to the anabolic and catabolic of muscle have been proposed [45]. Firstly, it is reported that higher levels of interleukin-6 and tumour necrosis factor-alpha are significantly associated with lower muscle mass and strength in older people, indicating an important role of systemic inflammation in improvement in muscle health [46, 47]. Similarly, in animal experiments, Heidrun et al. found that increased systemic inflammatory response can lead to muscle weakness [48]. N-3 PUFA and their derivatives can exert anti-inflammation effect through activating intracellular fatty acid receptors, leading to a reduced level of systemic inflammation and an inhibited activation of pathways involved in protein degradation eventually [49, 50]. On the other hand, Smith et al. found that dietary n-3 PUFA supplementation for 8 weeks can significantly increase the rate of muscle protein synthesis in older adults, and demonstrated that the action was partially mediated via increased activation of the mTOR-p70s6k signalling pathway (an integral control point for muscle cell growth) [31]. In addition, it is possible that the beneficial effects of n-3 PUFA supplementation on muscle health were due to a diminution of mitochondrial reactive oxygen species emission [32, 51].
Compared with previous studies, our study has following strengths: the sample size in each group was relatively large, which provided a robust basis for a reliable conclusion. Besides, during the 6-month intervention period, the patients were required to maintain their usual diet to make sure that the conclusion will not be confounded by potential factors (e.g. nutrients intake and physical activity, seen in Supplemental files, Supplementary Tables S3 and S4). There are some limitations that should be taken into consideration. Firstly, we did not detect the serum fatty acid profiles in the subjects after n-3 PUFA supplementation; consequently, whether the changed serum fatty acids levels led to the improvements in muscle health need to be further understood. Another limitation refers to the generalisability of our results. Since the subjects participating in the present trial were not clinically diagnosed with sarcopenia, whether the beneficial effects of n-3 PUFA supplementation on skeletal muscle could be observed in patients with sarcopenia need to be further explored.
Conclusion
A 6-month fish oil-derived n-3 PUFA supplementation could confer significant improvements on skeletal muscle mass, muscle strength and physical performance in older Chinese people. However, we should note that more well-designed trials are necessary before applying the nutritional supplements in clinical therapy.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The project was funded by the National Natural Science Foundation of China (Grant No. 81872618).
Ethics Statement
The study protocol involving human participants was reviewed and approved by the ethic committee of Zhongda Hospital Affiliated Southeast University (Grant number: 2014ZDSYLL064.1 and 2015ZDSYLL089.0), and registered in the China Ethics Committee of Registering Clinical Trials (ChiCTR-TRC-14005084 and ChiCTR-IOR-16008435). The patients/participants provided their written informed consent to participate in this study.
Reference
Author notes
Dengfeng Xu and Yifei Lu authors contributed equally as co-first authors.




Comments