-
PDF
- Split View
-
Views
-
Cite
Cite
Mathias Brix Danielsen, Elisa Worthington, Jesper Scott Karmisholt, Jørn Munkhof Møller, Martin Gronbech Jørgensen, Stig Andersen, Adverse effects of subcutaneous vs intravenous hydration in older adults: An assessor-blinded randomised controlled trial (RCT), Age and Ageing, Volume 51, Issue 1, January 2022, afab193, https://doi.org/10.1093/ageing/afab193
Close - Share Icon Share
Abstract
Hydration therapy is essential in the care of the older patient. Subcutaneous (SC) hydration is a relevant method for parenteral hydration, but clinical trials on the subject have methodological shortcomings compared to updated standards.
Assessor-blinded, non-inferiority RCT to explore if SC is a safe alternative to intravenous (IV) hydration.
Eligible patients were: Admitted patients 65 years or older with a need for parenteral hydration. The targeted sample size was 67 patients in each group.
Patients were randomised to parenteral hydration via an IV or SC catheter during a 24 hours observation period. The non-randomised catheter (inactive) was placed as a sham on the patient, thereby blinding the caregivers and outcome assessors.
Our primary outcome was the proportion of patients reporting at least one adverse effect with a non-inferiority calculation using a 20% margin.
We included 51 patients, with 24 randomised to SC and 27 to IV. We were unable to reach our target sample size due to challenges in recruitment, time limitation, and COVID-19. For the outcome of adverse effects, SC was non-inferior to IV (p = 0.012). Time spent on inserting the catheters was shorter with SC (p = 0.001). The groups did not differ by pain of insertion, discomfort during infusion, or the risk of developing delirium.
SC is a safe alternative to IV hydration, is faster to place and should be an available method for parenteral hydration wherever older adults are cared for.
ClinicalTrials.gov Identifier: NCT03710408
Key Points
This is the first high-quality randomised controlled trial on subcutaneous hydration in older adults with assessor blinding.
Subcutaneous hydration is a safe alternative to intravenous hydration in older adults.
It is significantly faster to place a subcutaneous catheter than an intravenous catheter.
Introduction
Adequate hydration is essential in treating older patients as dehydration is a common and potentially dangerous condition in our patient group [1–3]. There are two main methods for parenteral hydration; intravenous (IV) is a common choice, but subcutaneous (SC) hydration is an alternative that deserves further attention. Our recent comprehensive systematic review reported a limited number of randomised controlled trials on the subject [4] that were conducted and reported before the introduction of current guidelines, leading to several methodological shortcomings [5–10]. As a parenteral hydration method, SC has potential advantages compared to IV as the literature suggests fewer adverse effects with subcutaneous hydration than with IV. However, none of the previous trials had blinded outcome assessors or were registered with a description of outcomes, limiting their results’ validity. Furthermore, it may be faster to place the SC catheters than the IV catheters, but this result had a high risk of bias. Finally, the risk of delirium may be lower when using SC hydration compared to IV [4].
The limitation of previous trials on the subject led us to perform a randomised controlled trial (RCT) comparing SC with IV hydration. Our trial adheres to current methodological guidelines, including blinding of the outcome assessors to strengthen the quality of the literature on the subject. Our trial’s primary outcome is the risk of adverse effects. We used a non-inferiority design as the trials research question was if SC hydration is a relevant alternative to IV rather than whether SC hydration should replace it. Our RCT use a non-inferiority margin of 20%. This margin means that the proportion of patients experiencing a minor adverse effect in the SC group must not exceed an upper limit of 20% above the proportion reported in the IV group. This margin was settled based on a protocol for a Cochrane review on achieving access for hydration [11] and through discussions with consultants in geriatric medicine.
Additional outcomes were the time spent on inserting the catheters, the patient’s experience of insertion and infusion of fluid, and the risk of developing delirium. We included older adults with mild dehydration or at risk of dehydration during either admission to hospital or short-term care.
Methods
Trial design
This trial was a randomised controlled, parallel-group, assessor-blinded, non-inferiority trial registered on ClinicalTrials.gov (NCT03710408). The reporting follows the CONSORT guidelines [12] with the harms [13] and non-inferiority extensions [14]. Ethical approval was granted by the local Committee on Health Research Ethics (Project ID: N–20180014) in the North Denmark Region.
Participants
We conducted the trial at Aalborg University Hospital, Denmark, and at a short-term care facility in Aalborg. During the trial, the number of locations was increased to enhance recruitment. The inclusion criteria for the trial were: age 65 years or older, a prescription of 1–2 litres of parenteral fluid over the next 24 hours (mild dehydration or at risk of dehydration), and admission to either acute assessment unit, an orthopaedic ward with a hip fracture, or admission to a short-term care facility. Exclusion criteria were: Severe dehydration (expected to need more than 2 L of parenteral fluid over the next 24 hours), fluid restriction, unable to give informed consent, severe general oedema, or planned discharge from the hospital or care facility within the next 24 hours.
Patients were only allowed to receive parenteral fluid through the trial setup but were encouraged to drink fluid; IV medication, such as antibiotics, were allowed using a different IV access.
Interventions
Baseline measurements were obtained before randomisation, and eligible patients were randomised in the ratio of 1:1 to receive parenteral fluid through either an IV or SC placed catheter. The IV catheters were ‘BD Venflon™ Pro Safety – 22G (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA)’ placed in a vein on the dorsal side of the hand or forearm. The SC catheters were ‘BD Saf-T-Intima™ - 22G (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA)’ (butterfly needle with a plastic catheter) placed in the lower right or left quadrant of the abdomen. A sham catheter not piercing the skin was placed on the non-randomised location to achieve blinding of the care personnel and outcome assessors. A small non-transparent gauze square was placed on top of both the randomised and non-randomised catheter (inactive) to hide whether the catheter pierced the skin. Infusion lines primed with infusion fluid were connected to both the catheters, and the line connected to the randomised catheter was inserted into a fluid bag. The infusion lines were intertwined, and this entanglement was covered with opaque fabric. This setup prevented the outcome assessors from knowing which catheter had pierced the patient’s skin (Figure 1). Details of the trial setup can be found in the supplementary data. The fluid flow rate was roughly 3 mL per minute, and a litre of fluid was infused in 6 to 8 hours. The setup allowed the nursing staff to change the infusion bag and flow speed without knowing the patient’s randomisation.
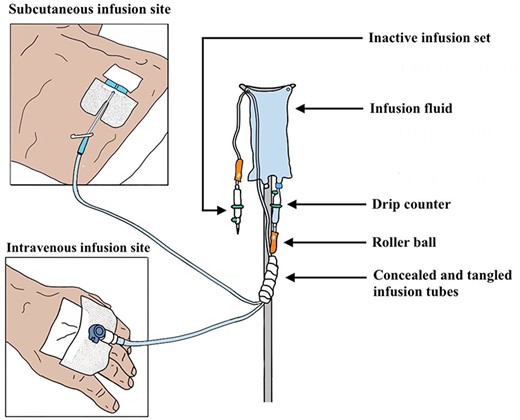
Primary outcome
The primary outcome was adverse effects. The Cochrane handbook [15] defines an adverse effect as ‘An adverse event for which the causal relation between the intervention and the event is at least a reasonable possibility.’ The following minor adverse effects were recorded: reddening of the skin at the insertion site, painful swelling, itching, phlebitis, infusion-related pain, termination of flow, need for reinsertion of the catheter, accidental catheter removal by the patient, need for a reduction of flow speed, and prolong swelling at the infusion site (>2 hours). Short-term swelling without discomfort was not recorded as an adverse effect. After the observation period, the patient’s charts were inspected for signs of severe adverse effects such as pulmonary oedema, cardiac failure, hyper/hyponatremia, and infection at the insertion site. The patients were observed for 24 hours. This short observation period reduced the risk of violating the blinding and patients changing treatment groups. Outcome assessors were the nursing staff, who recorded adverse effects three times during the observation period.
Secondary outcomes
Secondary objectives were delirium at the end of the observation period assessed by the patients attending nurse based on the Confusion Assessment Method (CAM) [16]. The CAM is used in clinical practice, but no formal training was given as part of this trial. We recorded if the patients died during the admission. The patients were also asked to evaluate the pain of inserting the randomised catheter and the discomfort from fluid infusion using a visual analogue scale from 0–100. Finally, the time spent on inserting the randomised catheter was recorded in categories 1 to 6 (1: less than three minutes, 2: 3 to 5 minutes, 3: 5 to 10 minutes, 4: 10 to 20 minutes, 5: need assistance from another staff, 6: need assistance from an intensive care nurse). Categorisation was chosen over a continuous recording of time to allow the two latter groups to be included. Biochemical markers of hydration (haemoglobin, sodium, potassium, urea nitrogen, creatinine, osmolality, albumin, eGFR (CKD-EPI [17])) were collected at the beginning and the end of the 24 hours observation period.
Sample size
Sample size calculation was based on previous trials on this topic with an observation time of fewer than 48 hours. They reported an incidence of adverse effects of 17% in both the SC and IV groups [6, 7]. With a significance level of 5%, a power of 90%, and a non-inferiority limit of 20%, a non-inferiority sample size calculation with a binary outcome resulted in 61 participants required in each group [18]. We expected an attrition rate of 10%, giving us a sample size of 67 patients in each group.
Randomisation
The included patients were randomised with a 1:1 allocation without stratification after baseline measurements using a web form (REDCap version 7.0.11) [19]. A REDCap data manager generated the randomisation sequence as block randomisation with unknown block sizes.
Statistical analysis
Before the completion of recruitment and any data analysis, a statistical analysis plan was made with a biostatistician’s support and uploaded to clinicaltrials.gov NCT03710408. All analyses were performed as intention-to-treat. For the primary outcome (dichotomous, blinded, non-inferiority), a one-sided z-test for non-inferiority was used [20]. If the primary outcome was found to be significantly non-inferior, we performed a superiority test (Fisher’s exact test).
For further analyses of the primary outcome, counting all adverse effects (discrete, non-inferiority), we used a Wilcoxon rank-sum test. All further analyses are superior analyses. Dichotomous and ordered categorical data were analysed using Fisher’s exact test and discrete data with a t-test.
Groups were merged in case of fewer than one or multiple groups with fewer than five patients. Biochemical markers are displayed as mean + SD at baseline, endpoint, and change (Supplementary Table S1). Statistical tests on the biochemical markers of hydration were not performed due to the risk of multiple comparisons and the indirectness of these markers on the outcome of hydration status. All statistical analyses were performed by MBD using STATA (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.). MBD was blinded to group allocation during data analysis.
Results
We screened patients for eligibility from January 2019 through November 2020 and found 51 eligible that accepted inclusion.
We intended to include patients for assessment at the acute assessment unit. An author EW working in the emergency department randomised and included patients before transfer to the acute assessment unit. However, shortly after commencing the trial, the local municipality started offering out-of-hospital intravenous hydration therapy, thereby vastly reduced the number of eligible patients. To enhance enrolment, we added recruitment of patients with a hip fracture at the orthopaedic ward from November 2019. This patients group had to stay in the hospital for the next 48 hours as the ethics committee required a 24 hours right to reconsider before inclusion in addition to the 24 hours of trial observation time. An author (MBD) assessed all patients on the ward for eligibility. Enrolment was feasible during the first four days of the working week only. When COVID-19 hit, no patients were included. Recruitment commenced in May 2020, and we added a short-term care facility, where patients were assessed for eligibility by an attending nurse. However, according to legal requirements, these patients’ general practitioners had to provide consent prior to inclusion.
We have no complete data on the total number of patients assessed for eligibility, found ineligible, or reasons to decline in the emergency room and the short-term care unit.
In the orthopaedic ward, 106 patients were assessed for eligibility, and 32 were eligible to participate and accepted enrolment. Most exclusions were due to an inability to provide informed consent. Based on data previously collected on our orthopaedic ward [21], 180 potentially relevant patients passed through the ward during the year we assessed patients for eligibility. This number includes those scheduled for transfer out of the ward.
Of the 51 patients randomised, 14 patients were recruited at the acute assessment unit, 32 from the orthopaedic ward, and five from the short-term care facility. The trial was terminated before reaching the sample size target due to time restrictions, the challenges faced in recruitment, and the restrictions imposed by the COVID-19 pandemic.
Twenty-four of the included patients were randomised for SC and 27 for IV. The discrepancy between the numbers recruited in the two groups is due to ending the recruitment in the middle of a randomisation block. See Figure 2 for the flow of patients. The principal admission diagnosis was a hip fracture followed by dehydration. The mean age of the included patients was 79 (SD 7.3) years in the subcutaneous group and 83 (SD 6.8) years in the IV group. The included patients had an average of 4 comorbidities, were all mildly dehydrated or at risk of dehydration, and received one litre of parenteral fluid during the trial period of 24 hours. See all baseline data in Table 1.
![Flow diagram of patients. Abbreviations: CAM: Confusion Assessment Method [16].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ageing/51/1/10.1093_ageing_afab193/1/m_afab193f2.jpeg?Expires=1748500034&Signature=uzumsHUucHezMTwBhCi6I19EXwCd5p0amVXsb3G9JanDIWp1Y4hu0301YCzwUtgX1n3tARoub8AzUyFMQKr~Uqgq9MtCfZzrpZr86~bg4WAA3JP5GmwaT083aMrvcJ9RUNYprXTK~ouVoBrFyeiIupvVxsJQ-8BRL6G0LEJW5ums1AwLyGKyRJsgQj5c2Em3yoSBquYbmOUmfcnk2aag4qhxkt1IZiEC6wOEogXkmFiwYeSUr-jVYd5i38J8uFxT4RjNL2ScLxIUOdZAGrcZ5br6kCnk--TgRTdjF4gjzVEQLTBfo1vghbwZZRZB4GcZfZBvYKA8bIL3kSutas~nVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Flow diagram of patients. Abbreviations: CAM: Confusion Assessment Method [16].
| . | Subcutaneous group . | Intravenous group . | |
|---|---|---|---|
| Age | 79 (7.3) | 83 (6.9) | |
| Sex (female)a | 16 (66%) | 17 (62%) | |
| Site of recruitmenta | ER: Orto: Short-term: | 7 (29%) 14 (58%) 3 (13%) | 7 (26%) 18 (67%) 2 (7%) |
| Number of known comorbidities | 4.6 (1.9) | 3.9 (1.4) | |
| Charlson Comorbidity Index [22] Median (25–75 range) | 1 (0–2) | 0 (0–2) | |
| Treated with anti-coagulant medicationa | 8 (35%) | 9 (33%) | |
| Systolic Blood Pressure (mm Hg) | 136 (28) | 129 (21) | |
| Diastolic blood Pressure (mm Hg) | 68 (10) | 69 (12) | |
| Pulse (/min) | 83 (18) | 79 (12) | |
| Hemoglobin (g/dl)b | 10.5 (2.3) | 11.3 (2.5) | |
| Sodium (mEq/l) | 137 (3.5) | 137 (3.7) | |
| Potassium (mEq/l) | 3.8 (0.6) | 3.8 (0.6) | |
| Urea (mg/dl)c | 50 (25) | 56 (46) | |
| Creatinine (mg/dl)d | 1.1 (0.46) | 1.0 (0.46) | |
| eGFR (ml/min/1.73m2) | 61 (23) | 63 (24) | |
| Albumin (g/dl)e | 2.7 (0.38) | 2.9 (0.43) | |
| Osmolality (mmol/kg) | 294 (18) | 290 (11) | |
| . | Subcutaneous group . | Intravenous group . | |
|---|---|---|---|
| Age | 79 (7.3) | 83 (6.9) | |
| Sex (female)a | 16 (66%) | 17 (62%) | |
| Site of recruitmenta | ER: Orto: Short-term: | 7 (29%) 14 (58%) 3 (13%) | 7 (26%) 18 (67%) 2 (7%) |
| Number of known comorbidities | 4.6 (1.9) | 3.9 (1.4) | |
| Charlson Comorbidity Index [22] Median (25–75 range) | 1 (0–2) | 0 (0–2) | |
| Treated with anti-coagulant medicationa | 8 (35%) | 9 (33%) | |
| Systolic Blood Pressure (mm Hg) | 136 (28) | 129 (21) | |
| Diastolic blood Pressure (mm Hg) | 68 (10) | 69 (12) | |
| Pulse (/min) | 83 (18) | 79 (12) | |
| Hemoglobin (g/dl)b | 10.5 (2.3) | 11.3 (2.5) | |
| Sodium (mEq/l) | 137 (3.5) | 137 (3.7) | |
| Potassium (mEq/l) | 3.8 (0.6) | 3.8 (0.6) | |
| Urea (mg/dl)c | 50 (25) | 56 (46) | |
| Creatinine (mg/dl)d | 1.1 (0.46) | 1.0 (0.46) | |
| eGFR (ml/min/1.73m2) | 61 (23) | 63 (24) | |
| Albumin (g/dl)e | 2.7 (0.38) | 2.9 (0.43) | |
| Osmolality (mmol/kg) | 294 (18) | 290 (11) | |
Abbreviations: ER: Emergency room, Orto: Orthopedic ward;
Unless otherwise indicated, data are expressed as mean (standard deviation)
aData expressed as number (per cent),
bTo convert the values for haemoglobin to mmol/l multiply by 0.62,
cTo convert the values for urea to mmol/divide by 6,
dTo convert the values for creatinine to μmol/l multiply by 88.42,
eTo convert the values of albumin to g/l multiply by 10.
| . | Subcutaneous group . | Intravenous group . | |
|---|---|---|---|
| Age | 79 (7.3) | 83 (6.9) | |
| Sex (female)a | 16 (66%) | 17 (62%) | |
| Site of recruitmenta | ER: Orto: Short-term: | 7 (29%) 14 (58%) 3 (13%) | 7 (26%) 18 (67%) 2 (7%) |
| Number of known comorbidities | 4.6 (1.9) | 3.9 (1.4) | |
| Charlson Comorbidity Index [22] Median (25–75 range) | 1 (0–2) | 0 (0–2) | |
| Treated with anti-coagulant medicationa | 8 (35%) | 9 (33%) | |
| Systolic Blood Pressure (mm Hg) | 136 (28) | 129 (21) | |
| Diastolic blood Pressure (mm Hg) | 68 (10) | 69 (12) | |
| Pulse (/min) | 83 (18) | 79 (12) | |
| Hemoglobin (g/dl)b | 10.5 (2.3) | 11.3 (2.5) | |
| Sodium (mEq/l) | 137 (3.5) | 137 (3.7) | |
| Potassium (mEq/l) | 3.8 (0.6) | 3.8 (0.6) | |
| Urea (mg/dl)c | 50 (25) | 56 (46) | |
| Creatinine (mg/dl)d | 1.1 (0.46) | 1.0 (0.46) | |
| eGFR (ml/min/1.73m2) | 61 (23) | 63 (24) | |
| Albumin (g/dl)e | 2.7 (0.38) | 2.9 (0.43) | |
| Osmolality (mmol/kg) | 294 (18) | 290 (11) | |
| . | Subcutaneous group . | Intravenous group . | |
|---|---|---|---|
| Age | 79 (7.3) | 83 (6.9) | |
| Sex (female)a | 16 (66%) | 17 (62%) | |
| Site of recruitmenta | ER: Orto: Short-term: | 7 (29%) 14 (58%) 3 (13%) | 7 (26%) 18 (67%) 2 (7%) |
| Number of known comorbidities | 4.6 (1.9) | 3.9 (1.4) | |
| Charlson Comorbidity Index [22] Median (25–75 range) | 1 (0–2) | 0 (0–2) | |
| Treated with anti-coagulant medicationa | 8 (35%) | 9 (33%) | |
| Systolic Blood Pressure (mm Hg) | 136 (28) | 129 (21) | |
| Diastolic blood Pressure (mm Hg) | 68 (10) | 69 (12) | |
| Pulse (/min) | 83 (18) | 79 (12) | |
| Hemoglobin (g/dl)b | 10.5 (2.3) | 11.3 (2.5) | |
| Sodium (mEq/l) | 137 (3.5) | 137 (3.7) | |
| Potassium (mEq/l) | 3.8 (0.6) | 3.8 (0.6) | |
| Urea (mg/dl)c | 50 (25) | 56 (46) | |
| Creatinine (mg/dl)d | 1.1 (0.46) | 1.0 (0.46) | |
| eGFR (ml/min/1.73m2) | 61 (23) | 63 (24) | |
| Albumin (g/dl)e | 2.7 (0.38) | 2.9 (0.43) | |
| Osmolality (mmol/kg) | 294 (18) | 290 (11) | |
Abbreviations: ER: Emergency room, Orto: Orthopedic ward;
Unless otherwise indicated, data are expressed as mean (standard deviation)
aData expressed as number (per cent),
bTo convert the values for haemoglobin to mmol/l multiply by 0.62,
cTo convert the values for urea to mmol/divide by 6,
dTo convert the values for creatinine to μmol/l multiply by 88.42,
eTo convert the values of albumin to g/l multiply by 10.
At the trial’s termination, 21 patients in the SC group and 23 in the IV group had completed the observation for adverse effects. No participant had a serious adverse effect, changed their treatment group during the observation period, or left the trial because of adverse effects. Six (28%) and 10 (43%) patients experienced at least one adverse effect in the SC and IV groups, respectively. Our primary outcome of adverse effect (non-inferiority, blinded outcome assessor) showed that SC was significantly non-inferior to IV (p = 0.012) (Figure 3).
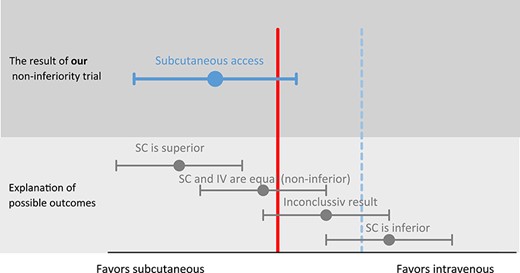
Graphical presentation of the non-inferiority of subcutaneous vs intravenous hydration. Footnote: The solid vertical line represents the line of no difference between subcutaneous (SC) and intravenous (IV). The dashed vertical line represents our pre-specified non-inferiority margin. p-value for non-inferiority = 0.012. The risk ratio between subcutaneous and intravenous is RR 0.66 (95% CI 0.29–1.49) favouring subcutaneous hydration.
A superiority calculation of adverse effects shows that SC is not significantly superior to IV with a risk ratio of 0.66 (95% CI: 0.29–1.49, p = 0.36). When including all reported adverse effects, and not just the first, SC was still not superior to IV (p = 0.19). There were no reports of bleeding or hematoma related to the catheters during the observation, and no patient died during their admission. See Supplementary Figure S1 for details of the observed adverse effects.
When patients experienced an adverse effect that caused the infusion to stop, it was assessed by the nursing staff if the patient needed to complete the hydration treatment or had received sufficient fluid. This is the reason for the discrepancy between the number of terminated flow and accidental catheter removal by the patients and the number of reinsertions reported.
SC catheters were significantly faster to place than IV (p = 0.001, Table 2, Supplementary Figure S2). Most SC catheters took less than five minutes to place, where the placement of IV catheters often took longer. Three patients in the IV group had delirium at the end of observation compared to 0 in the SC group (p = 0.23). The patients randomised to IV reported a mean pain score for insertion of catheter of 13.0 (SD 13.4) compared to 7.3 (SD 10.4) in the SC group on a scale from 0–100 (p = 0.13). The mean reported discomfort during infusion was 4.7 (SD 7.5) and 4.5 (SD 11.8) in the IV and SC group, respectively, again on a scale from 0–100 (p = 0.74). All secondary outcomes are reported in Table 2.
| Outcome . | Subcutaneous group n(%) . | Intravenous group n(%) . | Difference (95% CI) . | p-value for difference . | |
|---|---|---|---|---|---|
| Time spend on insertiona | < 5 min: 5–20 min: > 20 minb: | 18 (85%) 2 (10%) 1 (5%) | 7 (32%) 9 (41%) 6 (27%) | N/A | 0.001 |
| Death during hospitalization | 0 (0%) | 0 (0%) | N/A | N/A | |
| Delirium | 0 (0%) | 3 (14%) | 0.23 | ||
| n, mean (SD) | n, mean (SD) | ||||
| .......................... | |||||
| Pain of insertion (0–100 VAS) | n = 21, 7.3 (10.4) | n = 20, 13.0 (13.4) | 5.7 (−1.9; 13.2) | 0.13 | |
| Discomfort during infusion (0–100 VAS) | n = 18, 4.5 (11.8) | n = 18, 4.7 (7.5) | 0.2 (−6.9; 4.5) | 0.74 | |
| Outcome . | Subcutaneous group n(%) . | Intravenous group n(%) . | Difference (95% CI) . | p-value for difference . | |
|---|---|---|---|---|---|
| Time spend on insertiona | < 5 min: 5–20 min: > 20 minb: | 18 (85%) 2 (10%) 1 (5%) | 7 (32%) 9 (41%) 6 (27%) | N/A | 0.001 |
| Death during hospitalization | 0 (0%) | 0 (0%) | N/A | N/A | |
| Delirium | 0 (0%) | 3 (14%) | 0.23 | ||
| n, mean (SD) | n, mean (SD) | ||||
| .......................... | |||||
| Pain of insertion (0–100 VAS) | n = 21, 7.3 (10.4) | n = 20, 13.0 (13.4) | 5.7 (−1.9; 13.2) | 0.13 | |
| Discomfort during infusion (0–100 VAS) | n = 18, 4.5 (11.8) | n = 18, 4.7 (7.5) | 0.2 (−6.9; 4.5) | 0.74 | |
Abbreviations: VAS: Visual analogue scale, N/A: Not applicable
aOriginal groups are collapsed due to the low number of events in some groups.
bRequiring assistance from another staff member
| Outcome . | Subcutaneous group n(%) . | Intravenous group n(%) . | Difference (95% CI) . | p-value for difference . | |
|---|---|---|---|---|---|
| Time spend on insertiona | < 5 min: 5–20 min: > 20 minb: | 18 (85%) 2 (10%) 1 (5%) | 7 (32%) 9 (41%) 6 (27%) | N/A | 0.001 |
| Death during hospitalization | 0 (0%) | 0 (0%) | N/A | N/A | |
| Delirium | 0 (0%) | 3 (14%) | 0.23 | ||
| n, mean (SD) | n, mean (SD) | ||||
| .......................... | |||||
| Pain of insertion (0–100 VAS) | n = 21, 7.3 (10.4) | n = 20, 13.0 (13.4) | 5.7 (−1.9; 13.2) | 0.13 | |
| Discomfort during infusion (0–100 VAS) | n = 18, 4.5 (11.8) | n = 18, 4.7 (7.5) | 0.2 (−6.9; 4.5) | 0.74 | |
| Outcome . | Subcutaneous group n(%) . | Intravenous group n(%) . | Difference (95% CI) . | p-value for difference . | |
|---|---|---|---|---|---|
| Time spend on insertiona | < 5 min: 5–20 min: > 20 minb: | 18 (85%) 2 (10%) 1 (5%) | 7 (32%) 9 (41%) 6 (27%) | N/A | 0.001 |
| Death during hospitalization | 0 (0%) | 0 (0%) | N/A | N/A | |
| Delirium | 0 (0%) | 3 (14%) | 0.23 | ||
| n, mean (SD) | n, mean (SD) | ||||
| .......................... | |||||
| Pain of insertion (0–100 VAS) | n = 21, 7.3 (10.4) | n = 20, 13.0 (13.4) | 5.7 (−1.9; 13.2) | 0.13 | |
| Discomfort during infusion (0–100 VAS) | n = 18, 4.5 (11.8) | n = 18, 4.7 (7.5) | 0.2 (−6.9; 4.5) | 0.74 | |
Abbreviations: VAS: Visual analogue scale, N/A: Not applicable
aOriginal groups are collapsed due to the low number of events in some groups.
bRequiring assistance from another staff member
Discussion
We performed an assessor-blinded, non-inferiority, RCT, adhering to current guidelines, including trial registration and uploading of the statistical analysis plan. Our primary outcome of adverse effects showed that SC hydration was non-inferior to IV. Furthermore, the time it took to place an SC catheter was statistically significant shorter than placing an IV catheter.
Our trial was successful in its aim, providing high-quality evidence that subcutaneous hydration appears to be a safe alternative to IV. The incidence of adverse effects in our trial was higher than reported in other trials on SC hydration [6–9]. This could be due to our scrutinising observation for adverse effects since this was our primary outcome. Both IV and SC hydration appear to be safe hydration methods as we found no serious adverse effects. The main adverse effects reported were minor nuisances such as termination of flow and accidental catheter removal by the patient.
A low number of patients developed delirium during the observation period, with zero in the SC group and three in the IV group. However, the low number of delirium cases was expected as one of the inclusion criteria were: ‘being able to provide informed consent’. The non-significant difference in risk of delirium between groups contrasts with the findings in our recent systematic review. Here we found a reduced risk of agitation in patients receiving subcutaneous hydration [4]. However, the trials on this outcome included patients with cognitive impairment, being more vulnerable patients than those included in our trial [7, 9, 10].
We found that SC catheters were significantly faster to place than IV, which is in line with the findings reported in our systematic review, and our results raise the confidence in this estimate. In general, patients reported minimal discomfort from the placement of the catheters and discomfort during the infusion. This conforms to findings by a previous trial that showed the patient had a mean discomfort score of two on a six point Likert-like scale [8].
Limitations
A significant limitation of our trial is the intended sample size and the actual sample size. Nonetheless, it is intriguing that our main result still was statistically significant despite this shortcoming. Furthermore, many patients were not eligible due to an inability to provide informed consent. These vulnerable individuals are frequent visitors to hospitals and short-term care facilities, and their absence limits our results’ applicability. Less than one-third of the patients assessed for eligibility in the orthopaedic ward were eligible, and nearly half could not provide informed consent. Additionally, the 24 hours considerations time imposed by the ethic committee restricted recruitment in modern accelerated admissions. This trial highlights why ethically correct clinical trials are challenging to perform in the geriatric patient.
We did not perform a pilot study. This could have increased our recruitment by highlighting some of the challenges we faced.
Our observation period of 24 hours is shorter than the average duration of parenteral catheters. This observation time was chosen primarily to reduce the risk of violation of the blinding and prevent cross-over of patients between randomisation groups. The latter violation was reported by previous trials, in which a large proportion of patients swapped group during the trial, thus blurring the interpretation of results [8].
We did not perform adjusted analyses as the groups were similar. Moreover, adjusted analyses were not included in our uploaded statistical analysis plan.
Our trial’s main strengths are the registration with a description of all outcomes before the inclusion of the first patient and the registration of a detailed statistical analysis plan. These factors reduce the risk of bias of selective outcome reporting. Furthermore, the blinding of the outcome assessor reduces the risk of bias in our primary outcome. These factors contribute to a raised confidence in the estimates and strengthen the recommendation to use SC hydration.
In conclusion, SC hydration is non-inferior to IV for the outcome of adverse effects, and no serious adverse effects were reported. The overall discomfort was minimal from both hydration treatments, but SC catheters were significantly faster to place than IV. Based on our results, clinicians should consider SC hydration as an alternative in patients with mild dehydration or at risk of dehydration and maybe even preferred in patients at risk of delirium.
Acknowledgements
We would like to thank Kirsten Duch, Msc, Unit of Clinical Biostatistics, Aalborg University Hospital, for statistical assistance. We acknowledge the support of the departments of Geriatric Medicine, Orthopedic Surgery, and Emergency Medicine, Aalborg University Hospital. All who contributed significantly to this work have been mentioned. Reproducible research statement: ClinicalTrials.gov registration and statistical analysis plan: NCT03710408. Statistical code and data set: available from M. Danielsen MD, Department of Geriatric Medicine, Aalborg University Hospital, Aalborg, Denmark. E-mail: [email protected]
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
None.




Comments