-
PDF
- Split View
-
Views
-
Cite
Cite
Najma Siddiqi, Francine Cheater, Michelle Collinson, Amanda Farrin, Anne Forster, Deepa George, Mary Godfrey, Elizabeth Graham, Jennifer Harrison, Anne Heaven, Peter Heudtlass, Claire Hulme, David Meads, Chris North, Angus Sturrock, John Young, The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people, Age and Ageing, Volume 45, Issue 5, September 2016, Pages 652–661, https://doi.org/10.1093/ageing/afw091
Close - Share Icon Share
Abstract
Background and objectives: delirium is a distressing but potentially preventable condition common in older people in long-term care. It is associated with increased morbidity, mortality, functional decline, hospitalization and significant healthcare costs. Multicomponent interventions, addressing delirium risk factors, have been shown to reduce delirium by one-third in hospitals. It is not known whether this approach is also effective in long-term care. In previous work, we designed a bespoke delirium prevention intervention, called ‘Stop Delirium!’ In preparation for a definitive trial of Stop Delirium, we sought to address key aspects of trial design for the particular circumstances of care homes.
Design: a cluster randomized feasibility study with an embedded process evaluation.
Setting and participants: residents of 14 care homes for older people in one metropolitan district in the UK.
Intervention: Stop Delirium!: a 16-month-enhanced educational package to support care home staff to address key delirium risk factors. Control homes received usual care.
Measurements: we collected data to determine the following: recruitment and attrition; delirium rates and variability between homes; feasibility of measuring delirium, resource use, quality of life, hospital admissions and falls; and intervention implementation and adherence.
Results: two-thirds (215) of eligible care home residents were recruited. One-month delirium prevalence was 4.0% in intervention and 7.1% in control homes. Proposed outcome measurements were feasible, although our approach appeared to underestimate delirium. Health economic evaluation was feasible using routinely collected data.
Conclusion: a definitive trial of delirium prevention in long-term care is needed but will require some further design modifications and pilot work.
Introduction
Delirium is a distressing but potentially preventable condition common in older people. It is associated with increased morbidity, mortality, functional decline, hospitalization and significant healthcare costs [1–3].
Most delirium research has focussed on hospital patients. Another expanding group [4], for whom the burden of delirium is likely to be considerable, is residents of care homes, with a clustering of delirium risk factors [5], especially older age and dementia [6, 7]. We use the term ‘care home’ to refer to all residential long-term care settings providing group living and personal and/or nursing care for older people.
Delirium point prevalence in the care home population has been estimated to be 14% [8], and 33% for residents with advanced dementia [9]. Multicomponent interventions that target modifiable risk factors have been shown to reduce delirium by approximately one-third in hospitals [10–12]. These are areas of care that should be equally applicable to long-term care settings, but the effectiveness of delirium prevention in care homes is not yet known [13].
Delirium has been linked to quality of care [3, 14]. A focus on delirium prevention may, therefore, present an opportunity not only to reduce delirium but also to improve quality of care for older people living in care homes, with potential additional benefits that include reducing morbidity, hospital admissions and healthcare costs. The NICE Delirium guideline, therefore, includes a research recommendation to develop such evidence [13].
In previous work, we used the UK Medical Research Council framework for evaluating complex interventions [15] to design an intervention to prevent delirium in care homes (entitled Stop Delirium!) and demonstrated its feasibility [16, 17]. ‘Stop Delirium!’ is an enhanced educational package, incorporating additional strategies to change practice, designed to support staff to target common risk factors for delirium in residents. The intervention has been described in previous publications [17, 18], and materials can be viewed on the European Delirium Association website [19].
Building on this work, and to address key aspects of future trial design and intervention implementation for the particular circumstances of care homes, we report a feasibility study to test and optimize the protocol for a definitive trial of Stop Delirium! The specific objectives of the study were to (i) estimate recruitment and attrition rates; (ii) estimate the sample size for a subsequent trial with data on the proposed primary outcome, delirium occurrence, its variability and the intraclass coefficient (ICC), and to report data on hospital admission rates (as a potential alternative primary outcome); (iii) explore feasibility of collecting proposed baseline and outcome measures; (iv) test feasibility and refine the strategy for collecting resource use and quality-of-life data to inform the health economics evaluation; and (v) assess implementation, adherence and sustainability of the Stop Delirium! intervention.
Methods
A summary of methods is given here with full details available in the published protocol [18].
Design and setting
We conducted a parallel group, cluster randomized controlled feasibility trial in 14 care homes providing care for older people. Changes to the original protocol included the introduction of a second phase of resident recruitment 12 months after randomization because of a high attrition rate, and conducting structured case note reviews in order to explore the possibility that reliance on face-to-face assessments alone might be underestimating delirium.
Eligibility criteria
Independent sector care homes providing nursing or residential care for older people in one metropolitan district were eligible. All residents were eligible unless they had severe communication difficulties, were unable to communicate in English or were receiving end-of-life care.
Outcomes
For the future definitive trial, the proposed primary outcome is delirium occurrence. Delirium detection ideally requires repeated daily assessments, which would be challenging in multiple care homes. We therefore assessed the feasibility of research staff detecting delirium during a single month, 16 months after randomization. Feasibility of collecting the following secondary outcomes was also examined: severity and duration of delirium episodes; hospital admissions (number, length of stay and time to first admission), falls and mortality during previous 6 months and the number of medications.
Sample size
The number of homes was determined to allow a maximum range of homes within the research resources available to this feasibility study.
Participant recruitment and consent
Informed, written consent was obtained from participants, or for those lacking capacity to consent, agreement from a relative or professional caregiver, following requirements of the UK Mental Capacity Act, 2005 [20].
Randomization
Homes were randomized to intervention or control on a 1:1 basis using a computer-generated minimization programme by the Leeds Clinical Trials Research Unit that stratified by the size of the home in terms of the number of residents (<20, ≥20) and percentage of residents with dementia (<62%, ≥62%). Randomization took place after completion of the first phase of resident recruitment and baseline assessment. Thereafter, blinding of participants, staff or researchers to allocation was not feasible given the nature of the intervention and resource limitations.
Stop Delirium! intervention
The study intervention comprised a multifaceted enhanced educational package incorporating multiple strategies to change practice delivered to each care home over 16 months. The intervention has been described in detail elsewhere [16–19]; in brief, it consisted of a specialist Delirium Practitioner (a mental health nurse with expertise in delirium and in providing interactive education and training) who delivered three interactive education sessions and facilitated Working Groups of care home staff. Working Groups identified targets for delirium prevention and developed bespoke solutions for each home. The Delirium Practitioner also trained a Delirium Champion in each home. This was supported by a Delirium Box containing resources designed to support learning and act as reminders.
Control
Care homes randomized to be controls continued with care as usual. Control homes were offered the Stop Delirium! package after the end of the study, and all seven opted to implement the intervention.
Data collection
At recruitment, resident demographics, medications, activities of daily living (ADL) (Barthel index [21]) and co-morbidity (Charlson index [22]) were collected from care home records. Tests for visual (Snellen test card), hearing (Whisper test) and cognitive impairment (6-CIT [23]) were conducted. Trained research assistants also assessed for delirium using the short version Confusion Assessment Method (CAM) [24] and, for those screening positive, the Delirium Rating Scale-Revised-98 (DRS-R-98) [25]. Collateral information to inform completion of the CAM was sought from care home staff.
At follow-up, residents were assessed for delirium on alternate days (except Sundays) over a 1-month period starting 16 months after randomization. Inter-rater reliability was assessed by a second researcher observing the interview and scoring the CAM independently. Delirium severity was assessed using the DRS-R-98 (score >15.25) and duration using the number of days CAM positive for each delirium episode. Structured case note reviews were also undertaken for the same 1-month period using an established method to identify delirium cases [26].
Medications, hospital admissions, falls and deaths in a 6-month period starting 10 months after randomization were collected for each resident from care home records. We also obtained data for hospital admissions for the same 6-month period from hospitals in the catchment area, both for individual consented residents, and by care home postcode. These data are routinely collected by hospitals for ‘Hospital Episode Statistics’ (HES), a data warehouse containing details of all admissions, outpatient appointments and Accident and Emergency department attendances at NHS hospitals in England.
Economic evaluation
We explored the feasibility of a number of approaches to capture resource use: care-home-level monthly diaries; resident-level care home record review and hospital record capture, including data obtained directly from hospitals or through a request to the Health and Social Care Information Centre (a provider of national NHS hospital data).
We administered the EQ-5D and EQ-5D proxy [27] and Social Care Related Quality of Life (SCRQoL) [28] at baseline and the EQ-5D and Dementia Quality of Life [29] (DEMQOL and DEMOQOL proxy) at follow-up to test alternative modes of capturing utility values for this population. Utility is used to weight survival in the calculation of quality-adjusted life years, the recommended outcome measure used in cost-effectiveness analysis [30].
Records were kept of resources used in delivering the intervention and conducting research assessments (staff time). Unit financial costs for health and social care resources were obtained from national sources [Personal Social Services Research Unit (PSSRU) Costs of Health and Social Care, British National Formulary (BNF) and NHS reference cost databases] and used to determine costs of the Stop Delirium! intervention and costs for health and social care use by participants.
Implementation, adherence and sustainability of the intervention
A process evaluation was conducted alongside the trial, using the Normalisation Process Theory Framework [31] to describe the process of implementation, integration and sustainability of the intervention and to identify barriers and facilitators. Details of this will be reported in a separate publication.
Analysis
Quantitative data were summarized using descriptive statistics; analyses focussed mainly on confidence interval estimation rather than formal hypothesis testing. The ICC and its confidence interval were estimated for delirium occurrence using data from the post-intervention period.
Although determining differences between groups was not the main purpose in this feasibility study, we constructed the Kaplan–Meier plots [32] for time to hospital admission and mortality for the whole population and by study arm.
All analyses were conducted using Statistical Package for the Social Sciences (SPSS) (BM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY:IBM Corp.) and R [R Core Team (2012). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Version 2.15.2. Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/].
The study was approved by an NHS Research Ethics Committee (12/YH/0018).
Results
Recruitment and attrition
Figure 1 presents a CONSORT diagram of participant flow in the study.
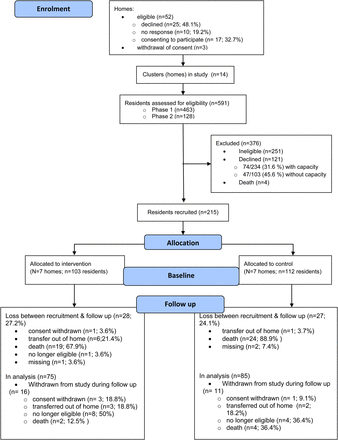
The 14 participating care homes were of similar size to homes that declined. Supplementary data, Appendix Table 1, available in Age and Ageing online describes their size, resident population and staffing.
Out of 639 registered residents, 591 (92.4%) could be screened for eligibility within the time available [463/486 (95.3%) in Phase 1 and 128/153 (83.7%) in Phase 2]; 340 (57.5%) were found to be eligible. The most common reason for ineligibility was severe communication problems (166/251, 63.4%), followed by end-of-life care (27/251, 10.3%).
Two-thirds (215/340) of eligible residents were recruited. The recruitment rate was higher in residents with capacity than without capacity [159/234 (67.9%; 95% CI: 61.9, 73.9) and 56/103 (54.4%; 95% CI: 44.8, 64.0), respectively]. The mean cluster size was 15.4 (SD 4.1). Supplementary data, Appendix Table 2, available in Age and Ageing online describes characteristics of all residents and of those recruited to the study by recruitment phase and by allocation. There were differences between allocation arms at baseline, with the Stop Delirium! group having a higher proportion of residents who were male, in nursing (as opposed to residential) care, lacking capacity to consent and with a dementia diagnosis.
Attrition
Overall, the attrition rate was 38.1% (82/215) (Figure 1); attrition was, as expected, higher among residents recruited in Phase 1 [45.6% (73/160)] compared with those recruited in Phase 2 [16.4% (29/55)] because of the longer interval between recruitment and follow-up.
Sample size
Delirium occurrence
There were three cases of delirium identified in the 215 residents at recruitment, giving a point prevalence of 1.4%.
Over the 1-month follow-up period, there were 13 CAM-positive assessments (3 in the intervention and 10 in the control arm). Consecutive positive assessments were counted as a single episode. Delirium period prevalence was, therefore, estimated as 4.0% (3/75 residents; 95% CI: 0, 8.4) in intervention and 7.1% (6/85 residents; 95% CI: 1.6, 12.6) in control homes. Delirium incidence was estimated as 4.9 (95% CI: 0.7, 15) per 100 resident-months at risk in intervention homes and 7.9 (95% CI: 1.4, 22.0) per 100 resident-months at risk in control homes (taking account of incomplete follow-up due to withdrawal from the study or death part-way through the month).
The DRS-R-98 was completed for 12 of the 13 instances of CAM-positive assessments; all were rated as high severity.
Structured care home records reviews identified 23 residents with delirium from 130 records reviewed, 20.3% (14/69) in intervention and 14.8% (9/61) in control homes. All but one of the nine cases of delirium identified by the CAM were also identified by case note review. In two homes, records could not be accessed within the study period.
Intraclass coefficient
The ICC for the proposed primary outcome, the proportion of residents with at least one CAM-positive assessment during the 1-month follow-up period, was estimated as 0.04 (95% CI: −0.02, 0.2).
Sample size estimate
Using this ICC to estimate the design effect, an expected delirium 1-month period prevalence of 15% in control and 8% in intervention groups, and an average cluster size of 26, the sample size for a definitive cluster trial is 36 homes (926 residents) per group to give 90% power at 95% significance level (two sided). The estimated delirium rate is taken from a review of previous long-term care studies [8] and the cluster size from the size of homes in a large national trial in care homes currently underway [33], applying our recruitment and attrition rates.
Hospital admission rates
At recruitment, using data obtained directly from hospitals, the 6-month hospital admission rate per 100 residents was 54.1 (SD 38.6) in intervention and 56.2 (SD 33.0) in control homes. At follow-up, the rates were 42.9 (SD 28.8) in intervention and 64.2 (SD 26.3) in control homes. Figure 2 presents a Kaplan–Meier plot for time to hospital admission by allocation arm, starting 10 months following randomization.
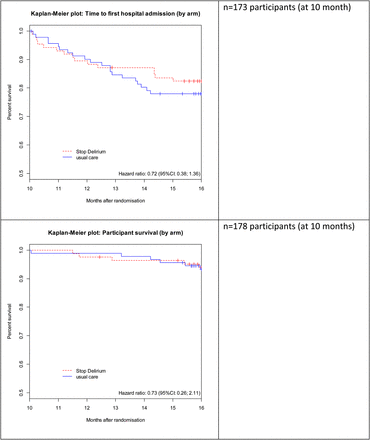
Time to hospital admission and mortality by randomization arm.
Other outcome measures
Medications
The mean number of medications per resident was 8.7 (SD 3.9) in the intervention and 9.1 (SD 3.9) in the control homes at follow-up.
Deaths
In 215 residents recruited, there were 49 deaths by study end, 20.4% (21/103) in intervention and 25% (28/112) in control homes. A Kaplan–Meier plot gave no indication of difference in survival at 16 months between the two study arms (Figure 2).
Feasibility of baseline and outcome measurements
Baseline
High rates of completion were achieved for most assessments (100% for Barthel index, Charlson index and the CAM; and more than 80% for cognitive and visual impairment tests). However, only two-thirds of hearing tests were conducted due to lack of appropriate space in the care home.
Delirium assessments
In total, 69.6% (1,389/1,996) of the planned CAM assessments [66.7% (913/1,368) for those recruited in Phase 1 and 75.8% (476/628) in Phase 2] were conducted during the 1-month follow-up period. Supplementary data, Appendix Table 3, available in Age and Ageing online summarizes assessments and reasons for non-completion by randomization group.
The inter-rater reliability for CAM was high (100%), although only 20 paired assessments were conducted because of limited researcher time. The DRS-R-98 could be used to assess delirium severity. Delirium duration, however, was difficult to estimate. Alternate-day CAM assessments could not differentiate between 1 and 3 days' duration, and case note entries were insufficiently precise to determine duration of discrete delirium episodes.
Hospital admissions
Summary home-level data for hospital admissions (obtained from care homes) were missing for two homes, and rates were lower than estimated from other sources. Admissions were recorded in individual resident records, but this was a resource-intensive source, requiring individual consent from participants.
Data obtained directly from hospitals were the most readily accessible source of information for hospital admissions.
Falls
Recording of falls differed markedly with some homes recording any instances where a resident was found on the floor as a fall, and others limiting recording to observed falls only; falls data were not, therefore, analysed further.
Resources for research assessments
Recruitment and conduct of baseline assessments required two full-time researchers for 7 months, with a similar requirement for outcome assessments.
Health economics
Health and social care resource use
Resident-level data collection diaries were found not to be feasible for completion by residents and too burdensome for staff. Care-home-level diaries were also only partially completed by staff due to time pressures. It was uncommon for friends and family members to be either present to complete proxy forms or to have spent sufficient time observing the resident to be able to comment.
We were unable to obtain a timely response to the request for Health and Social Care Information Centre data because of an embargo in place at the time. However, we were able to confirm that the data requested in our application were available from the centre. Data obtained directly from hospitals offered a robust way of capturing secondary care resource use. Using these, the overall cost for residents in the intervention arm was estimated as £3,281 and in the control homes £7,210. In addition, there were lower monthly costs per resident for homes in the intervention arm (£219.72 compared with £253.01, a saving of £33.29).
Quality of life
At baseline, the EQ-5D and SCRQoL were administered with only 4 and 2% missing assessments, respectively. At follow-up, non-completion rates for EQ-5D and DEMQoL-5D were 20 and 12%, respectively. However, there was a ceiling effect (a high proportion of residents had ‘full health’) in both the SCRQoL and DEMQoL limiting their usefulness. In addition, the SCRQoL was prohibitively resource intensive in terms of researcher time.
The mean EQ-5D score was similar at baseline for residents in intervention and control arms [0.50 (SD ± 0.40) and 0.51 (SD ± 0.37), respectively] and as expected deteriorated over time in both [to 0.42 (SD ± 0.39) in intervention and 0.38 (SD + 0.42) in control homes].
Stop Delirium! costs
The total cost of delivering Stop Delirium! was ∼£138 per care home resident, including costs for care home staff time and for the Delirium Practitioner.
Given the difficulties with capturing resource use at a resident level, we were unable to estimate the costs for an episode of delirium.
Intervention delivery
Overall, 84.4% of staff completed at least one education session; in four homes, over 90% of staff completed all three sessions. Working Groups were established in all homes. A Delirium Champion was identified in four, and there was evidence of outputs from Working Groups being used in five out of the seven homes.
Delivery of the intervention was compromised in the later part of the study due to first sickness absence and then maternity leave of the Delirium Practitioner (9 out of 22 months).
Discussion
Our findings have a number of implications for delirium research in care homes in general and specifically for a definitive trial of delirium prevention.
Study design
Our approach to recruitment was successful, securing representation of residents both with and without capacity in the study sample. However, our selection criteria excluded residents with severe communication difficulties because of the nature of the study assessments, thus excluding some of the very residents most vulnerable to delirium. Attrition was high when there was a 16-month interval between recruitment and follow-up. This is consistent with the reported finding of a median survival rate of 12 months for nursing and 16 months for residential care home residents [34]. A cross-sectional design recruiting trial participants nearer to follow-up could address this; we achieved a 96% follow-up rate using this approach for participants recruited in Phase 2. Potential differential recruitment influenced by knowledge of allocation could be addressed by blinding researchers involved in recruitment, although maintaining blinding is challenging with an intervention that is highly visible.
Measuring delirium
Rates for delirium prevalence and incidence in this study were lower than expected from previous research. It is possible that they are an underestimate of the true rate for a number of reasons. First, although our inclusion criteria were broad, as mentioned above, we excluded residents with severe communication difficulties. Assessments required the participation of residents, excluding those who were too unwell or unwilling to be seen by a researcher. These groups include the very people most likely to be at risk of delirium.
Second, our study population had a high prevalence of dementia and pre-existing cognitive impairment. Diagnosing delirium superimposed on dementia is challenging, even for experienced clinicians [35]. Although researchers had undergone training in using the CAM, they were often reliant on information from care home staff who tended to ascribe any deterioration to the underlying dementia. We anticipated that care home staff would have a good understanding of residents' usual health state and be well placed to report changes. However, this was frequently not the case because of high staff turnover and limited handover of information between shifts. Third, researchers were only able to assess residents during the day, potentially missing changes that manifested during the evening or night and making it more difficult to identify a fluctuating course. As residents were only assessed on alternate weekdays, there were potentially 4 days between some assessments, which may have also missed episodes of short duration.
Finally, it is also possible that the low observed delirium rate may have been due to higher quality of care or inclusion of residents at lower risk of delirium. However, our rates of dementia and hospitalization were similar to those reported in the published literature.
The view that our face-to-face assessments underestimated delirium appeared to be supported by care home records reviews, which identified additional cases of delirium. A combination of interview and records review has been recommended previously [36]. However, recording of delirium by staff may itself be influenced by the intervention; care home records reviews identified considerably more delirium episodes in the intervention homes, despite a lower observed rate from face-to-face assessments in the present study.
McCusker et al. in a multisite cohort of long-term care residents found a substantial increase in the reported prevalence of delirium when care home nurse-observed symptoms (structured interviews and care home records) were combined with symptoms observed by research staff alone [37]. However, there is still the possibility that increased awareness of delirium by staff as a result of the intervention may lead to increased availability of such informant information, influencing delirium detection.
An alternative approach would be to restrict outcome assessments to those most at risk of delirium (i.e. those with dementia or acutely unwell [38]) or to use ‘whole home’ assessments. Delirium screening instruments for use by non-specialists have been developed [39, 40], which could be administered by care home staff as part of routine care. Using anonymized data from such measures in research would also avoid the problem of excluding residents who are unable to be assessed for reasons that may be related to their delirium risk. However, the validity of these instruments in the care home setting is yet to be established. Again, it is likely that completion of such a measure by staff would be influenced by the intervention, making differentiation between intervention delivery and outcome measurement difficult.
Delirium detection in long-term care is challenging. Daily review by an experienced clinician using operationalized diagnostic criteria is the ‘gold standard’ but would be prohibitively resource intensive in a large trial. Daily CAM administration by a researcher with information from care home staff using structured interviews and review of care home records, despite its limitations, may be the optimum approach. Training and availability of supervision by experienced clinicians for research staff conducting CAM assessments would be important in view of the uncertainties in diagnosing delirium, particularly in people with dementia.
Other outcomes
An alternative is to use acute hospital admission as a primary outcome (as preventing delirium should reduce the need for hospital admission). An additional attraction in a trial where blinding is not possible is that hospital admission rates are less likely to be affected by observer bias. We were readily able to determine hospital admission rates for care home residents using routine data. However, this approach which relies on postcodes to identify admissions from care homes may not be reliable as homes may share a postcode with other private residences. In a study of hospital use by long-term care residents, 69% of hospital admissions identified as being from a care home using routine data were actually from a nursing or residential home [41]. A feasible alternative is to obtain hospital admission data directly from care homes, as these are increasing collected routinely as part of required reporting to commissioners.
The routinely collected hospital data also provide the most promising route to assess secondary care resource use. It is anticipated that data linkage with General Practice datasets will additionally provide primary care resource use in future.
We found that tools to capture health state utility were limited by lack of validity for the long-term care population. The only tool specifically developed for use in this setting (the SCRQoL) will probably not be practicable within the resources available in a large trial. Given the NICE recommendations [30], future studies should incorporate the EQ-5D. However, they should also consider additional measures such as the ICECAP-O (ICEpop CAPability measure for Older people) [42, 43], for which there is a recent and growing validation evidence in this group.
There were significant challenges in conducting this feasibility trial. Nevertheless, we were able to recruit residents successfully and conduct baseline and outcome assessments to a relatively high level of completion. On the basis of this study, we think that a future definitive trial is feasible but will require some modifications to the trial design in light of experience gained in this study.
Delirium is a distressing but potentially preventable condition common in older people in long-term care.
Delirium is associated with increased morbidity, mortality, functional decline, hospitalization and significant healthcare costs.
Multicomponent interventions can prevent delirium in hospitalized patients, but their effectiveness in long-term care is not known.
Detection of delirium in long-term care research is challenging and resource intensive.
Routine screening for delirium by care home staff may offer a way forward but requires further validation studies.
Conflict of interest
None declared.
Funding
This study was funded by the National Institute of Health Research (NIHR) Research for Patient Benefit Programme (PB-PG-0610-22068). The views expressed in this document are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The sponsor of this study is Bradford District Care NHS Foundation Trust. The sponsor and the funding agency had no role, and the authors retained full autonomy in the preparation of this manuscript.
Acknowledgements
Trial registration: ISRCTN27972532. The authors would like to acknowledge the following for their assistance in the conduct of this feasibility study: members of the Project Advisory Board, in particular Professor Finbarr Martin, Chair and Rita Exley, lay member on the Board; Chung Fu; Anna Burnham; and all staff in study care homes.
References




Comments