-
PDF
- Split View
-
Views
-
Cite
Cite
Lars Vilhelmsen, Brendon Boudinot, Josh Jenkins Shaw, Jörg U Hammel, Vincent Perrichot, Echoes from the Cretaceous: new fossils shed light on the evolution of host detection and concealed ovipositor apparatus in the parasitoid wasp superfamily Orussoidea (Hymenoptera), Zoological Journal of the Linnean Society, Volume 202, Issue 3, November 2024, zlae021, https://doi.org/10.1093/zoolinnean/zlae021
Close - Share Icon Share
Abstract
†Kryptovelona carstengroehni gen. et sp. nov. and †Orussus juttagroehnae sp. nov. are the first female members of the parasitoid wasp family Orussidae recorded from Baltic amber. We describe them, including relevant parts of the internal anatomy examined with synchrotron scanning. The fossils display a number of modifications in the antennae and foreleg correlated with the specialized host-detection mechanism, and in the ovipositor apparatus, as well as in the thorax and abdomen for accommodating the internalized ovipositor. The presence of these and other features places them as crown-group members of the Orussidae, as demonstrated by Bayesian phylogenetic analyses. The recently described stem-group orussoid fossils from Burmese amber, the probable female †Cretorussus vilhelmseni, and probable male †Burmorussus mirabilis (both placed in Burmorussidae), were also included in the dataset. By comparing the new Baltic amber taxa with †Cretorussus, it is possible to trace the progressive refinement of the echolocation mechanism through reductions in the number of antennomeres and foreleg tarsomeres. Unfortunately, †Cretorussus does not have the posterior part of the abdomen with the ovipositor preserved. Nevertheless, it is possible to infer that the putative echolocation mechanism for host detection evolved at least 100 Mya, whereas the concealed ovipositor apparatus has not been documented in fossils older than approx. 35 Mya.
Introduction
Orussidae are a small family of parasitoid wasps, comprising some 90 extant species distributed in all major biogeographic regions (Vilhelmsen 2004). They are unique among parasitoid wasps in not having a wasp-waist, but otherwise share a number of morphological traits with their putative sister-group, the Apocrita (e.g. Vilhelmsen et al. 2010). The Orussidae–Apocrita sister-relationship is also frequently retrieved by molecular analyses (e.g. Branstetter et al. 2017, Peters et al. 2017, Nyman et al. 2019, Blaimer et al. 2023), corroborating a scenario where the parasitoid lifestyle evolved only once within basal Hymenoptera, in the common ancestor of the Orussidae–Apocrita clade (Vilhelmsen and Turrisi 2011).
Orussidae are parasitoids of wood-living insect larvae, e.g. Buprestidae (Coleoptera) (Vilhelmsen 2003), a lifestyle that is probably very similar to that of the common ancestor of all parasitoid wasps. Extant female orussids have evolved specializations to target potential hosts inside their galleries in wood (Vilhelmsen et al. 2001); this involves vibrational sounding by tapping the wood with their modified antennae, and detecting the reflected vibrations by enlarged subgenual organs in the fore tibia through an elongate basitarsomere. The ovipositor is also unique in being concealed inside the body when not in use, extending all the way into the thorax (Vilhelmsen et al. 2001, Vilhelmsen 2020); extension and retraction is effectuated by small apodemes on the abdominal sternites.
A number of fossils have been associated with Orussidae (Jouault et al. 2021: table 1); these are included in the superfamily Orussoidea. Apart from the extant Orussidae and associated crown- and stem-group fossils, Orussoidea comprise †Paroryssidae (Martynov 1925) and †Burmorussidae [Zhang et al. 2020]. †Paroryssidae comprise compression fossils from the mid/Late Jurassic Karabastau Formation from Kazakhstan (age: approx. 160 Ma). †Burmorussidae comprise the recently described †Burmorussus Zhang, Kopylov and Rasnitsyn in Zhang et al. (2020) and †Cretorussus Jouault et al. 2021; both genera are from Cretaceous Burmese amber (age: approx. 100 Mya). The remaining fossils assigned to Orussoidea have been placed in Orussidae. Some are stem-group fossils: †MesorussusRasnitsyn 1977, Cretaceous Taimyr amber (age: approx. 95 Mya) and †MinyorussusBasibuyuk et al. (2000), Cretaceous New Jersey amber (age: approx. 90 Mya); others are in the orussid crown group (see: Vilhelmsen and Zimmermann 2014): †BaltorussusSchedl 2011, Eocene Baltic amber (age: approx. 35 Mya) and †Ophrynopus peritusEngel 2008, Miocene Dominican amber (age: approx. 17 Mya).
In the present paper, we describe two fossil female orussids from Baltic amber, contributing to the increasing knowledge of the fossil diversity of Orussoidea. Our examinations are based on light microscopy and synchrotron micro-computed tomography. We place the fossils in a phylogenetic context, and discuss the evolution of the morphological adaptations associated with host detection and ovipositing in Orussoidea in the light of observations across the fossil record of the superfamily.
Materials and methods
Material examined
The holotypes of †Kryptovelona carstengroehni Vilhelmsen and Perrichot, gen et sp. nov. (GPIH 5078, CCGG nº 3811) and †Orussus juttagroehnae Vilhelmsen and Perrichot, sp. nov. (GPIH 5081, CCGG nº 1288) are deposited in the amber collection of Carsten Gröhn in the Museum of Nature—Geology (formerly Geologisch-Paläontologischen Institut of University Hamburg, GPIH) at the Leibniz-Institut for the Analysis of Biodiversity Change, Hamburg, Germany. Each of these specimens are preserved in a piece of Baltic amber of unknown locality but that probably originated from the Samland Peninsula and should be dated as Late Eocene, Priabonian (34–38 Mya; for discussion on the age of Baltic amber, see, e.g. Sadowski et al. 2017). The holotype of †Cretorussus vilhelmseni Jouault et al. 2021 (IGR.BU-020), from mid-Cretaceous Burmese amber, is deposited in the collection of the Geological Department and Museum of the University of Rennes, France (IGR). Additional material of extant orussids from the collection of the Natural History Museum of Denmark (NHMD) was examined for comparison.
Microscopy and imaging
Specimens were examined with a Leica M205 C dissection microscope. Digital images were produced using a Canon EOS 5D, Canon EF 100mm Macro lens, Cognisys StackShot with flash lighting. A cylinder of semi-transparent plastic was placed around the amber pieces to disperse the light. For each final photograph, 25 images were obtained and stacked using Zerence Stacker v.1.04 by implementing the Pyramidal stacking method (PMax).
Synchrotron scanning
The amber specimens GPIH 5078, CCGG nº 3811 and GPIH 5081, CCGG nº 1288 (i.e. the newly described species) were scanned using synchrotron radiation based micro-computed tomography (SR-µCT), which was performed at the Imaging Beamline P05 (IBL) (Haibel et al. 2010, Greving et al. 2014, Wilde et al. 2016) operated by the Helmholtz-Zentrum Hereon at the storage ring PETRA III (Deutsches Elektronen Synchrotron—DESY, Hamburg, Germany). For imaging, a photon energy of 18 keV and a sample to detector distance of 30 cm was used; part of the amber piece containing GPIH 5081, CCGG nº 1288 was slightly darkened during the procedure. Projections were recorded using a 50 MP CMOS camera system with an effective pixel size of 0.46 µm. A total of 4001 projections were recorded for each tomographic scan at equal intervals between 0 and π, with an exposure time of 350 ms. Tomographic reconstruction was done by applying a transport of intensity phase retrieval and using the filtered back projection algorithm (FBP) implemented in a custom reconstruction pipeline (Moosmann et al. 2014) using MATLAB (Math-Works) and the Astra Toolbox (Palenstijn et al. 2011, van Aarle et al. 2015, 2016). For further processing, raw projections were binned two times resulting in an effective pixel size of the reconstructed volume of 0.913 µm.
Data segmentation and rendering
To ease data processing, the 32-bit .tif image sequence was converted to 8-bit files and downsampled twofold with FIJI (Schindelin et al. 2012), resulting in an effective pixel (voxel) size of 1.826 µm. The data were either segmented using Dragonfly (Comet Technologies Canada Inc., Montreal, Canada) or with AMIRA 6.1 (Visage Imaging GmbH, Berlin, Germany) using thresholding and manual segmentation, particularly to correct artefacts due to crystallization in the mesosoma and metasoma. For work in AMIRA 6.1, the segmented labels were exported as .tif image stacks with the plugin script ‘multiExport’ (Engelkes et al. 2018). Rendering was performed in VG-Studio Max 3.4 (Volume Graphics GmbH, Heidelberg, Germany).
Phylogenetic analysis
The dataset is a successively expanded version of the morphological matrix originally compiled in Vilhelmsen (2003). The current version was assembled in MESQUITE 3.70 (Maddison and Maddison 2021); the matrix can be downloaded from KryptovelonaMatrix2024 (figshare.com).
The following characters have been added to the dataset:
177. Number of fore tarsomeres, female: five = 0; three = 1.
178. Mesoscutum, notauli: at most very short lines developed anteriorly = 0; present, developed for at least half the length of mesoscutum = 1.
The final dataset included 178 characters scored for 102 taxa. The new taxa were scored from observations in a dissection microscope and the scan renderings. For †Kryptovelona carstengroehni gen. et sp. nov., 68 characters were inapplicable or could not be scored, meaning 62% of the characters were scored; for †Orussus juttagroehnae sp. nov., the corresponding numbers are 46 characters missing, scoring 74% complete. †Cretorussus was scored just from observations in a dissection microscope, with 119 characters missing (33% complete). Finally, †Burmorussus was scored from the description/illustrations in Zhang et al. (2020), with 129 characters missing (28% complete).
The morphological dataset was analysed using Bayesian inference in MrBayes 3.2.6 (Ronquist et al. 2012). The data were analysed using the Mkv model (Lewis 2001) with a gamma distribution and default settings for priors. The analyses used four chains (one cold and three heated) and six runs of 40 million generations. Urocerus gigas was set as the outgroup. The convergence of the six runs was visualized in TRACER v.1.6 (Rambaut et al. 2018) and by examining the potential scale reduction factor (PSRF) values and the average standard deviation of split frequencies in the MrBayes’ output. Computation was performed on the National Life Science Supercomputing Center—Computerome 2.0 (www.computerome.dk). We used R/RoguePlots (Klopfstein and Spasojevic 2019, R Core Team 2021) to explore phylogenetic placement of the three fossil taxa in all output trees of the Bayesian analysis. The online blog by Mario Coiro (https://mariocoiro.blog/2020/12/02/how-torepresent-uncertainty-in-phylogenies-rogueplots-to-the-rescue/) was also useful when implementing RoguePlots. Statistical support for placements of the eight fossil taxa was summarized using a majority-rule consensus tree and the post burn-in combined runs from the Bayesian analyses.
Results
Systematic palaeontology
†Kryptovelona carstengroehni Vilhelmsen and Perrichot, gen. et sp. nov.
(Figs 1–5)
ZooBank registration
: urn:lsid:zoobank.org:act:FCDEC20D-16ED-46AF-9866-4E792B0E7B1F (for Kryptovelona); urn:lsid:zoobank.org:act:DCE94EBD-773F-438A-A441-A059C79149F8 (for carstengroehni).
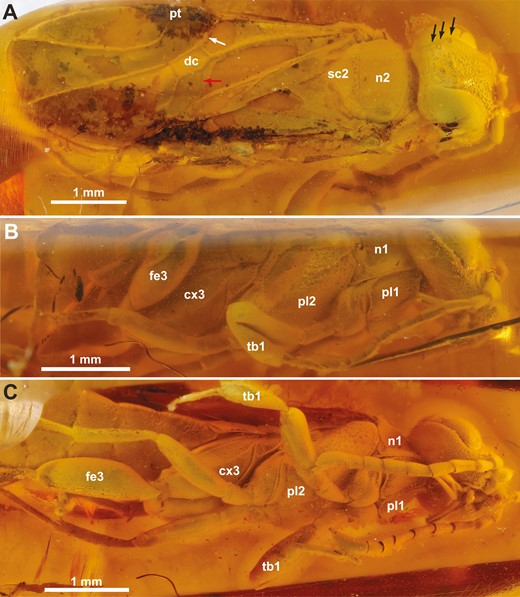
†Kryptovelona carstengroehni gen. et sp. nov., female holotype (GPIH 5078, CCGG nº 3811). A, dorsal habitus. B, lateral habitus. C, ventral habitus. White arrow = vein 1-Rs; red arrow = vein cu-a; black arrows = coronal teeth; cx3 = hind coxa; dc = discal cell; fe3 = hind femur; n1 = pronotum; n2 = mesoscutum; pl1 = propleuron; pl2 = mesopleuron; pt = pterostigma; sc2 = mesoscutellum; tb1 = fore tibia.
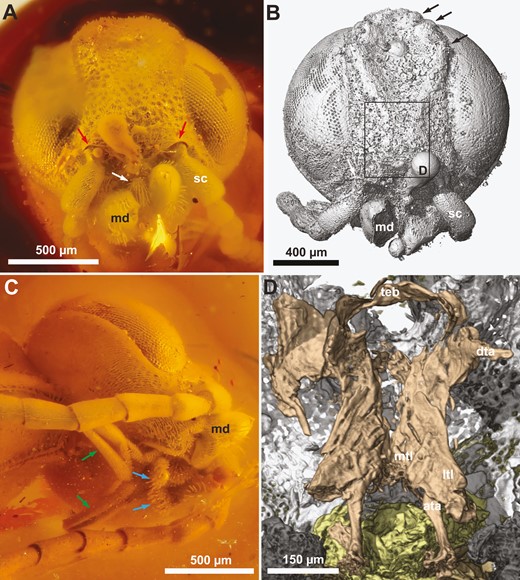
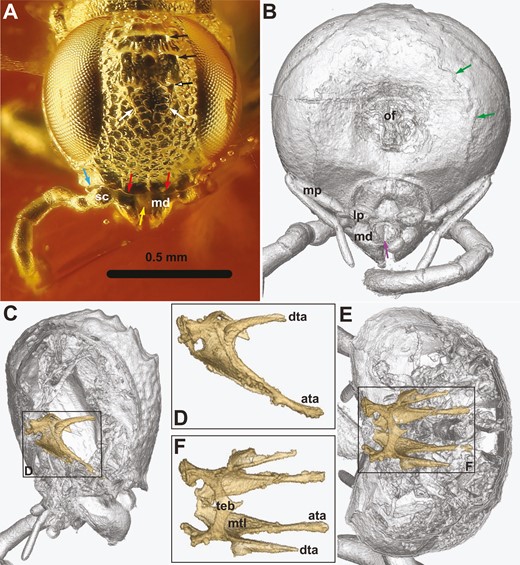
†Kryptovelona carstengroehni gen. et sp. nov., female holotype (GPIH 5078, CCGG nº 3811). A, C, photomicrographs. B, D, rendered from synchrotron scans. A, B, head, anterior view. C, head, lateroventral view. D, anterior view of tentorium. Red arrows = ventral transverse frontal carinae; white arrow = labrum; black arrows = coronal teeth; green arrows = maxillary palps; blue arrows = labial palp; ata = anterior tentorial arm; dta = dorsal tentorial arm; md = mandible; ltl = lateral tentorial lamella; mtl = median tentorial lamella; sc = scapus; teb = tentorial bridge.
![A, B, †Cretorussus vilhelmseniJouault et al. 2021, female holotype IGR.BU-020. C, †Kryptovelona groehni gen. et sp. nov., female holotype (GPIH 5078, CCGG nº 3811). Photomicrographs. A, distal part of antenna. B, fore tibia and tarsus. C, distal part of antenna and fore tibia and tarsus. Red arrow = tarsal spur; blue arrow = tibial spur (slightly displaced); green arrow = groove in fore tibia; A[x] = antennomere [x]; t[x] = fore tarsomere [x] (putative composite structures and structures distal to them in †Kryptovelona in italics, with homologues in †Cretorussus in brackets); tb1 = fore tibia.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/zoolinnean/202/3/10.1093_zoolinnean_zlae021/1/m_zlae021_fig3.jpeg?Expires=1750272344&Signature=N6TloSYE6praf~k3CWszL1oVwofOwQFCBYgMJhRWXce3AXQoN4kp6tcarQCXQGLlciQ7W2H1Qqf8HM0lU-GSErEa~zx4UH~U8HVWDFbRBDzNFPI6ZEG-bCVt6DY3NGZNoIMHwfcgqAyN84TFk6ZDpMmz49t2gw0E2Ztml~k2vDOT1mSP39X8dmRWeGA9ofy0JdR0McKfmPAqF-~AREr-wj9-2fxWOYQ-Im8BmdjI8dnmhmZ~no2XYWq0YKGQFTBfH3qOcBZ9dk6HkgDK-~jnQx-V8oBd2BXAbZsTZlwhr18AwxnSDZYQApCwp9DO2r6mzQaR3ikbI82OmnMwgR5R9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
A, B, †Cretorussus vilhelmseniJouault et al. 2021, female holotype IGR.BU-020. C, †Kryptovelona groehni gen. et sp. nov., female holotype (GPIH 5078, CCGG nº 3811). Photomicrographs. A, distal part of antenna. B, fore tibia and tarsus. C, distal part of antenna and fore tibia and tarsus. Red arrow = tarsal spur; blue arrow = tibial spur (slightly displaced); green arrow = groove in fore tibia; A[x] = antennomere [x]; t[x] = fore tarsomere [x] (putative composite structures and structures distal to them in †Kryptovelona in italics, with homologues in †Cretorussus in brackets); tb1 = fore tibia.
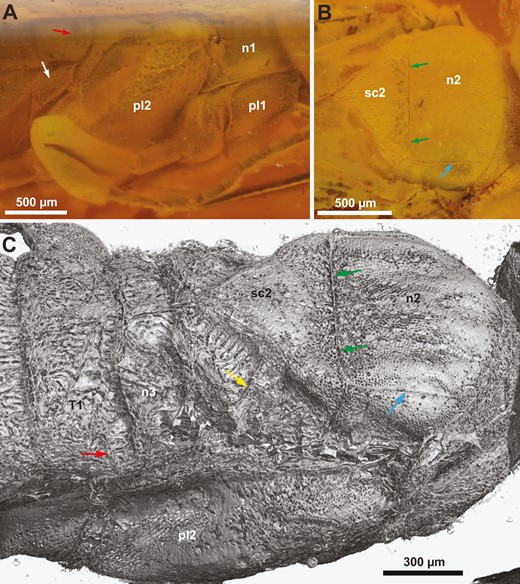
†Kryptovelona carstengroehni gen. et sp. nov., female holotype (GPIH 5078, CCGG nº 3811). A, B, photomicrographs. C, rendered from synchrotron scans. A, thorax, lateral view. B, thorax, lateral view. C, thorax and anterior abdomen, dorsolateral view. White arrow = metapleuron; red arrow = spiracle on 1st abdominal tergum; green arrows = transscutal articulation; blue arrow = parapside; yellow arrow = scutellar arm; n1 = pronotum; n2 = mesonotum; n3 = metanotum; pl1 = propleuron; pl2 = mesopleuron; sc2 = mesoscutellum; T1 = 1st abdominal tergum.
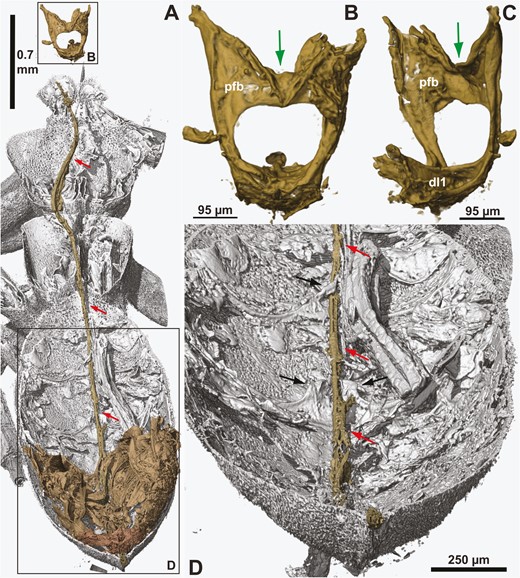
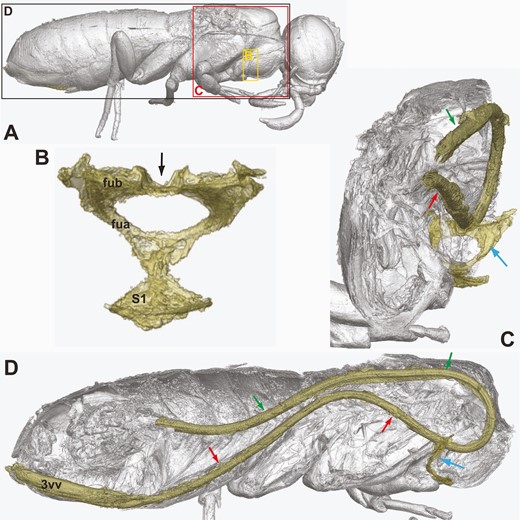
†Kryptovelona carstengroehni gen. et sp. nov., female holotype (GPIH 5078, CCGG nº 3811). A, dorsal overview of extent of ovipositor inside the body; anterior to the top. B, profurca in posterior view. C, profurca in anterolateral view. D, posterior part of abdomen, dorsal internal view. All views rendered from synchrotron scans. Red arrows = ovipositor; black arrows = medain apodemes on abdominal sternum 6 and 7; green arrows = median groove on profurcal bridge; dl1 = prodiscrimenal lamella; pfb = profurcal bridge.
Material examined
Holotype GPIH 5078 (CCGG no. 3811), a complete female preserved in a piece of Baltic amber dated as Late Eocene (Priabonian).
Diagnosis
Ventral coronal teeth absent, fully developed ventral transverse longitudinal carina absent. Antennomeres 4 and 5 not conspicuously shortened, maxillary palp elongate, five-segmented. Median mesoscutal sulcus at most developed for a short distance anteriorly, notauli absent, mesoscutellum triangular, acute, raised compared to surrounding sclerites. Forewing vein 1r arises from approximately middle of pterostigma; vein 1r-Rs well developed, elongate, discal cell rectangular, proximal part not broader than distal part. Ovipositor internalized, extending into thorax, profurca with median groove and abdominal sterna with median apodemes for handling ovipositor.
Description
Female (Fig. 1). Body length 6.8 mm. Specimen generally well preserved; body with opaque film somewhat obscuring sculpture and pilosity, as well as original coloration; cracks in matrix around specimen also obscures certain features. Right hind tarsus cut off during trimming.
Head
Ocellar corona narrow, distance between median ocellus and lateral coronal tooth subequal to ocellar width; ventral coronal teeth absent (Figs 1A, 2B). Dorsal transverse and longitudinal frontal carinae absent. Ventral transverse frontal carina not continuous medially, extending across toruli and laterally continuous with lateral carina of subantennal groove (Fig. 2A, B). Frons and vertex areolate-punctate, genae with scattered punctures; frons, vertex, and genae covered with fine, slender hairs (Fig. 2A, C). Internally, median and lateral frontal septa well developed. No conspicuous hairs just posteriorly of eyes. Postocular and occipital carinae absent (Fig. 2C). Lateral carina of subantennal groove low and short, not extending beyond posterior margin of eye. Tentorium with broad median and lateral lamellae on anterior arm, lamellae terminating some distance from anterior tentorial pit; tentorial bridge narrow, arched, with short anterior process; dorsal tentorial arms arise posteriorly on anterior arms, short, apparently do not reach head capsule (Fig. 2D). Antenna with 10 antennomeres, covered with short hairs; scapus elongate, cylindrical, slightly curved; combined length of antennomeres 4 and 5 longer than antennomere 6; antennomere 9 swollen subapically, as long as combined length of antennomeres 7 and 8, without carina laterally; antennomere 10 short, cylindrical, inserted subapically on antennomere 9 (Figs 1C, 3C). Labrum visible between opened mandibles, small, globose, with distinct ventrally directed hairs (Fig. 2A). Mandibles chisel-like, no teeth developed, with conspicuous distally pointing hairs laterally (Fig. 2A, B). Maxillary palps elongate, five-segmented, labial palps short, distal palpomere swollen (Fig. 2C).
Thorax
Pronotum dorsally of equal length throughout, only shallowly incurved posteriorly; lateral sculpture obscured by pilosity/film (Fig. 4A, B). Prosternum mostly concealed by propleura, trapezoid, lateral margins converging anteriorly, discrimen and discrimenal lamella distinct; profurcal arms extend obliquely anteriorly from posterior corners of prosternum, profurcal bridge high and straight, with distinct median groove, not bent posteriorly (Fig. 5B, C). Fore coxa not expanded medially (Fig. 1C); fore femur without ventral carina; fore tibia swollen distally and subdivided by groove; fore tarsus with three tarsomeres, basitarsus elongate and terminating in spur overlapping tarsomere 2 (Fig. 3C). Mesoscutum finely punctate, mostly covered with short hairs, with scattered punctures laterally, median mesoscutal sulcus at most developed anteriorly, notauli absent, parapsides present laterally and transscutal articulation posteriorly (Fig. 4B, C); axillar flanges well developed; scutoscutellar sulcus well developed; mesoscutellum finely punctate, covered with hairs, acutely triangular posteriorly, raised and with carinate lateral margins; mesoscutellar arm raised, without pit anteriorly; mesonotum continuous posterior to mesoscutellum (Fig. 4C). Mesopleuron areolate—punctate laterally, mesosubalar carina present, mesepisternal carina absent; mid-coxa subdivided, with distinct lateral carina. Metanotum with metascutellum not developed, median metanotal carina not observed, lateral metanotal carina present, reaching posterior margin of metanotum; metapleuron with few hairs, mostly glabrous (Fig. 4A); hind coxae not especially hairy laterally, with rounded medioventral margin and well-developed posterolateral carina; hind femur without ventral carina or denticles ventrally; hind tibia with distinct pegs dorsally, longitudinal and ventral carinae not developed; apical hind tibial spurs short, of unequal length.
Wings
Forewing vein 2r-rs arise from approximately middle of pterostigma; vein 1-Rs well developed, elongate, discal cell rectangular, proximal part not broader than distal part; vein cu-a inserts on Cu1 close to middle of cell M (Fig. 1A). Hindwing venation cannot be observed.
Abdomen
Dorsally covered by wings, hence obscuring structural detail via light microscopy; laterally with dense pilosity of short hairs. Tergum 1 and 2 coarsely areolate (Fig. 4C), postspiracular and subspiracular carinae on tergum 1 absent, median carina on tergum 2 absent; tergum 3–8 finely punctate. Median apodemes observed on several abdominal sterna (Fig. 5D); sternum 7 externally with narrow median part delimited laterally and projecting posteriorly. Tergum 9 covered with short hairs, without longitudinal carinae, smooth areas and depressions; internally, prominent anterior flange and chordate apodemes present. Ovipositor apparatus apparently not fully preserved (Fig. 5A); second valvifer with traces of ventral T9-second valvifer muscles [muscle 6 in Vilhelmsen et al. (2001)] observable, possible base of ovipositor shaft with remains of processus medianus present; only parts of internalized ovipositor loop traceable, ventral/distal part extends anteriorly at least to mesofurca, manner of coiling in prothorax not observed; tip of ovipositor slightly protruding from tip of abdomen, third valvulae not visible. Composite tergum 10/cercus triangular, not continuous medially.
Etymology
The genus name is a combination of krypto and velona, the Greek words for hidden and needle, respectively, alluding to the concealed ovipositor of the family. The species’ epithet honours Mr Carsten Gröhn (Glinde, Germany) who generously made the type specimen available for study.
Comments
Although both †Kryptovelona and †Baltorussus occur in Baltic amber, there are sufficient differences to justify recognizing them as separate genera. Besides, they do not group together in the phylogenetic analyses, where †Kryptovelona is placed closer to the clade comprising the majority of the extant taxa. The RoguePlots (Supporting Information, Figs S1, S4) flag a possible †Kryptovelona–†Baltorussus sister-relationship, but with lower probability than in the preferred Bayesian tree; other alternative placements of †Kryptovelona closer to the base of extant Orussidae have even lower support.
![†Orussus juttagroehnae sp. nov., female holotype (GPIH 5081, CCGG nº 1288). Dorsal habitus (A). Photomicrograph. B, rendered from synchrotron scans (head and wings removed in reconstruction). White arrow = vein 1-Rs; red arrow = vein cu-a; green arrow = median metanotal carina; blue arrow = lateral metanotal carina; yellow arrow = mesoscutellar arm; dc = discal cell; n1 = pronotum; n2 = mesoscutum; pt = pterostigma; sc2 = mesoscutellum; t[n] = abdominal tergum [n].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/zoolinnean/202/3/10.1093_zoolinnean_zlae021/1/m_zlae021_fig6.jpeg?Expires=1750272344&Signature=IzpbFuzqIRg23jKTTHp5r1VMcobMvkVpm-yhINrY~z8n48SECb6HDVqf70Pk~5uuZ8ZUA8uO~6DxI4sYwAKYiiU1MgaOivvhGi53GDsGIoaNJqIWsvEEdDkY5VhnsA8FeMn5~ouBQbeiFPDbFwaNk5xFvsMcehPL63KFVa~gX2Z3Sa-18NG0E3-AjJqicVEBfVQfuFWC-dq2Ejgj33~bWzVccZc0~hGLtQ~wSzVrGsX1wEm8yOcpaXCmXqdzea-KkiNjFYxzgrb~peF4cSvm04FMpQcRUHm5U4ENwd71igLtIjhqCvImV4PBS6ibY7HtYhgt86XEoIM0WzE38ctGLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
†Orussus juttagroehnae sp. nov., female holotype (GPIH 5081, CCGG nº 1288). Dorsal habitus (A). Photomicrograph. B, rendered from synchrotron scans (head and wings removed in reconstruction). White arrow = vein 1-Rs; red arrow = vein cu-a; green arrow = median metanotal carina; blue arrow = lateral metanotal carina; yellow arrow = mesoscutellar arm; dc = discal cell; n1 = pronotum; n2 = mesoscutum; pt = pterostigma; sc2 = mesoscutellum; t[n] = abdominal tergum [n].
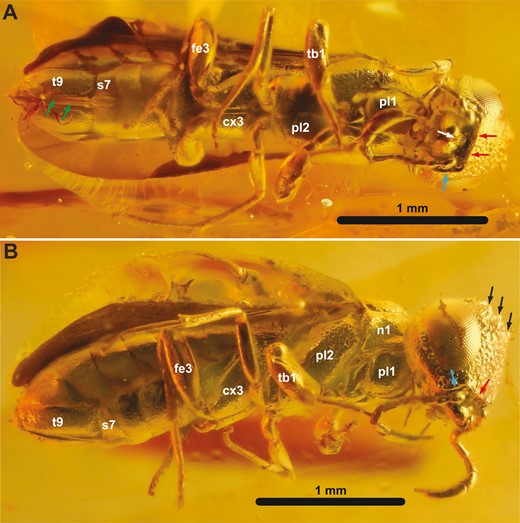
†Orussus juttagroehnae sp. nov., female holotype (GPIH 5081, CCGG nº 1288). A, ventral habitus. B, lateral habitus. Green arrows = 3rd valvulae; white arrow = labrum; red arrows = ventral frontal carina; blue arrows = lateral carina of subantennal groove; black arrows = coronal teeth; cx3 = hind coxa; fe3 = hind femur; n1 = pronotum; pl1 = propleuron; pl2 = mesopleuron; s7 = abdominal sternum 7; t9 = tergum 9; tb1 = fore tibia.
Orussus juttagroehnae sp. nov., female holotype (GPIH 5081, CCGG nº 1288). A, photomicrograph. B–F, rendered from synchrotron scans. A, head, anterior. B, head, posterior. C, head, lateral, showing position of tentorium. D, tentorium in lateral view. E, head, dorsal, showing position of tentorium. F, tentorium in dorsal view. Black arrows = coronal teeth (ventral tooth with white head); white arrows = median longitudinal frontal carina; blue arrow = lateral carina of subantennal groove; red arrows = ventral frontal carina; yellow arrow = labrum; green arrows = occipital carina; violet arrow = glossa of labium; ata = anterior tentorial bridge; dta = dorsal tentorial bridge; lp = labial palp; md = mandible; mp = maxillary palp; mtl = median tentorial lamella; of = occipital foramen; sc = scapus; teb = tentorial bridge.
Orussus juttagroehnae sp. nov., female holotype (GPIH 5081, CCGG nº 1288). Ovipositor; Rendered from synchrotron scans. A, habitus, lateral with position of views indicated. B, profurca, posterior view. C, profurca and ovipositor loop, posterolateral view. D, complete ovipositor loop, lateral view. Black arrow = median groove on profurcal bridge; green arrows = dorsal branch of ovipositor loop; red arrows = ventral branch of ovipositor loop; blue arrows = profurca; fua = profurcal arm; fub = profurcal bridge; S1 = prosternum; 3vv = 3rd valvula.
The sculpture of the frons is highly diagnostic in †Baltorussus (Vilhelmsen and Zimmermann 2014: fig. 3A, B); this is not observed in †Kryptovelona. The latter has much denser pilosity over large areas of the body, in particular the dorsal part of the thorax. The median mesoscutellar sulcus is well developed in †Baltorussus, extending all the way to the transscutal articulation (Vilhelmsen and Zimmermann 2014: fig. 7), whereas it is absent in †Kryptovelona; the notauli are also more developed in the former, extending approximately halfway to the articulation, whereas they are not developed in †Kryptovelona. In the forewing of †Kryptovelona, vein cu-a arises from approximately the middle of the discal cell; in †Baltorussus it is placed much closer to vein M.
†Orussus juttagroehnae Vilhelmsen and Perrichot, sp. nov.
(Figs 6–9)
ZooBank registration
urn:lsid:zoobank.org:act:2195267A-2F76-4A67-A2BD-893D44C90CD2.
Material examined
Holotype GPIH 5081 (CCGG no. 1288), a complete female preserved in a piece of Baltic amber dated as Late Eocene (Priabonian).
Diagnosis
Ventral coronal teeth present, connected by faint U-shaped carina (Fig. 8A). Ventral transverse longitudinal carina fully developed, with median notch (Figs 7A, 8A). Antennomeres 4 and 5 combined approximately the same length as antennomere 6, maxillary palp elongate, five-segmented (Fig. 8B). Fore coxa expanded medially. Median mesoscutal sulcus and notauli at most weakly developed, mesoscutellum triangular, acute, raised compared to surrounding sclerites (Fig. 6A, B). Forewing vein 1r arises from approximately middle of pterostigma; vein 1r-Rs well developed, elongate, discal cell trapezoid, proximal part broader than distal part (Fig. 6A).
Description
Female (Figs 6, 7). Body length 3.8 mm. Original body coloration not observable. Specimen very well preserved and well observable from most angles except for distal margin of right forewing, which is obscured by narrow cracks extending from the wings. Forewings folded above abdomen, obscuring it and the hindwings in dorsal view.
Head
Ocellar corona narrow, distance between median ocellus and lateral coronal tooth less than ocellar width; ventral coronal teeth present, situated slightly medial to median coronal teeth; faint median frontal carinae extend ventrally from ventral coronal teeth and converge medially, forming U-shaped loop (Fig. 8A). Dorsal transverse and longitudinal frontal carinae absent. Ventral transverse frontal carina continuous medially and with slight incision, covering toruli in anterior view (Fig. 8A); carina laterally continuous with lateral carina of subantennal groove (Fig. 7). Frons and vertex areolate (Fig. 8A), genae with scattered punctures; frons, vertex, and genae without conspicuous pilosity. Postocular carina well developed for most of height of eye, occipital carinae present (Fig. 8B), continuous with distinct lateral carina of subantennal groove ventrally. Tentorium with median lamellae on anterior arm broadening posteriorly (Fig. 8E, F), lateral lamellae hardly developed; tentorial bridge narrow, arched, with distinct anterior process (Fig. 8F); dorsal tentorial arms arise posteriorly on anterior arms, short, apparently do not reach head capsule (Fig. 8C, D). Antennae with 10 antennomeres, covered with short hairs; scapus short, subcylindrical (Fig. 8A); combined length of antennomeres 4 and 5 approximately equal to antennomere 6; antennomere 9 longer than antennomeres 7 and 8 combined, swollen subapically, without carina laterally; antennomere 10 short, cylindrical, inserted subapically on antennomere 9 (Fig. 7). Labrum visible between mandibles (Figs 7A, 8A), small, globose, with distinct ventrally directed hairs. Mandibles chisel-like, no teeth developed, with conspicuous distally pointing hairs laterally. Maxillary palps elongate, five-segmented, labial palps short, three-segmented (Fig. 8B); glossa of labium folded longitudinally, concave in posterior view.
Thorax
Pronotum dorsally short, of equal length throughout, deeply incurved posteriorly (Fig. 6B), laterally evenly punctured. Prosternum mostly concealed by propleura, diamond-shaped, lateral margins diverging anteriorly, discrimen and discrimenal lamella distinct; profurcal arms extend obliquely anteriorly from posterior corners of prosternum, profurcal bridge low and straight, with distinct median groove accommodating lower branch of curved ovipositor (Fig. 9B), bridge not bent posteriorly. Fore coxa expanded medially; ventral carina on fore femur not observed; fore tibia swollen distally and subdivided by groove (Fig. 7); fore tarsus with three tarsomeres, basitarsus elongate and terminating in spur overlapping tarsomere 2. Mesoscutum finely punctate, not densely covered with hairs, median mesoscutal sulcus absent, notauli only developed as shallow grooves not reaching transscutal articulation posteriorly, parapsides present laterally and transscutal articulation posteriorly (Fig. 6); axillar flanges well developed; scutoscutellar sulcus well developed; mesoscutellum areolate-punctate, rounded triangular posteriorly (Fig. 6), raised and with smooth carinate lateral margins; mesoscutellar arms curved posteriorly, area anterior to arms raised to same level as arms; mesonotum continuous posterior to mesoscutellum. Mesopleuron areolate–punctate lateroventrally, mesosubalar carina present, mesepisternal carina absent; mid-coxa subdivided, with distinct lateral carina. Metanotum with median and lateral metanotal carinae well developed (Fig. 6B); metapleuron areolate across most of its surface; hind coxae not especially hairy laterally, with angled medioventral margin and well-developed posterolateral carina; ventral part of hind femur concealed; hind tibia with distinct pegs dorsally, longitudinal and ventral carinae not observed; apical hind tibial spurs short, of approximately equal length.
Wings
Forewing apparently without marked bands of infuscation; vein 2r-rs arise from approximately middle of pterostigma; vein 1-Rs well developed, elongate, discal cell trapezoid, proximal part higher than distal part (Fig. 6A); vein cu-a inserts on Cu1 only slightly distally to vein M. Hindwing venation cannot be observed.
Abdomen
Dorsally covered by wings, hence obscuring structural detail; laterally not particularly hairy, sculpture finely imbricate. Tergum 1 with weakly developed postspiracular carina, subspiracular carinae absent. Tergum 2 without median carina (Fig. 6B), with rectangular smooth area laterally abutting antecosta. Median apodemes observed on several abdominal sterna; sternum 7 externally with narrow median part delimited laterally and projecting posteriorly (Fig. 7A). Tergum 9 punctate, with low, longitudinal carinae delimiting concave depression medially, smooth areas and depressions absent. Tip of ovipositor slightly protruding from tip of abdomen, third valvulae exposed ventrally (Fig. 7A). Internally, complete ovipositor loop can be traced (Fig. 9D), loop simple, hairpin-like, anterior part of ventral branch runs through dorsal longitudinal groove of profurcal bridge (Fig. 9C); traces of ovipositor sack containing loop observed. Composite tergum 10/cercus triangular, not continuous medially.
Etymology
The species epithet honours Mrs Jutta Gröhn, wife of Carsten Gröhn and longterm supporter of his passion for amber insects.
Comments
†Orussus juttagroehnae displays a character combination that unequivocally places it in Orussus Latreille 1796 (see Diagnosis); this is corroborated by the phylogentic analyses (Fig. 10), which places the new species nested well inside Orussus, as sister to Orussus hanumanus Vilhelmsen and Blank 2014. The RoguePlots (Supporting Information, Fig. S8) in a single instance places †O. juttagroehnae outside Orussus, but with low probability. A putative autapomorphy of Orussus that could not be confirmed in †O. juttagroehnae is the presence of white spots on at least some legs (Vilhelmsen 2003) and also on other body parts (e.g. face, antennae, pronotum, abdominal tip) in some species; given that the natural colour of the fossil specimen is not observable, white spots might well be present. †Orussus juttagroehnae can be separated from all other Orussus spp. by the unique U-shaped configuration of the short median frontal carinae and the comparatively well-developed vein 1-Rs in the forewing. †Orussus juttagroehnae is the first fossil species described from the genus, and the oldest member of an extant orussid genus recorded so far; †Ophrynopus peritusEngel 2008 is from the much younger Dominican amber.
The holotype of †O. juttagroehnae is also among the smallest specimens of Orussus spp. recorded; its body length of 3.8 mm ties with Orussus minutus Middlekauff 1983 at the lower end of the size range for this genus; the upper limit observed by Vilhelmsen (2004: table 2) being 16.0 mm (e.g. Orussus abietinus Scopoli 1763). This is the more remarkable as the †O. juttagroehnae specimen is a female and females tend to be significantly larger than males; the smallest specimen of Orussus minutus measured by Vilhelmsen (2004) was a male. There is, of course, preservational bias favouring smaller insect fossils in amber, but †O. juttagroehnae is not the smallest orussid recorded. Among extant taxa, a female Orussobaius minutissimusSchmidt and Vilhelmsen 2002 is the smallest with a body length of 2.0 mm (see: Blank and Vilhelmsen 2016: fig. 11); among fossils, †Minyorussus luzziBasibuyuk et al. 2000 (male, 2.2 mm) and possibly †Mesorussus taimyrensisRasnitsyn 1977 [female, possibly 1.75 mm—body length of incomplete fossil estimated by Vilhelmsen (2004)] are the smallest recorded.
Bayesian analyses
The Bayesian analysis achieved convergence with an average standard deviation of split frequencies well below 0.02 after 40 million generations. Effective sample sizes (ESS) for the posterior probability were much higher than 200 and observed PSRF was 1.00. The 50% majority rule consensus tree from the Bayesian analysis is presented in Figure 6.
Overall, the topology is very similar to earlier studies (e.g. Vilhelmsen 2003, Vilhelmsen and Zimmermann 2014) and, therefore, we focus on the placements of the new fossil taxa. The sister-relationship of †Kryptovelona carstengroehni to a large clade of extant taxa (Fig. 10), i.e. all except Orussella, Orussonia and †Baltorussus, was not well supported (PP = 0.55), and the RoguePlots reveal alternative positions, either as sister to †Baltorussus or closer to the base of crown group Orussidae. †Orussus juttagroehnae is placed well inside Orussus in most RoguePlots, but in one instance falls outside the genus as sister to Chalinus and Mocsarya, albeit with low probability.
The RoguePlots (Supporting Information, Figs S2, S3, S5, S6) show that the Cretaceous stem-group fossils (†Burmorussus, †Cretorussus, †Mesorussus, and †Minyorussus) cluster at the base of Orussoidea and are placed with high probability, despite having many missing entries in the data matrix; this could be because both the Orussoidea and crown-group Orussidae are well-supported nodes (Fig. 10), leaving the Cretaceous fossils little possibility for ‘floating’ beyond these branches. In contrast, the RoguePlots of the Cenozoic fossils, in particular those in the large extant genera Orussus (†O. juttagroehnae; Supporting Information, Fig. S8) and Ophrynopus (†O. peritus; Supporting Information, Fig. S7) show them to have many alternative, poorly supported positions, even a few outside the genera to which they have been assigned. This might be because the internal relationships of Orussus and Ophrynopus are poorly resolved and supported.
Discussion
Phylogenetic placement of the new fossil taxa and early diversification of the Orussoidea
†Kryptovelona is unequivocally placed as a crown-group member of Orussidae by the phylogenetic analyses. Within the crown clade, its placement is less certain; †Baltorussus comes out as the sister to the clade including †Kryptovelona. The Cretaceous fossil taxa (†Burmorussus, †Cretorussus, †Mesorussus, and †Minyorussus) are all placed as stem-group orussoids, with †Burmorussus being the sister to all the rest; †Minyorussus is the sister to the crown group (Fig. 10).
Notably, the inclusion of the Baltic amber fossils does not induce any major changes to the topology of the extant taxa [compare the results of the present paper and Vilhelmsen and Zimmermann (2014) with, e.g. Vilhelmsen (2003)]. †Kryptovelona and †Baltorussus are placed as successive sister-groups to the clade comprising Orussobaius, Leptorussus, and the three major clades: (i) Pedicrista—Mocsarya—Chalinus; (ii) Pseudoryssus—Orussus; and (iii) the ophrynopine clade. Previous analyses resolve the relationships between these three clades with, at most, weak character support; in the Bayesian tree presented here (Fig. 10), the trichotomy is unresolved.
The weakly supported sister-group relationship of †Kryptovelona occurs in spite of the comparatively high number of characters scored for this fossil, i.e. 62% (see above); this is approximately the same as that for †Baltorussus (73 characters missing out of 178, score completeness 59%) and about twice as high as for the stem-group fossils, e.g. †Burmorussus and †Cretorussus, which were not subjected to scanning techniques and in the case of †Cretorussus is incompletely preserved. The poor support for the node leading to †Kryptovelona might be caused by the proximity of the two Baltic amber fossils on the tree, leading to uncertainty as the two fossil taxa still have fewer characters scored than the extant taxa surrounding them.
In the following, we will focus on the character support for the basal splits within Orussoidea; characters of particular interest, e.g. those concerned with the echolocation mechanism and the ovipositor apparatus, will be discussed in more detail separately (see below: Character evolution). The interpretation of character evolution in Orussoidea is constrained by many of the characters being sex-specific, and all of the fossil taxa (as well as many of the extant) being known from only one sex (female: †Cretorussus, †Mesorussus, †Kryptovelona, and †Orussus juttagroehnae; male: †Burmorussus, †Minyorussus, †Cretorussus, †Baltorussus, and †Ophrynopus peritus).
Orussoidea are supported by the presence of an ocellar corona (char. 1:1, Figs 1A, 2B; paralleled in Schlettererius (Stephanidae); see: Jouault et al. 2021), female with 12 antennomeres (char. 164:1, Fig. 3A; not observed in †Burmorussus), apical antennomere tapered distally and with flattened apex (char. 40:1, Fig. 3A; not observed in †Burmorussus), female fore tibia swollen and subdivided medially (char. 57:1, Fig. 3B; not observed in †Burmorussus), and spur on third fore tarsomere present (char. 61:1, Fig. 3B; not observed in †Burmorussus). All orussoids, except †Burmorussus, are supported by the mandibles being narrowed distally and chisel-like (char. 45:1, Fig. 2A, B; paralleled in Schlettererius, not observed in †Mesorussus and †Minyorussus).
†Minyorussus plus the crown group are supported by the presence of the ventral transverse frontal carina present above the antennal bases, the carinae not being continuous medially (char. 19:1, Fig. 2A, B), and the absence of vein 2m-cu in the forewing (char. 122:0, Fig. 1A; not observed in †Mesorussus). Crown-group orussids are corroborated by the female having 10 antennomeres (char. 164:2, Fig. 1C; not observed in †Minyorussus), the apical antennomere being reduced and subcylindrical (char. 40:2, Fig. 3C; not observed in †Minyorussus), and the female fore tarsus having only three tarsomeres (char. 177:1, Fig. 3C; not observed in †Mesorussus and †Minyorussus).
The least inclusive clade containing all crown-group fossils is supported by the presence of a subantennal groove that is delimited by short carina laterally (char. 31:1; reversed in Orussobaius), the mid-coxa having a distinct lateral carina (char. 87:2), and the hind tibia having distinct pegs dorsally (char. 103:2; reversed in some Orussobaius and Orussus spp.). †Kryptovelona and its sister-group are weakly held together by the absence of the notauli (char. 178:0, Fig. 4B, C; also absent from Orussonia). The sister-group of †Kryptovelona is corroborated by having the scapus short and subcylindrical (34:0), the mesoscutellar arm with a deep pit anteriorly (79:1), and the hind coxa having the medioventral margins angled (96:1/2).
Character evolution
Tentorium
The dorsal tentorial arms of †Kryptovelona are apparently incomplete, not reaching the head capsule (Fig. 2D). This is an apomorphic trait, also being observed in the extant genus Orussus, including †O. juttagroehnae (Fig. 8C, D). In contrast, †Baltorussus has fully developed dorsal tentorial arms (Vilhelmsen and Zimmermann 2014: fig. 6), the plesiomorphic condition (see: Zimmermann and Vilhelmsen 2016). Given that no extant orussoid genera other than Orussus have been surveyed for this trait, it is premature to speculate on the potential phylogenetic significance of the occurrence of the dorsal tentorial arm within the superfamily.
Antennae
Contrary to statements in Jouault et al. (2021: 329, ‘†Burmorussidae females may have displayed this plesiomorphic, unmodified antennae [sic]’) and the drawing in their figure 3A which shows a rounded tip, we observe the tip of the apical antennomere to taper slightly distally and be somewhat flattened and darkened, indicating thickened cuticle (Fig. 3A). This configuration was also observed in †Mesorussus from Taimyr amber (Vilhelmsen 2004) and can be interpreted as an evolutionary intermediate with respect to the configuration of the apical antennomere 10 in crown-group Orussidae. The enlarged antennomere 9 in the latter could be a composite sclerite that evolved by the fusion of the three penultimate antennomeres (i.e. 9–11; Fig. 3C) in †Cretorussus and †Mesorussus, which are, at most, slightly longer and wider than the apical antennomere. It appears that the antennal tip in the female of †Cretorussus was already somewhat modified for generating vibrations in wood by tapping it.
Foreleg
Jouault et al. (2021) concluded that the single known specimen of †Cretorussus vilhelmseni is a female, based on observations of the foreleg, in particular; we concur with this. The foreleg is modified similarly to that of crown-group orussids, i.e. the fore tibia is swollen and has a prominent transverse furrow approximately halfway, and there is a distinct spur on the tarsomere proximal to the apical and penultimate fore tarsomere (Fig. 3B). The main difference is that in †Cretorussus the spur is on the third fore tarsomere, whereas in the crown group the spur is on the very elongate fore ‘basitarsomere’ (Fig. 3C). Vilhelmsen et al. (2001) have suggested that the fore basitaromere in Orussidae is actually a composite structure comprising tarsomeres 1–3; this seems to be corrobated by the configuration in †Cretorussus. There is apparently a slight inaccuracy in Jouault et al. (2021) in their fig. 2D showing a close-up of most of the fore tarsus; the structure labelled [tarsomere] ‘IV’ is actually the distal part of the third tarsomere, the fourth being very short, occupying only the distance between the third tarsomere and the kink that indicates the boundary between fore tarsomeres 4 and 5 (see also Fig. 3B). The spur on the fore tarsus is probably used for receiving the reflected vibrations generated in the wood by the antennae and transmitting them up the leg to the subgenual organ in the fore tarsus (Vilhelmsen et al. 2001); the elongate composite basitarsomere observed in †Kryptovelona (Fig. 3C) and other crown orussids possibly serves to transmit the vibrations more effectively, as they do not have to cross membraneous areas between the basalmost tarsomeres. As with the antennal tip, †Cretorussus presumably displays an early stage in the modification of the foreleg for host detection.
Meso- and metanotum
The mesonotum of all Orussoidea appears to have well-developed parapsides and transscutal articulation (Fig. 4B, C); these are apomorphic traits that evolved in the woodwasp grade of Hymenoptera (e.g. Gibson 1985, Vilhelmsen 2001). In contrast, the presence of the median mesoscutal sulcus and notauli are plesiomorphic traits that are weakly developed or absent in most extant orussids, as well as †Kryptovelona, but present in †Baltorussus (Vilhelmsen and Zimmermann 2014: fig. 7) and at least some Cretaceous fossils, e.g. †Burmorussus (Zhang et al. 2020: fig. 1A) and †Cretorussus (Jouault et al. 2021: fig. 3C); both the sulcus and the notauli appear to have been lost at least twice in Orussidae. All crown-group Orussidae have the mesoscutellar arms arising from the anterolateral corners of the mesoscutellum (Figs 4C, 6B), a potential autapomorphy for the family (Vilhelmsen 2003); in †Cretorussus, the mesoscutellar arms arise from the posterolateral corners of the mesoscutellum, the plesiomorphic condition for Hymenoptera. Zhang et al. (2020) stated that the forewing fixation apparatus consisting of the cenchri on the metanotum and the associated hairy area in the anal part of the forewing is absent from †Burmorussus, a statement repeated by Jouault et al. (2021) in their diagnosis of †Burmorussidae. However, we observe at least the left cenchrus to be present in †Cretorussus.
Wing venation
Crown-group Orussidae have fairly consistent wing venation, at least with regard to the veins that are present. Furthermore, the venation can easily be derived from that displayed by the Cretaceous stem-group orussoids (e.g. †Burmorussus and †Cretorussus) by the supposed loss of a few veins: 1r-rs, 2-Rs, 3-Rs, and 2m-cu in the forewing, and vein C in the hindwing [compare fig. 1B in Zhang et al. (2020) and fig. 3D in Jouault et al. (2021) with, e.g. figs 63–74 in Vilhelmsen (2003)].
Ovipositor apparatus
Given the absence of the posterior part of the abdomen in †Cretorussus and the incompletely preserved ovipositor apparatus in †Kryptovelona, the evolution of the uniquely internalized ovipositor in Orussidae (Vilhelmsen et al. 2001, Vilhelmsen 2020) is still shrouded in mystery. However, from what can be observed in †Kryptovelona it is clear that it has the same ovipositor mechanism as observed in modern taxa. The low, median apodemes (possibly somewhat distorted) for gripping the ovipositor when it is extended or retracted can be observed in the fossil (Fig. 5D); furthermore, the configuration of the profurca with a distinct median groove extending across the profurcal bridge (Fig. 5B, C) for accommodating the anterior part of the ovipositor loop indicates that the loop extended as far anterior in †Kryptovelona as in extant taxa and also that it was probably of the simple, plesiomorphic hairpin type (Vilhelmsen 2020: fig. 10). The hairpin type is directly observed in †Orussus juttagroehnae (Fig. 9D), in combination with the straight profurcal bridge with a dorsal groove accommodating the ovipositor (Fig. 9C); this is in contrast with the conditions observed in extant Orussus spp., which have the ovipositor coiled in the prothorax and the profurcal bridge bent posteriorly for accommodating it (Vilhelmsen 2020: figs 2a, 4b). Given the variation within Orussus and that only few extant members of the genus have been examined, it is likely that the ‘safety pin’ coiled loop arose independently within Orussus, as well as in Pedicrista. As for †Cretorussus/†Burmorussidae, clarification of the configuration of their ovipositor apparatus awaits the discovery of a complete fossil.
Intriguingly, Zhang et al. (2020: fig. 3) retrieved the terminals they placed in †Paroryssidae (†Praeoryssus, †Paroryssus, and †Microryssus) as more closely related to crown orussids than †Burmorussus/†Burmorussidae. Members of †Paroryssidae had an external ovipositor, indicating that perhaps the modern ovipositor mechanism evolved closer to the common ancestor of the extant taxa and possibly only long after the host detection mechanism. The latter would still be useful in conjunction with a long external ovipositor [compare with the putative host-detection mechanisms and ovipositor lengths in, e.g. Stephanidae (Vilhelmsen et al. 2008) and some Ichneumonidae (Broad and Quicke 2000]. Indeed, the development of the host-detection mechanism and the lengthening of the ovipositor might have reinforced each other in the early evolution of Orussoidea, more refined vibrational sounding allowing targeting hosts deeper inside wood and in turn driving the extension of ovipositor length to a point where special adaptations, i.e. internalization of the ovipositor and the associated structures (e.g. median groove on profurca, median apodemes) became essential for the female wasp to be able to perform efficient drilling in wood.
Conclusion
The traits displayed in the antennae and forelegs of †Cretorussus are all correlated with the putative vibrational detection system in Orussidae (Fig. 11), indicating that this mechanism for host detection evolved at least 100 Mya ago. By the Late Eocene (approx. 35 Mya ago) the more refined mechanism employed by extant orussids was already in place. Interestingly, both in the case of the antennae and the fore tarsus, it seems that the distal part, i.e. the contact point with the substrate (flattened tip of antennomere and spur on third fore tarsomere), was modified first as observed in †Cretorussus. This was followed by fusion of sclerites proximally to the contact point (the putatively composite antennomere 9 and fore basitarsus) in the crown group, as seen in, for example, †Kryptovelona. The ovipositor apparatus of the Eocene amber fossils is also essentially ‘modern’ in having the ovipositor fully internalized and operated by a similar mechanism as that observed in extant taxa. To elucidate the early evolution of this unique mechanism awaits the discovery of a well-preserved, complete fossil of a female orussoid from a Cretaceous locality. Toward this end, µCT scanning will be a valuable approach.
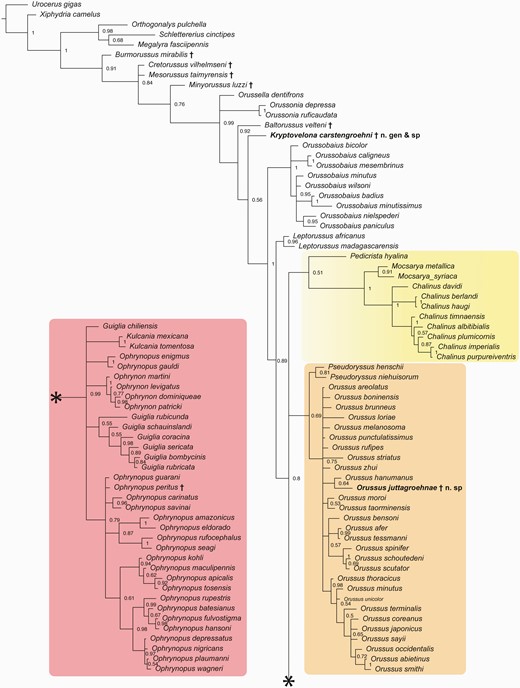
Fifty per cent majority rule consensus tree from the Bayesian inference analysis of the morphological matrix, showing relationships among extant and extinct Orussoidea. Fossil taxa indicated with †. Ophrynopine clade in pink box on the lower left connects to remainder of tree at *. Yellow box = Pedicrista—Mocsarya—Chalinus clade; orange box = Pseudoryssus—Orussus clade.
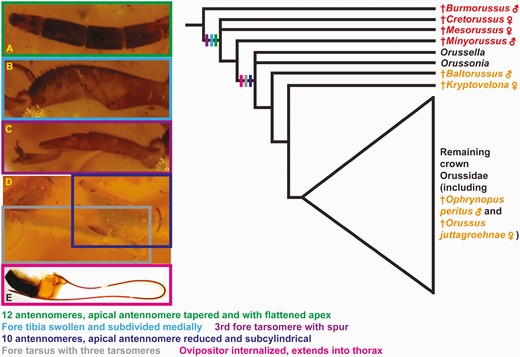
Summary of character evolution in female Orussoidea with regard to traits related to host detection and ovipositing. Tree based on Figure 10. Cretaceous stem-grade fossils indicated with red, Cenozoic crown-group fossils with orange. Characters mapped to the deepest node they can be traced to with certainty. A–C, †Cretorussus vilhelmseni; D, †Kryptovelona carstengroehni; E, Pseudoryssus henschii (Mocsáry 1910).
http://zoobank.org/urn:lsid:zoobank.org:pub:B2779F3E-BA14-4C8D-BBBE-125E69DDCD55
Acknowledgements
We are grateful to Carsten Gröhn who kindly facilitated access to the type specimens of the two newly described species for study and arranged for their deposition in the Museum of Nature—Geology of Hamburg. Vincent Perrichot also heartily thanks Carsten and Jutta Gröhn for the warm welcome and hosting during his visit in March 2023. We thank Alexey Solodovnikov for allowing us to use his imaging equipment. We thank Bernhard Bock and the Phyletisches Museum Entomology Group at the FSU Jena for facilitating scanning of the specimens at DESY. During this work, Brendon Boudinot was supported by an Alexander von Humboldt Research Fellowship (2020–22) and a Smithsonian Institution Peter S. Buck Fellowship (2023). Josh Jenkins Shaw is supported by the Villum Experiment grant ‘Baltic amber enigma’, managed by A. Solodovnikov.
Conflict of interest
None declared.
Data availability
The data matrix used to create the phylogenetic tree is available from KryptovelonaMatrix2024 (figshare.com)



