-
PDF
- Split View
-
Views
-
Cite
Cite
Matthew Hartog, Qing-Yu Zhang, Xinxin Ding, Role of Mouse Cytochrome P450 Enzymes of the Cyp2abfgs Subfamilies in the Induction of Lung Inflammation by Cigarette Smoke Exposure, Toxicological Sciences, Volume 172, Issue 1, November 2019, Pages 123–131, https://doi.org/10.1093/toxsci/kfz171
Close - Share Icon Share
Abstract
Many constituents of tobacco smoke (TS) require bioactivation to exert toxic effects; however, few studies have examined the role of bioactivation enzymes in the adverse effects of TS exposure. This knowledge gap is a major source of uncertainty for risk assessment and chemoprevention efforts. Our aim is to test the hypothesis that cytochrome P450 (P450) enzyme-mediated bioactivation is essential to the development of TS exposure-induced lung toxicity, by determining the contributions of P450 enzymes in the mouse Cyp2abfgs gene subfamilies to environmental tobacco smoke (ETS)-induced lung inflammation. Adult female wildtype (WT) and Cyp2abfgs-null mice (both on C57BL/6J background) were exposed to filtered air or ETS, intermittently, for 1 or 2 weeks. Lung inflammation was assessed by quantification of inflammatory cells, cytokines, chemokines, and proteins in bronchoalveolar lavage fluid (BALF) and histopathological analysis. Glutathione (GSH) conjugates of 2 ETS constituents, naphthalene (NA), and 3-methylindole (3MI), were measured in mice exposed to ETS for 4 h. Persistent macrophagic and neutrophilic lung inflammation was observed in ETS-exposed WT mice; the extent of which was significantly reduced in ETS-exposed Cyp2abfgs-null mice. Levels of proinflammatory cytokines and chemokines, along with the total protein concentration, were increased in cell-free BALF from ETS-exposed WT mice, but not Cyp2abfgs-null mice. Additionally, GSH conjugates of NA and 3MI were detected in the lungs of WT, but not Cyp2abfgs-null, mice following ETS exposure. These results provide the first in vivo evidence that the mouse Cyp2abfgs gene cluster plays an important role in ETS-induced lung inflammation.
Tobacco smoke (TS) is a complex mixture of over 4000 unique chemicals, including 69 known carcinogens, heavy metals, polycyclic aromatic hydrocarbons, heterocyclic aromatic amines, N-nitrosamines, and short chain alkanes/alkenes (IARC, 2004). Tobacco smoking is associated with the occurrence of multiple organ toxicities and pathologies, such as cardiovascular disease (Ambrose and Barua, 2004), bladder cancer (Freedman et al., 2011), and oropharyngeal cancers (La Vecchia et al., 1997), with the hallmark diseases being chronic obstructive pulmonary disease (COPD) and lung cancer (U.S. Department of Health and Human Services, 2014). COPD is a multifaceted lung disease that includes emphysema and chronic bronchitis. Additionally, patients with COPD have periods of exacerbations during which the pulmonary inflammation and associated symptoms temporarily become more severe. Although the specific etiologies of COPD and lung cancer following TS inhalation remain unknown, multiple toxic pathways, involving the generation of oxidative stress and inflammation, generation of reactive metabolites from TS constituents, and occurrence of DNA damage through the formation of DNA adducts, have been implicated (Adcock et al., 2011; Dela Cruz et al., 2011; Phillips and Venitt, 2012).
Some constituents of TS require bioactivation in order to exert their toxic effects, whereas other constituents do not. The xenobiotic-metabolizing cytochrome P450 (P450) enzymes have previously been shown to be key mediators of the in vitro or in vivo bioactivation process for multiple compounds found in TS, such as the polycyclic aromatic hydrocarbons benzo[a]pyrene (Singh et al., 2006) and naphthalene (NA) (Li et al., 2011) and the carcinogenic N-nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (Li et al., 2014; Zhou et al., 2012b) and N-nitrosodiethylamine (Verna et al., 1996). However, few studies have examined the role of P450 enzymes, or of bioactivation in general, in the various toxic effects induced by exposure to TS, an environmental mixture in which most of the toxic constituents studied to date are present at much lower levels than those utilized to demonstrate the toxicity of individual compounds, and many of the constituents may act as inhibitors of the bioactivation process for other toxicant ingredients. This knowledge gap is a major source of uncertainty for risk assessment, biomonitoring, and chemoprevention efforts.
In this study, we have utilized a knockout mouse model, named Cyp2abfgs-null (Li et al., 2014), to address the specific contributions of a subset of the mouse P450 enzymes to environmental tobacco smoke (ETS) induced lung inflammation. The Cyp2abfgs-null mouse lacks all P450 enzymes encoded by the Cyp2a, 2b, 2f, 2g, and 2s subfamilies, which are known to bioactivate many environmental chemicals that are also found in TS, such as 3-methylindole (3MI) (D'Agostino et al., 2009; Yan et al., 2007; Zhou et al., 2012a) and NA (Hu et al., 2014; Li et al., 2011, 2017). Wildtype (WT) and Cyp2abfgs-null mice were exposed to either HEPA-filtered air (FA) or a mixture of mainstream and sidestream TS (as a surrogate for ETS), intermittently, for 1 or 2 weeks using “nose only” inhalation. The severity of lung inflammation was assessed by quantification of inflammatory cells, cytokines, and chemokines present in bronchoalveolar lavage fluid (BALF). The presence of lung inflammation was confirmed histologically. Airway injury was also assessed by measuring the protein concentration of cell-free BALF. Finally, reactive metabolites (detected as glutathione [GSH] conjugates) of NA and 3MI, 2 known toxic substrates of the CYP2 enzymes, and ETS constituents, were measured using liquid chromatography-mass spectrometry (LC-MS). Our findings provide the first in vivo evidence for a major role of P450 enzymes in ETS-induced lung inflammation.
MATERIALS AND METHODS
Chemicals and reagents
3R4F research cigarettes were purchased from the University of Kentucky’s Center for Tobacco Reference Products (Lexington, Kentucky). Acetaminophen-glutathione conjugate standard was purchased from Toronto Research Chemicals (Ontario, Canada). 3-methylindole-glutathione (3MI-GSH) conjugate standards were generously provided by Dr Christopher Reilly of the University of Utah. Naphthalene-glutathione (NA-GSH) conjugate standards were generously provided by Drs Alan Buckpitt and Dexter Morin of the University of California at Davis. Water used for liquid chromatography was generated with a Milli-Q Integral 5 Q-Pod system equipped with an LC-Pack water polisher (EMD Millipore, Billerica, Massachusetts). The remaining ultrahigh performance liquid chromatography solvents used were of LC-MS grade and purchased from Fisher Scientific (Waltham, Massachusetts). Unless otherwise noted, all other chemicals and reagents were purchased from Sigma-Aldrich (St Louis, Missouri).
Animals
Homozygous Cyp2abfgs-null [Cyp2abfgs(−/−)] (Li et al., 2014) and WT mice, both on the C57BL/6J background, were obtained from breeding stock maintained at the Wadsworth Center. The animals were housed in a climate controlled environment with a 12 h light:dark cycle and ad libitum access to standard rodent chow and water. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. The animals were treated humanely and with due consideration to the alleviation of distress and discomfort.
ETS generation and nose-only inhalation exposure
ETS, a mixture of 89% sidestream and 11% mainstream smoke, was generated using filtered 3R4F research cigarettes. Cigarettes were stored in their original packaging and refrigerated. Cigarettes were conditioned to room temperature and 55 ± 5% humidity 1 day prior to use. Single cigarettes were smoked according to the Federal Trade Commission method (2 s, 35 ml cigarette “puff” every minute) using a Teague TE-10z cigarette smoking machine (Teague Enterprises, Woodland, California). Adult (8–12-week old) female WT and Cyp2abfgs-null mice were exposed to either FA or ETS via nose-only inhalation using an Oro-Nasal Aerosol Respiratory Exposure System (ONARES) from CH Technologies (Westwood, New Jersey). Exposures of WT and Cyp2abfgs-null mice were done simultaneously. This system utilizes a 24 port Jaeger rodent inhalation exposure chamber with an air flow rate of 0.3 l/min, measured at the nose port. Animals were acclimated to the exposure conditions for 1 week prior to exposure. The exposure protocol used to induce the development of lung inflammation was based on a previously developed regimen described in Takahashi et al. (2010). Mice were exposed to either FA or ETS for either 1 or 2 weeks. Exposures lasted 40 min/day for a total of 5 days (1 week) or 10 days (2 weeks). Animals in the 2 week group were exposed for 5 days, given a 2 day break, and then exposed for an additional 5 days. Animal body weight was recorded daily. The daily ETS exposure was performed intermittently using eight 5 min segments alternating between ETS and FA. The daily FA exposure group received FA continuously for 40 min. The concentration of ETS was determined by measuring suspended total particulate matter (TPM) at the nose port of the inhalation exposure chamber. TPM was measured in real time using a MicroDust Pro aerosol monitor (Casella, Buffalo, New York). The average TPM concentration for the 1-week FA and ETS groups were 1.21 ± 0.17 and 168.8 ± 3.8 mg/m3, respectively. The average TPM concentration for the 2-week FA and ETS groups were 1.08 ± 0.01 and 167.6 ± 2.4 mg/m3, respectively. The average level of carbon monoxide (CO) during the daily ETS exposure was 55.4 ppm (maximum concentration: 114.2 ppm) and was 0.8 ppm, on average, during the daily FA exposure. CO was measured in real time at the nose port using an IQ-604 Total Volatile Organic Compound Monitor containing a photoionization detector and electrochemical gas sensor (Graywolf Sensing Solutions, Shelton, Connecticut).
Animals were euthanized using carbon dioxide 24 h after completion of the last FA or ETS exposure. Bronchoalveolar lavage and lung tissue fixation were performed on separate sets of animals. To fix lung tissue, the trachea was exposed, cannulated, and tied with silk thread. The fixative, 10% neutral buffered formalin, was infused into the lung at a constant fluid pressure of 25 cm. After 10 min, the fixed lung tissue was removed and stored in 10% neutral buffered formalin. Paraffin-embedded lung tissue sections were cut and hematoxylin and eosin (H&E) stained by the Histopathology Core at the Wadsworth Center.
A modified ETS exposure protocol, with a higher ETS dose and shorter postexposure time, was used to enable the detection of GSH conjugates. Adult female WT and Cyp2abfgs-null mice were exposed to either FA or ETS for two 2 h sessions, with a 30 min break in between (included to reduce stress on the animals). Animals were euthanized immediately following completion of the exposure. Lung and liver were collected and stored at −80°C until processing and analysis. Blood was collected via cardiac puncture and used to prepare serum. Serum was stored at −80°C until processing and analysis. Serum levels of nicotine and cotinine were measured to verify similarity of exposure to ETS among the animals. The average ETS concentration for this exposure was 200 mg TPM/m3 and the average CO concentration was 139.3 ppm (maximum concentration: 195.8 ppm). The average CO concentration during the FA exposure was 0.8 ppm.
Bronchoalveolar lavage and cell counting
Bronchoalveolar lavage was conducted as described in Takahashi et al. (2010) using calcium- and magnesium-free phosphate-buffered saline (PBS), pH 7.1, as the lavage fluid (BALF). Cells were isolated from BALF by centrifugation at 1600 rpm in an Eppendorf 5425R centrifuge for 5 min and then resuspended in 100 µl of PBS containing 4% heat-inactivated fetal bovine serum (Gibco, ThermoFisher, Waltham, Massachusetts). The cells were counted manually with a hemocytometer and dead cells were identified by uptake of Trypan Blue dye. Cytopreparations were made using a Shandon Cytospin II centrifuge (ThermoFisher) and stained with Modified Wright-Giemsa. Cell types were identified by morphology. A total of 400 cells were counted and the total numbers of macrophages, neutrophils, and lymphocytes present in the BALF were calculated. Cell-free BALF was stored at −80°C for later measurement of total protein concentration, cytokines, and chemokines. The total protein concentration of the cell-free BALF was determined by the Pierce bicinchoninic acid assay (ThermoScientific, Waltham, Massachusetts) using bovine serum albumin as the calibration standard.
Quantification of cytokines and chemokines
Proinflammatory cytokines and chemokines in cell-free BALF collected following 1 week of FA or ETS exposure were analyzed using a custom mouse multiplex (U-Plex) assay from Meso Scale Discovery (MSD, Rockville, Maryland). The following chemokines and cytokines were measured using an MSD QuickPlex SQ 120 instrument: granulocyte macrophage-colony stimulating factor (GM-CSF), interferon gamma (IFN-γ), interleukin-1beta (IL-1β), interleukin-6 (IL-6), interleukin-17A and 17F (IL-17A/F), interleukin-23 (IL-23), keratinocyte chemoattractant-growth regulated oncogene (KC-GRO), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1α), and tumor necrosis factor alpha (TNF-α). Sample preparation and analysis were carried out according to the manufacturer’s instructions using undiluted BALF.
Histopathology evaluation of lung tissue
H&E-stained, formalin-fixed paraffin-embedded lung tissue sections were used to confirm the presence of lung inflammation in ETS-exposed mice. The entire lung was sectioned and a random set of 20, 5-µm thick, tissue sections from each animal exposed to either FA or ETS were evaluated for the occurrence of lung inflammation and other pathologies using a Nikon Eclipse 80i microscope with a ×4 objective (Nikon Instruments, Melville, New York). Images were taken with a QICAM Fast 1394 camera using QCapture software version 2.9.123 (QImaging, Surrey, British Columbia, Canada) and viewed using ImageJ software version 1.51p22 (National Institutes of Health, Bethesda, Maryland). The total number of peribronchiolar and perivascular nodules of inflammation (NIF) in FA and ETS-exposed WT and Cyp2abfgs-null mice was determined. NIFs are defined as localized collections of inflammatory cell infiltrates that have developed a nodular organization within the lung tissue (Arlt et al., 2015; Stahl et al., 2013). To avoid double counting of NIFs in adjacent sections, those that appeared to overlap, based on their location within the section, were not counted. The observer was not blinded; but each observed NIF was imaged and recorded with its size determined using image analysis in ImageJ software.
Analytical chemistry methods
LC-MS methods for detection of bioactivated ETS constituents, nicotine, and cotinine, and gas chromatography-mass spectrometry methods for quantification of NA and 3-MI, are included in the Supplementary Material.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 software. A two-way analysis of variance (ANOVA) with Tukey’s post hoc test for multiple comparisons was used to identify statistically significant differences (p ≤ .05) between exposure groups (FA vs ETS) and mouse strain (WT vs Cyp2abfgs-null). Fisher’s exact test was used to assess the statistical significance of differences in the number of peribronchiolar or perivascular NIFs observed in WT and Cyp2abfgs-null mice.
RESULTS
ETS-Induced Lung Inflammation
The development of lung inflammation following ETS inhalation was assessed in WT and Cyp2abfgs-null mice (n = 3) using a previously developed acute cigarette smoke inhalation model (Takahashi et al., 2010). There was no significant difference in animal body weights between exposure or genotypes groups (data not shown). WT mice exposed to ETS for 1 week were found to have significantly increased numbers of total cells present in the BALF as well as increased numbers of macrophages, neutrophils, and lymphocytes than their corresponding FA-exposed control group (Figs. 1A–D). Conversely, Cyp2abfgs-null mice did not develop marked lung inflammation following 1 week of ETS exposure. Cyp2abfgs-null mice were protected against increases in alveolar macrophages, neutrophils, and lymphocytes following ETS inhalation and had significantly fewer cells present in BALF than ETS-exposed WT mice had (Figs. 1A–D).
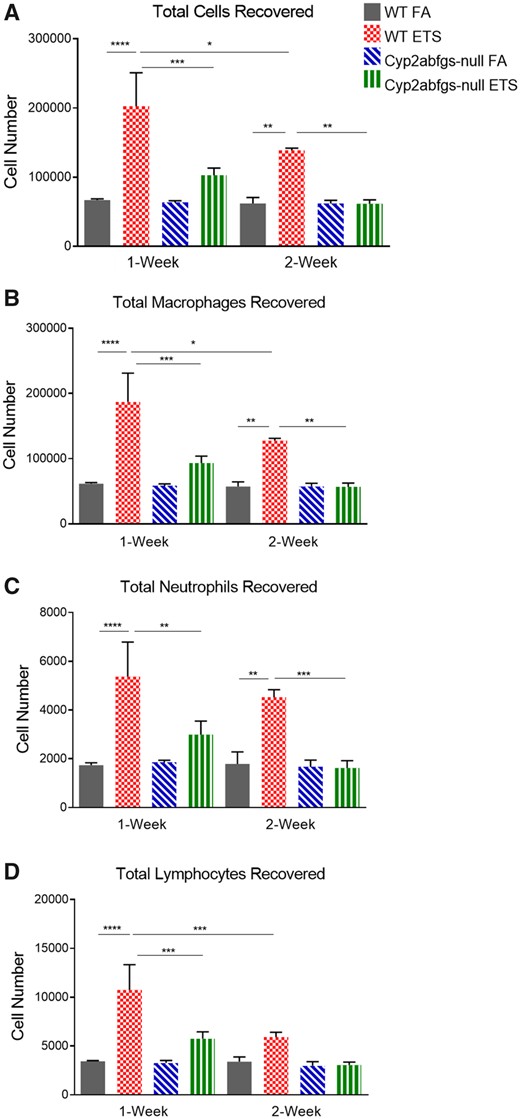
Abundance of inflammatory cells in bronchoalveolar lavage fluid (BALF) from wildtype (WT) and Cyp2abfgs-null mice after acute environmental tobacco smoke (ETS) inhalation. WT and Cyp2abfgs-null mice (adult female) were exposed daily (40 min per day) to filtered air (FA) or ETS for 1 or 2 weeks as described in Materials and Methods section. Bronchoalveolar lavage was performed 24 h after completion of the last exposure. A, Total cells were counted manually using a hemocytometer. Total numbers of macrophages (B), neutrophils (C), and lymphocytes (D) were determined using cytopreparations. Data represent means ± SD (n = 3). *p < .05, **p < .01, ***p < .001, ****p < .0001 (two-way ANOVA, followed by Tukey’s multiple comparisons test).
After 2 weeks of ETS exposure, the total number of cells and numbers of macrophages and neutrophils recovered in BALF from WT mice were still significantly higher compared to their corresponding FA control (Figs. 1A–C). However, the total number of cells and numbers of macrophages and lymphocytes recovered from WT mice were significantly lower in the 2-week ETS-exposed, compared to the 1-week ETS-exposed, group. There was no significant difference between the number of neutrophils recovered following 1 and 2 weeks of ETS exposure in WT mice. Notably, significant differences in the total number of cells and numbers of macrophages, neutrophils, or lymphocytes recovered in BALF of 2-week ETS-exposed, compared to FA-exposed, Cyp2abfgs-null mice were not observed (Figs. 1A–D). The 2-week ETS-exposed Cyp2abfgs-null mice had significantly lower numbers of total cells and numbers of macrophages and neutrophils recovered in BALF compared to the 2-week ETS-exposed WT mice (Figs. 1A–C).
Cytokines levels were determined in 1-week ETS-exposed mice (n = 3). The 1-week ETS-exposed WT mice were found to have significantly increased concentrations of the chemokines MIP-1α and KC-GRO (Figs. 2A and 2B) in cell-free BALF. Accompanying this finding, these mice also had a significantly increased concentration of the proinflammatory cytokine TNF-α (Figure 2C). The levels of MIP-1α, KC-GRO, and TNF-α in cell-free BALF were significantly lower in ETS-exposed Cyp2abfgs-null mice, compared to the ETS-exposed WT mice (Figs. 2A–C). Following ETS exposure, Cyp2abfgs-null mice had a significantly increased concentration of KC-GRO in cell-free BALF compared to their respective FA control group (Figure 2B). There was no significant difference among the various groups in the concentrations of the remaining cytokines or chemokines that were measured (GM-CSF, IFN-γ, IL-1β, IL-6, IL-17A/F, IL-23, and MCP-1) (data not shown).
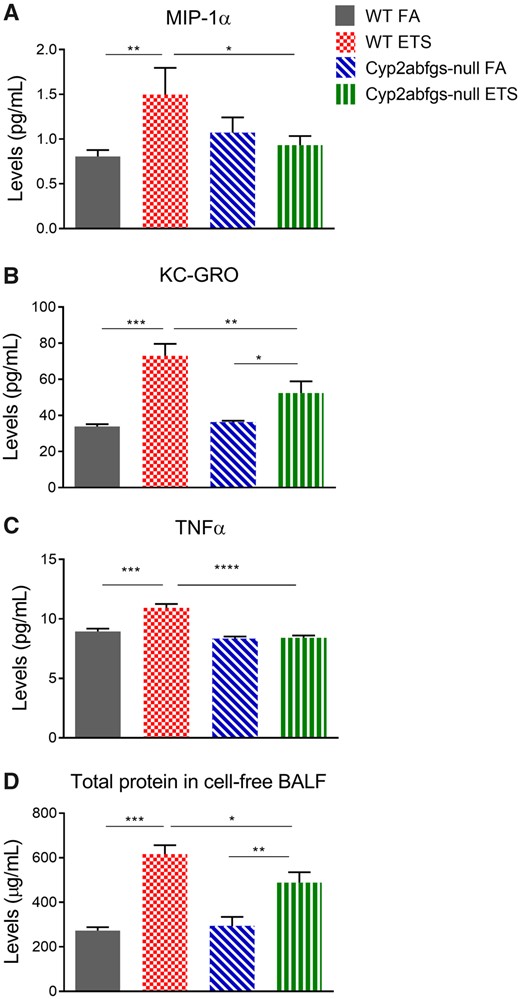
Levels of proinflammatory cytokines, chemokines, and total protein in cell-free bronchoalveolar lavage fluid (BALF) following acute ETS inhalation. Levels of macrophage inflammatory protein-1 alpha (MIP-1α) (A), keratinocyte chemoattractant-growth regulated oncogene (KC-GRO) (B), and tumor necrosis factor alpha (TNF-α) (C), as well as total protein (D), were measured in cell-free BALF collected from wildtype (WT) and Cyp2abfgs-null mice (adult female) that have been exposed daily to filtered air (FA) or ETS for 1 week as described in Materials and Methods section. Bronchoalveolar lavage was performed 24 h after completion of the last exposure. Cells were removed from BALF by centrifugation. Data represent means ± SD (n = 3). *p < .05, **p < .01, ***p < .001, ****p < .0001 (two-way ANOVA, followed by Tukey’s multiple comparisons test).
The total protein concentration of cell-free BALF was measured and used as a marker of alveolar and airway toxicity. ETS-exposed mice had significantly increased protein levels in cell-free BALF compared to their respective FA control group (Figure 2D). However, the protein concentration of BALF was significantly higher in ETS-exposed WT mice versus ETS-exposed Cyp2abfgs-null mice. There was no significant difference in BALF protein concentration between the FA-exposed WT and Cyp2abfgs-null mice (Figure 2D).
ETS-Induced Lung Pathology
Formalin-fixed, paraffin-embedded lung tissue sections were examined for the presence of pathological changes using light microscopy. ETS-exposed WT mice (n = 3) were found to have NIFs associated with both peribronchiolar and perivascular regions within the lung (Figure 3). NIFs observed in ETS-exposed Cyp2abfgs-null mice were reduced in size, compared to those in WT mice, and were only associated with perivascular regions (Figure 3). In ETS-exposed WT mice, a total of 6 peribronchiolar and 5 perivascular NIFs were observed per mouse, versus 0 peribronchiolar and 5 perivascular NIFs observed per mouse in the ETS-exposed Cyp2abfgs-null mice. The incidence of NIFs in ETS-exposed WT and Cyp2abfgs-null mice was 100% (3 of 3 mice) whereas the NIF incidence in the FA-exposed WT and Cyp2abfgs-null mice was 33% (1 of 3 mice). In the FA-exposed control groups, 2 perivascular NIFs were found in a WT mouse and 1 peribronchiolar NIF was found in a Cyp2abfgs-null mouse. No peribronchiolar NIFs were observed in the FA-exposed WT mice and no perivascular NIFs were observed in the FA-exposed Cyp2abfgs-null mice.
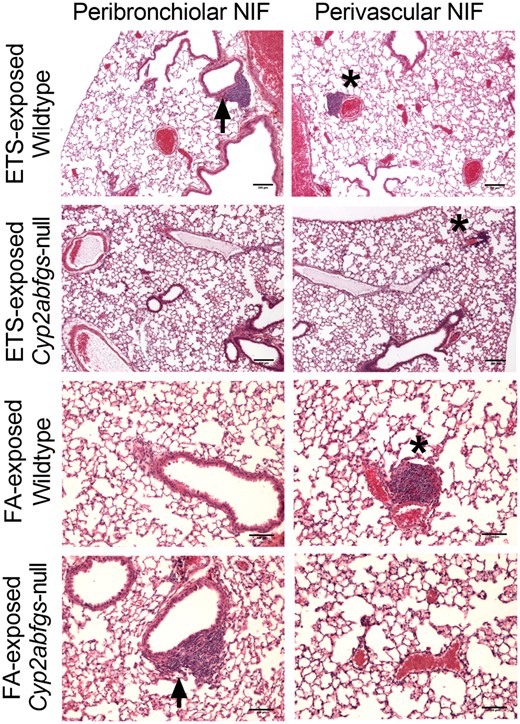
Histopathologic evaluation of lung tissue from wildtype (WT) and Cyp2abfgs-null mice following exposure to filtered air (FA) or environmental tobacco smoke (ETS) via inhalation. Paraffin-embedded, formalin-fixed lung tissue sections from WT and Cyp2abfgs-null mice (adult female, n = 3) were H&E stained and evaluated for the occurrence of pathological changes following 1 week of daily FA or ETS inhalation exposures as described in Materials and Methods section. Representative images of nodules of inflammations (NIFs) from ETS-exposed WT and Cyp2abfgs-null mice are shown at ×4 magnification. Scale bar is 200 µm. NIFs were observed in both peribronchiolar (arrow) and perivascular (*) regions of ETS-exposed WT mice. However, NIFs were observed only in perivascular, but not in peribronchiolar, regions of ETS-exposed Cyp2abfgs-null mice. Images of NIFs observed in FA-exposed WT and Cyp2abfgs-null mice are shown at ×10 magnification with a 100 µm scale bar.
Formation of GSH Conjugates With 2 Toxic ETS Constituents, 3MI, and NA
Successful exposure to ETS was confirmed by the measurement of nicotine and cotinine in serum of WT and Cyp2abfgs-null mice postexposure. The serum concentrations of nicotine and cotinine were 0.18 ± 0.03 and 3.68 ± 0.43 ng/ml, respectively, in WT mice and 0.78 ± 0.10 and 3.77 ± 0.16 ng/ml, respectively, in the Cyp2abfgs-null mice. Serum levels of NA and 3MI were below detection limits, 80 and 460 pg/ml, respectively, in ETS-exposed mouse.
GSH conjugates of NA (NA-GSH) and 3MI (corresponding to 2 previously described conjugates, 3MI-GS-A1 and 3MI-GS-A2; Zhou et al., 2012a) were detected in the lungs of WT mice following the 4 h ETS inhalation (n = 3). Representative chromatograms and mass spectra of NA-GSH and 3MI-GS-A1/A2 are shown in Figures 4 and 5, respectively. NA-GSH was detected at an average concentration of 17.2 ± 2.2 pg/g lung tissue and 3MI-GS-A1/A2 was detected at an average concentration of 194 ± 27 pg/g lung tissue. The concentration of NA-GSH and 3MI-GS-A1/A2 in the lungs of Cyp2abfgs-null mice was below the limits of detection of 0.6 and 6.7 pg/g tissue, respectively. The 3MI-GSH conjugate corresponding to 3MI-GS-A3 in D'Agostino et al. (2009) was not detected in ETS-exposed WT or Cyp2abfgs-null mice. The limit of detection for 3MI-GS-A3 was 26 pg/g tissue.
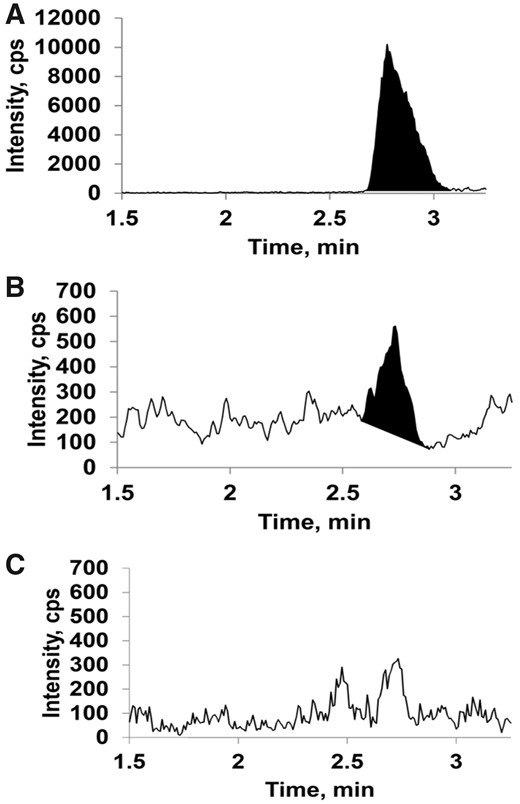
Liquid chromatography-mass spectrometry (LC-MS) detection of NA-GSH conjugates in environmental tobacco smoke (ETS)-exposed mouse lung tissue. Wildtype (WT) and Cyp2abfgs-null mice (adult female) were exposed to either filtered air (FA) or ETS for 4 h, and their lungs were processed for LC-MS analysis of naphthalene-glutathione (NA-GSH), as described in Materials and Methods section. A, A representative chromatogram of authentic NA-GSH standard. B and C, Representative chromatograms from ETS-exposed WT mice (panel B) and Cyp2abfgs-null mice (panel C). Corresponding mass spectra for relevant peaks in (A), (B), and (C) (at approximately 2.7 min) and the structure and predicted fragmentation of the NA-GSH standard are shown in Supplementary Figure 1. NA-GSH was detected in the lungs of WT mice but not Cyp2abfgs-null mice exposed to ETS, or in FA-exposed mice (Supplementary Figure 1).
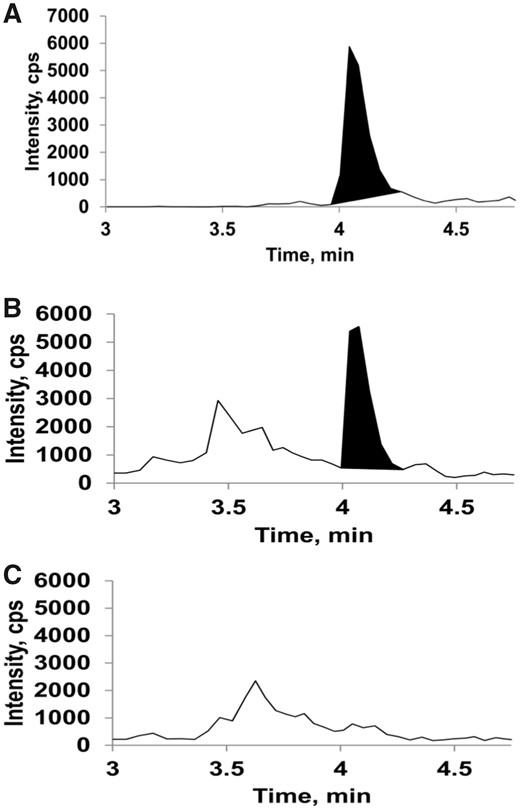
Liquid chromatography-mass spectrometry (LC-MS) detection of 3-methylindole-glutathione (3MI-GSH) conjugates in environmental tobacco smoke (ETS)-exposed mouse lung tissue. Lung tissues as described in the legend of Figure 4 were processed for LC-MS analysis of 3MI-GSH conjugates (3MI-GS-A1/A2 and 3MI-GS-A3) as described in Materials and Methods section. A, A representative chromatogram of a 3MI-GS-A1/A2 standard. B and C, Representative chromatograms from ETS-exposed wildtype (WT) mice (panel B) and Cyp2abfgs-null mice (panel C). Corresponding mass spectra for relevant peaks in (A), (B), and (C) (at approximately 4.1 min) and the structures and predicted fragmentation of 3MI-GS-A1 and 3MI-GS-A2 standards are shown in Supplementary Figure 2. 3MI-GS-A1/A2 was detected in the lungs of WT mice but not Cyp2abfgs-null mice exposed to ETS, or in filtered air (FA)-exposed mice (Supplementary Figure 2). 3MI-GS-A3 was not detected in any sample (data not shown).
DISCUSSION
P450 enzymes have previously been shown to bioactivate numerous TS constituents, but many of the TS constituents, such as aldehydes, do not require bioactivation to cause cytotoxicity. We hypothesized that the P450-mediated toxicating process and the bioactivated ETS constituents are key contributors to the development of lung inflammation. We observed that female WT mice developed significantly more pronounced lung inflammation following acute ETS inhalation than mice lacking CYP 2A, 2B, 2F, 2G, and 2S enzymatic activity. The significant increases in numbers of inflammatory cells recovered in the BALF of ETS-exposed WT mice, compared to ETS-exposed Cyp2abfgs-null mice, indicate that the bioactivation of ETS constituents by these CYP subfamilies played a critical role in the development of ETS-induced lung inflammation. Reactive metabolites generated by these enzymes from ETS ingredients may ultimately bind to cellular structures and biomacromolecules, such as proteins and DNA, thereby causing cell damage and resulting in the recruitment of inflammatory cells to the site of injury. This notion is further supported by evidence showing that ETS-exposed WT mice also had significantly increased levels of the proinflammatory cytokine TNF-α, and of the chemokines MIP-1α and KC-GRO, in cell-free BALF, compared to ETS-exposed Cyp2abfgs-null mice. Female mice were used in this study as published literature suggests that female smokers may be more prone than male smokers to developing lung cancer (Risch et al., 1993). However, it remains to be determined whether male and female mice differ in their sensitivities to ETS-induced lung inflammation.
The human equivalent dose of TS relative to the ETS concentration that was used in this study to generate pulmonary inflammation can be estimated using a published dose translation model (Phillips, 2017). In the mouse lung the maximal delivered dose of TPM, from inhaled ETS, was calculated to be 0.1 mg of TPM per day, based on (1) the daily exposure to 0.006 m3 of air containing 168.8 mg/m3 TPM (approximately 1 mg TPM per day) at the nose port, (2) an assumption that 100% of the airflow through the nose port enters the mouse airway, and (3) using a pulmonary deposition fraction of 10% for rodents. Using allometric scaling and assumed weights of 0.03 kg for a mouse and 65 kg for a human, we can estimate that the ETS dose of 0.1 mg TPM per day in mice is equivalent to a human dose of 17.7 mg of TPM per day. A 3R4F cigarette generates a total of 11 mg particulate matter (Roemer et al., 2012) and, if assuming a 40% pulmonary deposition fraction for humans, this amounts to smoking approximately 4 cigarettes per day. The ETS dose used to assess bioactivation of specific ETS constituents (NA and 3MI) was higher, at 1.44 mg of TPM per mouse, which is equivalent to 247 mg of TPM in humans. Using the conditions described above, this would amount to smoking approximately 56 cigarettes (2.8 packs) in humans.
These calculations of maximal delivered dose most likely overestimate the actual lung deposition of TPM and internalization of ETS constituents within the mouse lung following ETS inhalation, as they are based on the airflow rate at the nose port and not inhalation dynamics. Unexposed adult, female C57BL/6J mice have a respiratory minute volume (MV) of approximately 40 ml (Lim et al., 2014; Tsuji et al., 2011), which can decrease to approximately 13 ml following TS inhalation (Tsuji et al., 2011). In the exposure model used to generate pulmonary inflammation, an MV of 40 ml with a 10% pulmonary deposition fraction would result in 0.013 mg of TPM within the mouse lung, whereas having an MV of 13 ml would decrease TPM deposition to 0.0044 mg. These levels are equivalent to 2.23 mg TPM (smoking 0.51 cigarettes) and 0.756 mg TPM (smoking 0.17 cigarettes) in humans, respectively. In the exposure model to assess bioactivation of ETS constituents, an MV of 40 ml with a 10% pulmonary deposition fraction would result in 0.19 mg of TPM within the mouse lung, whereas having an MV of 13 ml would decrease TPM deposition to 0.062 mg. These levels are equivalent to 32.64 mg TPM (smoking 7.42 cigarettes) and 10.65 mg TPM (smoking 2.42 cigarettes) in humans, respectively. These dose considerations support the notion that the P450-mediated bioactivation of ETS constituents, at ETS concentrations relevant to the daily exposure of human smokers, contributes to the development and progression of lung inflammation following TS inhalation.
The specific occurrence of P450 genotype-related differences in the number of NIFs in the peribronchiolar region, but not in the perivascular region, suggests that the NIF formation was a result of local, rather than systemic, bioactivation of ETS constituents. In that regard, the bronchiolar and bronchial epithelia are known to have the highest expression of P450s within the mouse lung (Hukkanen et al., 2002), a feature that would make the peribronchiolar regions more prone than other lung regions to developing inflammation following ETS inhalation and bioactivation of specific ETS constituents. The similar development of perivascular NIFs between the 2 ETS-exposed groups may be a function of P450-independent vascular toxicity and related to the deposition and/or absorption of particulate matter or direct acting toxicants (eg, aldehydes and elemental metals) following TS inhalation. Additionally, although the occurrence of general alveolar toxicity was not evident when using histopathological evaluation techniques, the significant difference in protein concentration of cell-free BALF between ETS-exposed WT and Cyp2abfgs-null mice suggests that the Cyp2abfgs-null mice may be less susceptible to the occurrence of both alveolar and airway damage following ETS inhalation.
GSH conjugates of the respiratory toxicants NA and 3MI were detected in the lungs of ETS-exposed WT mice but not ETS-exposed Cyp2abfgs-null mice; this finding implies that NA and 3MI are among the ETS ingredients that contribute to the ETS-induced inflammatory process within the lung. Further studies are warranted to identify additional ETS ingredients that are bioactivated by the CYP enzymes in vivo, to more directly demonstrate their involvement in the mechanisms of toxicity, and to determine which ETS constituents are most relevant to the development of ETS-induced tissue pathologies in humans.
The ETS-induced lung inflammation observed in this study is relevant to pathogenesis of lung diseases in humans, as previous work has demonstrated increased numbers of inflammatory cells, cytokines, and chemokines in BALF samples collected from human COPD/emphysema patients, as well as in mouse models of these diseases (Barnes, 2016; O'Donnell et al., 2006; Takahashi et al., 2010; Tetley, 2002). The disease relevance is further supported by the persistence of the increases in the number of macrophages and neutrophils recovered in BALF, which are inflammatory effector cells likely involved in the development of chronic pathologies associated with TS-induced lung disease (Grivennikov et al., 2010; Knaapen et al., 2006), and the levels of the chemotactic factor KC-GRO, which may play a key role in the progression of lung inflammation (Traves et al., 2002). Our finding of the specific involvement of the P450 enzymes encoded by the mouse Cyp2abfgs gene cluster in ETS-induced lung inflammation is also relevant to humans as members of the same gene cluster in humans, including CYP2A6, CYP2A13, CYP2B6, CYP2F1, and CYP2S1, are also expressed in human lung and are active in the bioactivation of numerous toxicants contained in ETS (see Ding et al., 2018, for a recent review). Furthermore, the abilities of human CYP2A13 and CYP2F1 to mediate acute lung toxicity of inhaled NA (Li et al., 2017) and lung tumorigenesis induced by the tobacco-specific procarcinogen NNK (Megaraj et al., 2014) have been demonstrated using CYP2A13/2F1-humanized mice. Thus, our findings provide the basis for further studies that directly examine the ability of various human lung P450 enzymes to mediate ETS-induced lung inflammation.
The residual signs of lung inflammation observed in ETS-exposed Cyp2abfgs-null mice may reflect contributions by other enzymes, including non-Cyp2abfgs P450 enzymes and non-Cyp enzymes, as well as direct effects of nonbioactivated ETS constituents, including highly reactive aldehydes (Freeman et al., 2005; Seeman et al., 2002) and suspended particulate matter (Ghio et al., 2008). It was previously shown that there are no compensatory changes in the protein levels of CYP1A1/2, CYP2C, CYP3A, CYP2E1, or microsomal cytochrome P450-reductase (CPR) within the liver, lung, and olfactory mucosa of Cyp2abfgs-null mice. (Li et al., 2014). Induction of specific enzymes following ETS exposure was not assessed in WT or Cyp2abfgs-null mice; however, the observed protection of Cyp2abfgs-null mice against the development of ETS-induced lung inflammation suggests that induction of additional enzymes capable of bioactivating ETS constituents did not contribute significantly to the onset of pulmonary inflammation in this model of ETS-induced lung toxicity. Additionally, lung inflammatory effects from direct-acting xenobiotics appear to be minor, if any, compared to those mediated by Cyp2abfgs enzymes in the ETS exposure model utilized (featuring intermittent ETS exposure of relatively short duration [a total of 20 min of ETS exposure per day vs the 6 h per day exposure in Ghio et al., 2008]), which mimics the exposure pattern in human smokers. Further studies to examine contributions of P450-mediated bioactivation to ETS-induced chronic disease endpoints, such as COPD, which is a significant and independent risk factor for lung cancer (Durham and Adcock, 2015), are necessary.
CONCLUSIONS
The results of this study provide the first in vivo evidence that the mouse Cyp2abfgs gene cluster plays an important role in ETS-induced lung inflammation.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENTS
We gratefully acknowledge the use of the Pathology Core facilities of the Wadsworth Center at the New York State Department of Health. We thank Drs A. Buckpitt and D. Morin of the University of California at Davis for providing NA-GSH standards and Dr C. Reilly of the University of Utah for providing the 3MI-GSH standards. We would also like to thank Dr S. Sell of the Wadsworth Center for use of the Shandon Cytospin II, Drs J.A. Melendez and T. Begley for use of the MSD QuickPlex SQ 120, and Dr N. Cady for use of the Nikon 80i Eclipse microscope.
FUNDING
This work was supported in part by National Institutes of Health grants (CA092596, CA023074, ES006694, and ES020867). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
U.S. Department of Health and Human Services. (




Comments