-
PDF
- Split View
-
Views
-
Cite
Cite
Shawna L Ehlers, Kimberly Davis, Shirley M Bluethmann, Lisa M Quintiliani, Jeffrey Kendall, Raj M Ratwani, Michael A Diefenbach, Kristi D Graves, Screening for psychosocial distress among patients with cancer: implications for clinical practice, healthcare policy, and dissemination to enhance cancer survivorship, Translational Behavioral Medicine, Volume 9, Issue 2, April 2019, Pages 282–291, https://doi.org/10.1093/tbm/iby123
Close - Share Icon Share
Abstract
Accreditation standards are at the forefront of evolving healthcare systems, setting metrics for high-quality care. Healthcare outcomes (health, experience, cost, provider satisfaction/burn out) are becoming mutual goals of the patient, provider, payer, and healthcare system. Achieving high-quality outcomes in cancer care necessitates collaboration among interdisciplinary teams of clinical providers, administrators, patient advocates, caregivers, and researchers. Dissemination and implementation science provides necessary frameworks to organize the efforts of these implementation teams, inclusive of identifying facilitators and barriers to implementation of accreditation standards. Since 2015, cancer distress screening has been mandated for continued cancer center accreditation by the American College of Surgeon’s Commission on Cancer. Cancer centers have thus become real world implementation laboratories. We present the current context of distress screening, highlighting prior research and key areas of future research. We consider multiple levels of cancer care delivery and the use of interdisciplinary teams to help cancer center teams adopt, implement, and maintain efficient distress screening programs. Finally, we present a case study to identify methods for successful implementation of distress screening at one cancer center and then describe efficiencies that can be introduced using elements from human factors engineering, e- and m-health screening platforms, and community partnerships.
Practice: Providers of mental healthcare should lead teams of allied health professionals and other interested stakeholders in designing, implementing, and evaluating distress screening programs in oncology care using evidence-based approaches with attention to workflow and human factors approaches.
Policy: Enforcement of the Commission on Cancer distress screening mandate needs to include measures of effectiveness in terms of referral rates, distress reduction, patient and caregiver satisfaction, provider burden, and healthcare utilization, which may serve to deepen stakeholder alliances and further improvements in healthcare delivery.
Research: Multidisciplinary, implementation research is needed to evaluate the downstream effects of cancer distress screening in terms of measure selection and relevance to all stakeholders, including the “quadruple aim” of health outcomes, patient experience, provider burden, and costs/healthcare utilization.
INTRODUCTION
Since 2015, cancer distress screening has been mandated for continued cancer center accreditation by the American College of Surgeons Commission on Cancer (CoC) [1,2]. Standard 3.2 on psychosocial distress screening in the CoC “Cancer Program Standards 2012: Ensuring Patient-Centered Care” states: “The cancer committee develops and implements a process to integrate and monitor on-site psychosocial distress screening and referral for the provision of psychosocial care.” [1].
The purpose of the present paper is to describe the policies and procedures related to implementation of distress screening in oncology. We present evidence using the framework of the Triple Aim, which focuses on improving patient experiences, population health outcomes, and cost efficiency [3,4]. We then provide a case example and identify research gaps and policy implications to improve distress screening implementation.
Distress screening in cancer care
Screening rationale and distress prevalence
For over 30 years, the field of psycho-oncology has recognized the impact of distress in cancer patients through advocacy of distress screening during all phases of the cancer trajectory [5,6]. Consistent evidence suggests prevalent distress among cancer patients and their families, and associated unmet psychosocial health needs [7–10]. Research also demonstrates the effectiveness of interventions to reduce distress, and the positive downstream impact on patients, families, cancer outcomes, and the medical system when distress is addressed [11,12].
The term “distress” defines unpleasant psychological, social, and/or spiritual experiences that interfere with the ability to effectively cope with a cancer diagnosis, its symptoms, and subsequent treatment side-effects [13]. Distress was selected as the screening terminology because it was a multidimensional construct believed to be more acceptable to oncology patients, better understood by the lay person, and less stigmatizing than psychiatric diagnostic categories such as depression or anxiety [14]. Differences in terminology and approaches to screening exist however, as the American Society of Clinical Oncology guideline utilizes measures of depression and anxiety symptoms in its description of distress screening implementation [15].
Regardless of terminology, distress has serious implications for cancer patients, as 25–50% of cancer patients indicate significant levels of distress [16–20] and distress is a potential risk factor for nonadherence to treatment and poorer quality of life [21–23]. Depressive symptoms have received the most study and have been linked to lower adherence to medical treatment recommendations [24,25] and cancer mortality in a meta-analysis [26]. Intervention studies suggest that these effects are reversible (see below Efficacy of Psychosocial Interventions to Reduce Distress).
Distress screening implementation
Implementation of distress screening can be viewed as a multilevel intervention, involving behavior and system change across various levels (e.g., organizational change to work flow, provider change to administer and respond to results, patient willingness to complete more “paperwork”). Thus multiple types of screening and referral approaches may be effective within a comprehensive healthcare system to identify and treat distress. Within the last decade, researchers have developed and validated a number of distress screening tools (e.g., Distress Thermometer [DT]; Hospital Anxiety and Depression Scale; Patient Health Questionnaire-9; see refs. [15,16,27] for further examples of distress screeners). Many of these brief instruments are effective at detecting clinically significant elevations of distress among diverse oncology populations [16,28,29]. Furthermore, distress screening appears feasible, even among large patient populations, and clinical staff report generally positive perceptions of the screening process [8,18,30,31].
Research evidence
Distress screeners
Use of widely-available and no cost single-item distress measures could facilitate adoption, implementation, and maintenance of screening within healthcare systems, such as addressing patient and provider time burdens and increasing instrument acceptability [19,32], while also minimizing financial cost to the healthcare system. The DT is a common single-item distress screener available in the public domain. It can be utilized with or without a 38-item problem list [13]. The DT has demonstrated strong associations with established measures of depressive and anxious symptoms [16,27,33], and is found to be acceptable and feasible in busy clinical settings [18,30,31] (see section on Measures below). However, multi-item screening measures, such as the Hospital Anxiety and Depression Scale, offer greater psychometric rigor and diagnostic accuracy in detecting mental health conditions [15,27]. The downstream effects of these longer measures are understudied in terms of patient, provider, and healthcare system benefits and burdens pertinent to dissemination and implementation.
Efficacy of referral processes
After implementation of screening, the next step is to identify and implement an appropriate referral process for patients who receive a positive screen. Randomized controlled trials (RCTs) that specifically evaluated the impact of distress screening interventions (vs. psychosocial treatment interventions described below) have demonstrated benefits of screening among lung and breast cancer patients with elevated levels of distress, depression, and anxiety [7,30]. Other work has demonstrated lack of long-term benefits following distress screening [34] (see Gaps section below). The work by Carlson and colleagues has identified implementation factors that contribute to improved outcomes following distress screening, specifically how the screening information is used (e.g., uptake of referral vs. distress screening alone) [35]. Routine screening among lung cancer patients plus individualized referral yielded the most benefit for reducing physical and psychosocial problems [35]. As the field of distress screening interventions has grown, current efforts involve systematic reviews of RCTs specific to the impact of distress screening on improved patient-reported and health outcomes [36]. Initial reviews identified no consistent impact of distress screening alone on improved outcomes, leading some to oppose widespread distress screening implementation [37,38]. However, recent evaluations have demonstrated reductions in healthcare utilization when institutions adhere to distress screening protocols [9].
Efficacy of psychosocial interventions to reduce distress
Numerous RCTs of specific psychosocial interventions with cancer survivors have demonstrated efficacy in reducing cancer distress, most commonly measured as reductions in multi-item depressive or post-traumatic stress symptom measures [39–41]. Results from some RCTs also suggest potential improvements in nondistress endpoints such as telomere length [42] or increased survival associated with comprehensive cognitive behavioral interventions [15,40,41] and supportive–expressive interventions [43–45]. Meaning-centered psychotherapy has been associated with reduced distress specifically in advanced cancer populations [46]. While the above RCTs were led by doctoral-level interventionists, dissemination studies of psychosocial interventions show promise in distress reduction when facilitated by mid-level providers [47,48], patient navigators [49], community workers [50], and e- and m-health platforms (see Section below).
A recent meta-analysis identified survival benefits following psychosocial interventions but only among patients with early stage disease [51]. Other systematic reviews in specific cancer populations, such as prostate cancer, identified small and transient impacts of psychosocial interventions on outcomes such as cancer-related quality of life and physical components of general health-related quality of life [52].
Gaps in knowledge related to distress screening
While both research and policy have emphasized the importance of distress screening for identifying unmet needs of cancer patients, arguments against widespread distress screening focus on issues related to lack of operationalization, referral protocols, and evidence of screening impact [38,53,54]. First, although the term “distress” was selected because of its multidimensionality, distress remains a construct that is inconsistently operationalized. Since distress includes multiple domains (psychological, social, spiritual), it is difficult to measure with a single-item as recommended by some groups (e.g., DT recommended by NCCN). When screening for distress, it is unclear whether distress is the target condition or a presymptomatic state for another condition, such as depression or anxiety [54]. In 2014, the American Society for Clinical Oncology (ASCO) introduced a more specific guideline calling for screening of both depression and anxiety [15]. As noted in the CoC mandate, cancer centers can elect to screen for distress using the measures and procedures they prefer; such real-world implementation will result in various procedures and assessment tools. With the goal of ultimately reducing distress, research efforts focused on the referral and treatment procedures after a positive distress screen are still needed, no matter which measure is used.
Critical evaluation and need for research
Given the above concerns and gaps in existing knowledge, the mandate of distress screening in cancer populations is ripe with dissemination and implementation research questions that fall under the general question of “How do we best implement screening practices to benefit patient outcomes?” Within this general implementation question, specific research questions stem from uncertainty regarding optimal screening measure selection and complexity, criterion measure selection to demonstrate screening effectiveness, method and timing of screening administration, degree of individualization in referral mechanism, and patient interest, engagement, and benefit from referral. These questions should be considered from the perspective of all stakeholders (patient, family/caregiver, provider, organization/healthcare system) to include barriers and facilitators that may be conceptualized as secondary outcome measures (e.g., time and cost burdens). Processes to engage stakeholders from top down and bottom up perspectives are vital to optimize program reach and organizational adoption, implementation, maintenance and sustainability [55]. Integration of expertise from diverse fields, such as health information technology and human factors engineering, can help improve efficiency and effectiveness of distress screening [56]. Across dissemination and implementation efforts, reduction of patient distress should remain the primary outcome. Below we identify and describe the specific questions that will help translate the clinical mandates into evidence-based, efficient and sustainable distress screening programs. We conclude with a case example and recommendations for next steps.
Constructs important to optimizing distress screening
Key aspects of dissemination and implementation theory include understanding the context of outer setting (e.g., healthcare policy, accreditation standards such as the CoC distress screening mandate) and inner setting (e.g., local/regional healthcare system and organizational culture) (see Fig. 1). In terms of healthcare policy, the Department of Health and Human Service’s National Quality Strategy established by the Affordable Care Act [57] coincides with the Institute for Health Care Improvement Triple Aim of healthcare (improving the experience of care, improving health of populations, and reducing healthcare costs) [3,4]. These policies, along with adaptations such as consideration of a Quadruple Aim that includes attention to provider well-being [58], provide a common language that can be used to support interdisciplinary research that includes not only perspectives of the patient and provider, but the realities of healthcare as a business. Increasingly, improving patient health outcomes (e.g., survival, recurrence, behavioral and social determinants of health), patient experience (e.g., quality, satisfaction, reliability, accessibility, safety), and lowering healthcare costs are incentivized via reimbursement structures (e.g., incentivizing reduced 30-day hospital readmissions) and quality recognition (e.g., accreditation). Notably, the most all-cause 30-day readmissions for Medicaid patients are mood disorders (i.e., depression and bipolar disorders), and for private insured patients are maintenance chemotherapy/radiotherapy and then mood disorders [59].
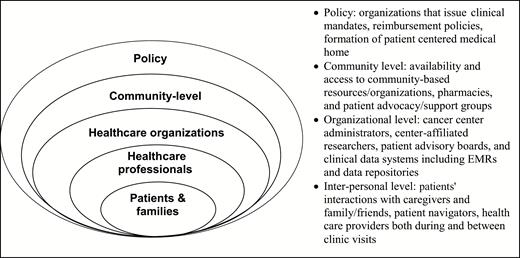
Multiple levels involved in cancer care delivery mandates at cancer centers
These healthcare outcomes (health, experience, cost, see Table 1.) thus become mutual goals of the patient, family/caregivers, provider, payer, and healthcare system, which will help bring together teams of clinicians, administrators, patient advocates/caregivers, and researchers to achieve excellence in cancer care. As healthcare activities increasingly rely on untrained family/caregivers (e.g., caring for surgical sites, monitoring for fever, administering medications), it is important to include family/caregiver stakeholders. Caregivers may be more aware of patient distress and also experience distress themselves [47]. Indeed, awareness of multiple stakeholder needs, with a particular emphasis on caregiver and family-level approaches, is important across the continuum of cancer care from diagnosis to treatment to survivorship. Identification, treatment and monitoring of distress throughout this continuum can complement efforts to improve quality of care and thus reduce the cancer burden on patients, families, and healthcare systems.
Distress and secondary outcomes to advance the dissemination and implementation science of distress screening
| Triple Aim of Healthcare . | ||
|---|---|---|
| Costs . | Patient Experience . | Healthcare outcomes . |
| Distress | ||
| Hospitalization days | Patient satisfaction with comprehensive/overall cancer care | Survival |
| ER visits | Social QOL | Recurrence |
| ICU days | Emotional QOL | Biomarkers |
| Readmission to hospital within 30 days | Functional QOL | Pain |
| Number of patient phone calls documented | Physical QOL | Fatigue |
| Billing data | Feasibility of navigating or managing care by patient or family | Depression |
| Staff time to administer screen | Patient perceives partnership in care | Anxiety |
| Provider time to interpret screen to determine referral need | Adherence to cancer monitoring visits | |
| False positive rate/unnecessary referral | Adherence to preventive screening guidelines | |
| False negative rate/ mental health needs fall to oncology providers | Tobacco cessation | |
| Number referred/Number screened | Physical activity | |
| Order for mental health visit placed | Nutrition quality | |
| Mental health visit documented | ||
| Number of mental health visits documented | ||
| Missed planned chemotherapy, radiation, or other cancer therapy session | ||
| Adverse healthcare-associated infection or other condition | ||
| Invasive procedures | ||
| Infections | ||
| Triple Aim of Healthcare . | ||
|---|---|---|
| Costs . | Patient Experience . | Healthcare outcomes . |
| Distress | ||
| Hospitalization days | Patient satisfaction with comprehensive/overall cancer care | Survival |
| ER visits | Social QOL | Recurrence |
| ICU days | Emotional QOL | Biomarkers |
| Readmission to hospital within 30 days | Functional QOL | Pain |
| Number of patient phone calls documented | Physical QOL | Fatigue |
| Billing data | Feasibility of navigating or managing care by patient or family | Depression |
| Staff time to administer screen | Patient perceives partnership in care | Anxiety |
| Provider time to interpret screen to determine referral need | Adherence to cancer monitoring visits | |
| False positive rate/unnecessary referral | Adherence to preventive screening guidelines | |
| False negative rate/ mental health needs fall to oncology providers | Tobacco cessation | |
| Number referred/Number screened | Physical activity | |
| Order for mental health visit placed | Nutrition quality | |
| Mental health visit documented | ||
| Number of mental health visits documented | ||
| Missed planned chemotherapy, radiation, or other cancer therapy session | ||
| Adverse healthcare-associated infection or other condition | ||
| Invasive procedures | ||
| Infections | ||
Distress and secondary outcomes to advance the dissemination and implementation science of distress screening
| Triple Aim of Healthcare . | ||
|---|---|---|
| Costs . | Patient Experience . | Healthcare outcomes . |
| Distress | ||
| Hospitalization days | Patient satisfaction with comprehensive/overall cancer care | Survival |
| ER visits | Social QOL | Recurrence |
| ICU days | Emotional QOL | Biomarkers |
| Readmission to hospital within 30 days | Functional QOL | Pain |
| Number of patient phone calls documented | Physical QOL | Fatigue |
| Billing data | Feasibility of navigating or managing care by patient or family | Depression |
| Staff time to administer screen | Patient perceives partnership in care | Anxiety |
| Provider time to interpret screen to determine referral need | Adherence to cancer monitoring visits | |
| False positive rate/unnecessary referral | Adherence to preventive screening guidelines | |
| False negative rate/ mental health needs fall to oncology providers | Tobacco cessation | |
| Number referred/Number screened | Physical activity | |
| Order for mental health visit placed | Nutrition quality | |
| Mental health visit documented | ||
| Number of mental health visits documented | ||
| Missed planned chemotherapy, radiation, or other cancer therapy session | ||
| Adverse healthcare-associated infection or other condition | ||
| Invasive procedures | ||
| Infections | ||
| Triple Aim of Healthcare . | ||
|---|---|---|
| Costs . | Patient Experience . | Healthcare outcomes . |
| Distress | ||
| Hospitalization days | Patient satisfaction with comprehensive/overall cancer care | Survival |
| ER visits | Social QOL | Recurrence |
| ICU days | Emotional QOL | Biomarkers |
| Readmission to hospital within 30 days | Functional QOL | Pain |
| Number of patient phone calls documented | Physical QOL | Fatigue |
| Billing data | Feasibility of navigating or managing care by patient or family | Depression |
| Staff time to administer screen | Patient perceives partnership in care | Anxiety |
| Provider time to interpret screen to determine referral need | Adherence to cancer monitoring visits | |
| False positive rate/unnecessary referral | Adherence to preventive screening guidelines | |
| False negative rate/ mental health needs fall to oncology providers | Tobacco cessation | |
| Number referred/Number screened | Physical activity | |
| Order for mental health visit placed | Nutrition quality | |
| Mental health visit documented | ||
| Number of mental health visits documented | ||
| Missed planned chemotherapy, radiation, or other cancer therapy session | ||
| Adverse healthcare-associated infection or other condition | ||
| Invasive procedures | ||
| Infections | ||
Measurement
Considerable variability exists in the tools used to measure distress. Research is needed to assist teams in the selection of measures most appropriate for their specific settings, patients and anticipated process of distress screening [60]. As the distress screening mandate includes a mechanism for referral to mental healthcare providers, it seems imperative that the cut-off score or triage system is effective in detecting treatable mental health conditions. Clear efforts are needed related to evaluation of effectiveness (criterion and diagnostic validity; screening complexity and predictive validity). Below we highlight key measurement issues and identify areas for future research.
Effectiveness: validity and generalizability
The most commonly studied cancer distress screening measure is the single-item DT [13]. In studies of the DT, criterion validity is often evaluated using the Hospital Anxiety and Depression Scale (HADS) or other depressive symptom measures [27]. In terms of effectiveness, a systematic review of cancer distress screening measures found that the sensitivity and specificity of the DT was lower than 80% compared to gold-standard criterion measures, most of which focused on depression [15,27]. Another systematic review identified other brief measures (e.g., HADS; “1-item structured interview”) [61] as having better positive predictive value compared to the DT [16]. Studies employing blinded, psychometrically rigorous diagnostic interviews are needed to test the criterion of mental health diagnoses such as adjustment, anxiety, and depressive disorders [15,38].
Finally, the predictive value of screening measures remains an emerging area of study in terms of reductions in distress [27] and utilization of care [9]. Carlson et al. demonstrated that multiple-item screening that used a printed summary and included the availability of personalized triage improved distress through referrals [30]. One study of the DT and an associated problem list laudably examined three outcomes relevant to the Triple Aim, yet did not result in improved mood, increased referrals to psychological services, or cost savings [34]. A recent evaluation across 55 CoC-accredited healthcare settings demonstrated that among institutions adherent to their full distress screening protocol, emergency room visits and patient hospitalizations decreased by 18–19% [9]. The following questions need to be addressed as organizations review which screening measures to use for distress screening in which patient populations—all while weighing screening efficiency with accuracy:
• How do shorter distress measures relate concurrently to mental health diagnoses, which the longer criterion measures were created to detect?
• What is the downstream effectiveness—impact on experience of care, healthcare outcomes, and cost—of distress screening measures that vary in psychometric strength?
• Do positive screens result in patients receiving evidence-based interventions?
• Do positive screens lead to reduced distress? By what mechanisms?
• How do psychometrics of screening measures vary by cancer population?
• How many false positive screens occur and what is the impact on experience of care, healthcare outcomes, and cost of distress screening?
Adoption: acceptability, feasibility, healthcare costs, and time burden
The debate surrounding distress measure selection is salient within the literature and numerous editorial commentaries. A common reason for adoption of short measures is to minimize patient and healthcare system burden; brief measures appear to be more acceptable to busy staff [17]. However with 30–50% of cancer patients screening positive on short measures and high false positive rates that approach 50%, the downstream burden of short measures may be greater (e.g., need for further screening and triage efforts in a large subsample) [16,35]. Thus, measurement selection may differentially impact provider behavior and the setting of criteria for when further assessment, evaluation, and treatment of distress is needed.
Longer measures, while often possessing stronger psychometric properties [27], are more likely to incur upstream health system costs (scoring time, documentation time, purchase costs for some) and upstream patient burden of time [27], although this issue can be mitigated through the use of tablets for data-assessment and the automatic computation of values indicating risk of distress which would trigger action by providers [62,63]. Likewise, recommendations have been made to include assessment of patients’ interest in referral at the time of distress screening [27,64]. Research questions related to adoption of distress screening include:
• Do the downstream effects of higher test sensitivity and specificity of longer screening measures translate into more efficient, targeted screening in terms of reduced healthcare costs and patient and provider burden (e.g., less number of patients screening positive, less need for further assessment and triage, less unnecessary referrals)?
• Can computerized adaptive testing (e.g., PROMIS), which tailors questions to each individual patient, be successfully implemented in the clinical setting to reduce the number of items that patients answer while maintaining rigorous psychometric properties?
• Does screening effectiveness improve with an additional item to query the patient’s interest in a mental health referral?
• In terms of emotional distress, is it more efficient to use a two- or three-tiered screening system (e.g., distress screen --> depression and anxiety screen --> mental health referral, vs. depression and anxiety screen --> mental health referral)?
Implementation
Implementation barriers
Previous research in primary care and oncology clinical settings has identified multiple barriers to the successful implementation of cancer distress screening [19,32]. At the patient level, individuals may encounter barriers in receiving screening or adequate referral and follow-up due to lack of insurance, being unaware of available resources, lacking in self-advocacy, being hesitant to discuss emotional issues with their providers for cultural reasons or due to stigma, and other demographic factors (e.g., age, language). At the provider-level, barriers include time availability, provider knowledge and skill in performing the screening, and trust between patients and providers. Provider-level barriers may further depend on the disciplinary background of the providers involved, buy-in from organizational leadership and depth of the allied healthcare team in terms of capacity to provide referrals, treatment, and follow-up. At the setting level, availability of provider training and adequate access to available referral resources are important factors. Psychologists and other behavioral and mental health providers are well-suited to lead teams of allied health professionals to deliver care or ensure appropriate referral of distressed patients. With the dearth of behavioral health specialists in medical settings, increased attention to training opportunities for other members of the cancer care team will be important with the growing number of cancer survivors. Examples include focused training in psychosocial oncology through National Cancer Institute-supported R25 training grants or preconference workshops led by professional organizations with depth and expertise in behavioral health (e.g., Society of Behavioral Medicine).
Implementation: facilitators and patient engagement
Postscreen activities of triage and patient engagement in care are critical to reducing distress [65]. Ultimately, an estimated 11–12% of the eligible screening population engages in referral care [7,63,66]. Carlson demonstrated that regardless of one-time triage type (personalized phone call, computerized triage), patients who engaged in care over the subsequent 1-year experienced greater decreases in distress, anxiety, and depression (assessed by single-item Likert scales). A study of computerized screening among over 800 mixed-type cancer patients found that patient interest in counseling/therapy was associated with history of mental health diagnosis, medications, or therapy, as well as physical symptom burden, and possibly ethnic minority status [67]. Identified gaps include the following questions:
• What are individual characteristics of patients interested in services? Is mental health history important?
• What methods engage patients who will use and benefit from intervention?
• Does screening with depression and anxiety symptom measures result in more efficient triage (e.g., less false positive and less downstream staff burden)? More efficient use of referrals?
• How do we ensure fidelity to screening, triage, and intervention processes across providers?
Implementation: partnering with health systems and case example
Cancer care is delivered through cancer centers located in the community, centers with National Cancer Institute designation, community-based and advocacy organizations, and ambulatory centers [68,69]. Only a small number of cancer patients (approximately 15%) receive care at comprehensive cancer centers, with more patients seen at community-based cancer centers [70]. Recent data suggest that community centers are having greater success in implementing the distress screening mandate [10]. Use of electronic health records (EHRs) can facilitate implementation and communication across partner disciplines [71]. For example, evaluation of an electronic patient-reported outcomes system (ePRO) to meet distress screening mandates was effective in identifying and providing the infrastructure for patient referral and treatment of distress [48]. Learning from settings with successful implementation and partnering with the local healthcare system and cancer care organizations is vital to implementation.
Case example at a comprehensive cancer center
At a comprehensive cancer center located in the mid-Atlantic region of the USA, we adapted the NCCN Distress Thermometer Screen and associated problem list to be administered via tablets. All new patients presenting for their first clinic visit complete a New Patient Assessment at check-in, which includes the DT, the PROMIS six-item Depression and Anxiety short forms [72,73] and several other questions. Patient scores on the DT automatically populate the notes section of the EHR. When patients are taken into an exam room, the medical assistant also enters the Distress score into the vital signs section with the other vital signs.
Between April 1, 2017 and April 30, 2018, approximately 1,750 patients completed a DT. Of these patients, 34.9% reported mild distress (1–3); 38.9% reported moderate distress (4–7); 7.4% indicated high to severe distress (7–10); and 18.7% reported no distress (0). The most commonly reported problems from the DT problem checklist included: fatigue (16%); coping with illness (14%); feeling anxious or fearful (11%); pain management (9%); and sleep disturbance (7%).
Presently, an e-mail alert is sent to all social workers when a patient indicates a distress score of 7 or above and/or if a patient indicates that he/she needs or wants additional information based on the problem list. Scores of 7 (moderate distress) trigger a follow-up call within 1 week, 8 or 9 (high distress) prompt a call within 5 business days, and a score of 10 (extreme distress) triggers an alert to the social worker who will see or talk with the patient within 24 hr. These cut-off scores were determined based on practical constraints. If the social worker deems the patient could benefit from additional services, the patient is referred to the clinical psychologist and/or psychiatrist in the cancer center. The experiences within this cancer center highlight the importance of having the distress screening efforts championed by someone with mental and behavioral health expertise, the challenges of implementation and integration into the EHR and the need for multidisciplinary input across mental health, digital health records and information technology, such as E- and m-health platforms.
Implementation and reach: E-health and m-health platforms
Generally, e-health and m-health (i.e., electronic and mobile health) technologies are of high interest in addressing the myriad challenges in the modern healthcare system. As part of the legislation known as HITECH (Health Information Technology for Economic and Clinical Health Act of 2009) [74], healthcare stakeholders have been encouraged to consider opportunities for “meaningful use” of EHRs, including optimizing access and use of health information in improving health outcomes. This priority was also reiterated by the National Institutes of Health in 2011, in conjunction with the National Science Foundation, calling for a concerted effort to build a “cyberinfrastructure,” which would support health data storage, management and integration, and facilitate the translation of scientific evidence into clinical practice [75].
Cancer survivors are consistently found to be receptive to electronic health information exchange with providers, especially for the purposes of receiving information about recommended tests, symptom management (including distress) and lifestyle advice [76,77]. A recent trial of a tablet/internet-based program for symptom monitoring decreased distress in oncology patients, when combined with opportunities for clinical consultation [78]. Ultimately, these tools need to be meaningfully integrated with EHRs, to address compliance with “meaningful use” objectives, facilitate practical use among medical teams, and reduce the potential for disparities in access or use of distress management tools (e.g., across education level, income, and age) [78,79]. Identified gaps and questions pertinent to e- and m-health platforms include:
• How can we optimally design the provider interface to best support distress screening and triage? Distress management?
• How can e-/m- health technology be seamlessly integrated with the EHR and other workflow processes to reduce the burden on clinicians and patients?
• How can providers be compensated for non-face-to-face consultation time?
• What level of provider interaction do patients prefer in distress management? Can e-health approaches work effectively across different patient populations?
• How can we best tailor patient-facing e-health or EHR tools to varying levels of patient health literacy?
Human factors engineering is a scientific discipline that can help address many of these questions within team science approaches. Human factors focus on understanding human capabilities and applying this knowledge to improve technology, workflow processes, and other aspects of the healthcare system to support both providers and patients [80]. It can be applied to specific aspects of the healthcare system, such as improving the interface of tablets to collect patient-reported outcome data more effectively by promoting design, development, and implementation that prioritizes end-users needs. Human factors engineering also provides a framework for considering multiple aspects of the complex healthcare system, through a socio-technical systems (STS) approach [81]. STS models provide a method to understand the interactions between multiple healthcare system levels inclusive of technologies, people, work processes, policies, and other factors so that these factors can be optimized for patient outcomes and provider experience and performance. Human factors engineering has been embraced by many high risk and complex industries such as aviation, nuclear energy, and defense; it is increasingly being applied to healthcare and has significant potential to improve quality of care and patient outcomes. Specific to distress screening, human factors approaches can help identify how to optimize the flow of information for improving efficiencies and quality of care along all of the CoC’s Standard 3.2 process requirements: timing of screening, method, tools, assessment, referral, and documentation [1]. Evaluation of distress screening approaches using a human factors approach can identify and correct system-level constraints that impede the process. For example, such an approach can be useful in terms of identifying ineffective alert systems: settings in which high distress scores have to be visually screened by a member of the team and then manually flagged and moved from a “notes” section in the EHR rather than relying on technology to have an automated process. In addition, a human factors approach can identify gaps in communication between healthcare providers: referrals for distressed patients may not be followed-up on by the patient or provider in a timely manner. These approaches can be helpful during implementation of a new distress screening program or as a way to improve efficiency and effectiveness of an existing distress screening program.
CONCLUSION
With the recent accreditation mandate for cancer distress screening, accredited cancer centers across the USA are implementing procedures to effectively screen for and treat distress. Although screening implementation procedures vary and a number of pivotal measurement issues need to be resolved, we have a growing body of literature to support and guide future efforts for effective distress screening interventions in terms of organizational and provider adoption and implementation. As providers and researchers continue to evaluate various distress screening tools, we anticipate that a few select screening measures will emerge as both efficient and effective. Careful attention to effectiveness and efficiency across different populations and various assessment platforms is needed. Efficient single-item screening approaches such as the DT may be of value, but the predictive validity and downstream costs of these brief tools need to be established. In addition to interventions that screen for distress, research is needed to optimize referral procedures, ensure effectiveness of treatment approaches, and understand the composition of the teams best suited to participate in screening, assessment and treatment. Behavioral interventions can be used not only to treat acute distress but also maintain reduced distress that remains evident among subgroups of cancer survivors [21]. Distress screening can include facilitation by mid-level providers with supervision from a licensed, perhaps doctoral-level trained, behavioral health specialist. One policy level recommendation is that evaluation of the standards for Distress Screening could include assessment of the appropriateness of the multidisciplinary expertise of the teams responsible for the implementation and follow-through of the CoC Distress Screening mandate. By utilizing multidisciplinary expertise within the complexities of the U.S. healthcare system, and relying on evidence-based approaches to dissemination and implementation of both distress screening and treatment, we can keep patient-centered care at the forefront. Improving outcomes for the millions of cancer survivors is the ultimate goal. We look forward to robust clinical and research efforts to inform distress screening policy.
Compliance with Ethical Standards
Authors Contributions: S.L.E. and K.D.G. conceived of the paper idea; S.L.E., S.M.B., L.M.Q., J.K., M.A.D., K.D.G. contributed to the initial drafting of the manuscript. All authors contributed to the interpretation and discussion and provided critical feedback on the final paper.
Conflicts of Interest: All authors declare that they have no conflicts of interest.
Ethical Approval: This article does not contain any studies involving human participants.
Acknowledgments:
We would like to thank Ms. Susan Marx and Dr. Abigail Montero for their assistance with manuscript preparation. We would also like to acknowledge the Cancer Special Interest Group at the Society of Behavioral Medicine as this manuscript stemmed from a pre-conference seminar hosted by the Cancer Special Interest Group. This work was supported in part by the P30CA051008-24 and the Survivorship Research Initiative at Lombardi Comprehensive Cancer Center, Georgetown University.



