-
PDF
- Split View
-
Views
-
Cite
Cite
Matjaž Kuntner, Klemen Čandek, Matjaž Gregorič, Eva Turk, Chris A Hamilton, Lisa Chamberland, James Starrett, Ren-Chung Cheng, Jonathan A Coddington, Ingi Agnarsson, Jason E Bond, Increasing Information Content and Diagnosability in Family-Level Classifications, Systematic Biology, Volume 72, Issue 4, July 2023, Pages 964–971, https://doi.org/10.1093/sysbio/syad021
Close - Share Icon Share
Abstract
Higher-level classifications often must account for monotypic taxa representing depauperate evolutionary lineages and lacking synapomorphies of their better-known, well-defined sister clades. In a ranked (Linnean) or unranked (phylogenetic) classification system, discovering such a depauperate taxon does not necessarily invalidate the rank classification of sister clades. Named higher taxa must be monophyletic to be phylogenetically valid. Ranked taxa above the species level should also maximize information content, diagnosability, and utility (e.g., in biodiversity conservation). In spider classification, families are the highest rank that is systematically catalogued, and incertae sedis is not allowed. Consequently, it is important that family-level taxa be well defined and informative. We revisit the classification problem of Orbipurae, an unranked suprafamilial clade containing the spider families Nephilidae, Phonognathidae, and Araneidae sensu stricto. We argue that, to maximize diagnosability, information content, conservation utility, and practical taxonomic considerations, this “splitting” scheme is superior to its recently proposed alternative, which lumps these families together as Araneidae sensu lato. We propose to redefine Araneidae and recognize a monogeneric spider family, Paraplectanoididae fam. nov. to accommodate the depauperate lineage Paraplectanoides. We present new subgenomic data to stabilize Orbipurae topology which also supports our proposed family-level classification. Our example from spiders demonstrates why classifications must be able to accommodate depauperate evolutionary lineages, for example, Paraplectanoides. Finally, although clade age should not be a criterion to determine rank, other things being equal, comparable ages of similarly ranked taxa do benefit comparative biology. [Classification, family rank, phylogenomics, systematics, monophyly, spider phylogeny.]
In phylogenetic systematics, classifications often need to account for the possibility that monotypic taxa—representing depauperate evolutionary lineages—are discovered as sister to well-defined, ostensibly more diverse, crown groups. This biological reality is due to asymmetric phylogenies and therefore, discovering such a new taxon (that lacks synapomorphies of its better-known sister clade) does not invalidate that clade nor its rank. For example, discovery of a monotypic lineage sister to mammals that lacks mammalian synapomorphies would not invalidate Mammalia.
The Tree of Life is rich with examples of monotypic representatives being sister clades to more speciose ones. For example, Ginkgo biloba is the sole representative of the family Ginkgoaceae and order Ginkgoales, and this lineage is sister to Cycadaceae and Cycadales, a clade that contains over a hundred species (Wu et al. 2013). The split between Ginkgo and cycads is estimated to have occurred during the late Carboniferous (Condamine et al. 2015). Similarly, the tuatara (Sphenodon punctatus) is the only representative of the family Sphenodontidae and the order Rhynchocephalia, and is sister to Squamata, which includes all remaining lizards and snakes. Here, the split between the clades is estimated to have occurred in the early Mesozoic (Gemmell et al. 2020). Other examples of depauperate evolutionary lineages are Welwitschia, Limnognathia maerski, and many more.
When depauperate lineages are discovered, the classical Linnean, ranked classification scheme requires naming of all intervening ranks, a so-called Gregg’s paradox (Buck and Hull 1966). A fully rankless higher taxonomic level classification scheme avoids this problem as it continues to accept well-defined clade names even in the light of new, sister lineage discoveries. The International Code of Phylogenetic Nomenclature, for example, does not mandate the use of ranks (Cantino and De Queiroz 2020). A sensible solution is the combination of the Linnean and phylogenetic systems (Kuntner and Agnarsson 2006), with a limited use of ranks of maximal information content (e.g. genera, families, orders) in a network of phylogenetically defined, unranked clades.
Whether ranked or unranked, named higher taxa must be monophyletic to be phylogenetically valid. Ranked taxa (i.e., above the species level) should also maximize information content, diagnosability, and utility (e.g., communication, comparative biology, cohesive natural history, preservation of traditional concepts, conservation prioritization, etc.). If many well-established depauperate lineages were to be classified into more inclusive taxa (e.g., if Ginkgoaceae were lumped into Cycadaceae), much phylogenetic diversity would be concealed, lowering the taxonomic, conservation, and practical utility of such classification.
EXAMPLE FROM SPIDER CLASSIFICATION
The circumscription of the spider family Araneidae has been debatable, even though the “classic” or “core” common orb weavers are widely and intuitively recognized as a natural group (as well as being the basionym of the order Araneae). The last comprehensive araneid classification was Simon (1895)—taxonomists have been amending it ever since. Araneidae continues to be difficult to diagnose due to the heterogeneity of its contents. Its history is overwhelmingly one of subtracting monophyletic groups to better circumscribe Araneidae, for example, Arkyidae, Cyatholipidae, Linyphiidae, Mimetidae, Nesticidae, Tetragnathidae, and Theridiosomatidae.
Because of downstream implications, the delimitation of family rank taxa in arachnology is more than a semantic issue. In the World Spider Catalog (WSC 2023), a vital, decisive, and universally used tool for arachnologists, the family rank is the terminal rank in which taxa are organized; incertae sedis is not considered. Other commonly used family group ranks, such as superfamily, subfamily, and tribe can be found only in the scattered primary literature. For arachnologists, therefore, it is particularly important that family-level taxa be well-defined and informative. Fifteen of the currently recognized spider families are monogeneric (11.3%).
Kuntner et al. (2019) published a phylogenomic study of the spider family Nephilidae recognizing seven genera that included 84% of the known species in the family. This phylogeny, along with earlier generic-level treatments that formed the basis for the removal of the group from Tetragnathidae (Kuntner 2005, 2006, 2007; Harvey et al. 2007; Kuntner et al. 2013), confirmed its monophyly and provided a differential diagnosis with respect to Araneidae, and therefore sharpened the definition of the latter (Kuntner et al. 2019). The argument for the 2019 classification was based on the criteria of monophyly, information content, diagnosability, and in part, inferred clade ages. Kuntner et al. (2019) also recognized Phonognathidae, thus three families: Nephilidae, Phonognathidae, and Araneidae, s.s. Recent phylogenies estimated by other researchers (Dimitrov et al. 2017; Kulkarni et al. 2020; Scharff et al. 2020) agreed that these three clades are mutually monophyletic, well-defined, and informative. Kuntner et al. (2019) named this unranked group of three families Orbipurae, the archetypal orb web spiders, preserving the family group name Araneidae for a classical, core set of diverse, coherent, widely recognized, and most studied groups of genera, including Araneus, the type genus of Araneae.
Kallal et al. (2020) revisited the findings of Kallal and Hormiga (2018), which presented a weakly supported topology not confirmed by any subsequent study. Their topology placed Nephilidae and Paraplectanoides inside Araneidae sensu stricto. Kallal et al.’s (2020) taxonomy creates an expanded Araneidae, which lumps three important, diagnosable families into one, rendering “Araneidae” ambiguous. Although Kallal et al. (2020) address the subfamilies Phonognathinae and Nephilinae, they ignore the implication of these actions, Araneidae sensu stricto, providing neither name, diagnosis, nor composition (we acknowledge these were not required). Kallal et al.’s (2020) Araneidae sensu lato is equivalent in composition to Orbipurae, but as a family, Araneidae s.l. is challenging to diagnose and loses information content and utility, for example, by uniting into a single family distinct taxa with disparate morphologies and biologies. Such lumping can have consequences. For example, Nephilidae is the only spider family for which all species have been scored for IUCN threat status (https://www.iucnredlist.org/) and retaining it as a family adds value to conservation biology. Further, in practice, this results in the loss of classical araneids, phonognathids, and nephilids as entities in WSC and the inability to track these important lineages as such. According to Kallal et al. (2020), a novel placement of the enigmatic Australian Paraplectanoides as sister to Nephilidae provides further arguments against Orbipurae, and Nephilidae as a family “renders the family Araneidae paraphyletic.”
Briefly recapped, Kallal et al.’s (2020) arguments for Araneidae s.l. are i) topological: Paraplectanoides + Nephilidae nested within Araneidae (and the misplacement of this clade within Araneidae s.s.) negates the family status of Nephilidae; ii) historical: because Paraplectanoides has been traditionally an araneid, nephilids are also to be classified as araneids; iii) utilitarian: lumping nontraditional families into a classical one despite diagnosability difficulties and profuse variation in biology simplifies the classification of Araneoidea. Here we use subgenomic data and extensive taxon sampling of Orbipurae to confirm Paraplectanoides + Nephilidae, however, that clade is placed outside Araneidae s.s. (as in Kulkarni et al. 2020; Scharff et al. 2020). We then argue that a better supported phylogeny, and the criteria of diagnosability, information content, and utility, eclipse the arguments put forth by Kallal et al. (2020). We show that the inclusiveness of a family-level taxon directly impacts the utility, information content, and stability of a group’s systematic structure. Even if portrayed as arbitrary, decisions whether to lump or split in higher-level classification have consequences that need careful consideration.
Phylogenetic Placement of Paraplectanoides
Kallal et al.’s (2020) hypothesized relationship of Paraplectanoides + Nephilidae restated results from earlier Sanger data-based hypotheses (Kallal and Hormiga 2018, 2019), placing the pair inside Araneidae s.s. Developing UCE data probes for spiders, Kulkarni et al. (2020) recovered the grouping Paraplectanoides + Trichonephila outside Araneidae s.s., but the data set contained only one nephilid terminal on a long branch. As the Paraplectanoides + Trichonephila relationship might plausibly have been an artifact of long-branch attraction (Felsenstein 1978; Bergsten 2005), and as Paraplectanoides was not used in the nephilid phylogeny (Kuntner et al. 2019), further testing of this relationship is important. We therefore combined data from the UCE matrix from Kulkarni et al. (2020) with additional UCE data from five nephilid genera, five araneid s.s. genera (selected to maximize subfamily coverage), and two phonognathid genera (See Supplementary material available on Dryad for methodological details and Supplementary Table S1 for taxon sampling). The strength of this analysis is the inclusion of all but one nephilid genus (Indoetra) rather than a select few (Kallal et al. 2020), or a single nephilid representative as in Kulkarni et al. (2020).
Our results (Fig. 1; Supplementary Figs S1–S4 available on Dryad) also recover the sister-group relationship Paraplectanoides + Nephilidae and corroborate that detail of the topology of Kallal et al. (2020) and Kulkarni et al. (2020). Unlike Kallal and Hormiga (2018, 2019) and Kallal et al. (2020), our results (and those of Dimitrov et al. 2017; Kulkarni et al. 2020; Scharff et al. 2020) unambiguously place this clade outside Araneidae s.s. The Caerostris placement in Kallal and Hormiga (2018, 2019), reused in Kallal et al. (2020), is not supported.
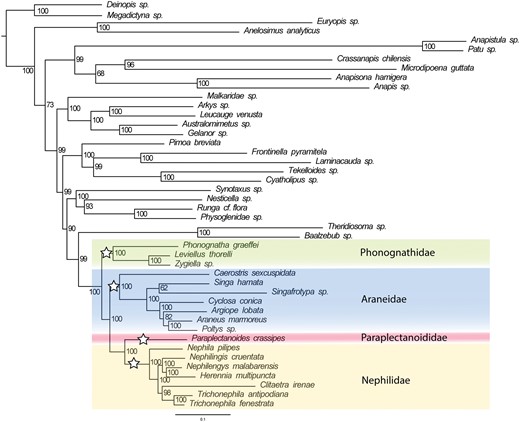
The maximum likelihood tree inferred from UCE data for Orbipurae, containing Phonognathidae, Paraplectanoididae, Nephilidae, and Araneidae. Orbipurae families are marked with white stars and their contents colored. Node values represent bootstrap support.
Orbipurae (Phonognathidae ((Paraplectanoides, Nephilidae) Araneidae)) is strongly supported in maximum likelihood (Fig. 1; Supplementary Fig. S2 available on Dryad) and coalescent-based (ASTRAL) analyses (Supplementary Fig. S3 available on Dryad). Gene concordance factors (gCF) and site concordance factors (sCF) are positively correlated and are generally high (gCF > 10%; sCF > 40%; Minh et al. 2020) when branch support is high (Supplementary Figs S3–S5 available on Dryad). The tree topology tests support the unconstrained tree over the three alternative tree topologies (Supplementary Table S2 available on Dryad). The relationships within nephilids match the results from Anchored Hybrid Enrichment data (Kuntner et al. 2019) except in the varying position of Clitaetra, probably due to low loci recovery, and the absence of Indoetra in the current analysis.
To Lump or to Split?
Kallal et al. (2020) state that the Kuntner et al. (2019) classification rendered Araneidae paraphyletic. For various reasons, the taxon sample of Kuntner et al. (2019) did not include Paraplectanoides, and Kallal et al.’s (2020) argument was based in part on the weakly supported incorrect placement of Nephilidae + Paraplectanoides. Second, the placement of Paraplectanoides in their analyses did not per se reject the monophyly of any groups we discussed. Therefore, the purported paraphyly was mischaracterized, and as discussed below, can be debated based on topological and logical arguments.
Lumping Nephilidae and Phonognathidae into Araneidae s.l. (Dimitrov et al. 2017; Scharff et al. 2020) reduces the information content of the classification. Kallal et al. (2020) reduced core araneids to the currently nameless, undiagnosed remainder of Araneidae—containing many of the most prominent genera of spiders—unrecognized and untraceable as a group in the World Spider Catalog (WSC 2023). We favor the relimitation of Araneidae to its core taxa and propose an unranked name for the four well-characterized families in Orbipurae. It thus contains one more component of information (Mickevich and Platnick 1989) making an additional statement that Araneidae s.l. does not. At 170 genera, Araneidae s.s. still contains disparate groups and could perhaps be improved in the future by the removal of even more groups, as has happened repeatedly over the last century or more (Scharff et al. 2020).
Kallal et al. (2020) dismiss the possibility of a monogeneric family ranked group for Paraplectanoides because that would create a “textbook example of Gregg’s Paradox.” However, Hormiga (1993, 1994) argued against the importance of Gregg’s paradox in the case of Pimoidae, another family that started as monogeneric; similarly, Bond et al. (2020) argued that “nomenclatural stability and abandoning well-established diagnosable genera in favor of a more inclusive polymorphic taxon” favored recognizing the monotypic genus Cryptocteniza.
The sister-group Paraplectanoides + Nephilidae illustrates Gregg’s paradox. Considering the Linnean requirement that all taxa are unequivocally classified in families, these two spider groups could be classified in different ways. One possibility is to recognize two families (one currently monogeneric). It is possible that other extant or extinct paraplectanoidids will be discovered, as has happened, for example, in Pimoidae, Macrothelidae, Liphistiidae, and Cithaeronidae. Paraplectanoididae, rejected by Kallal et al. (2020), allows for stability of the clearly monophyletic and diagnosable Nephilidae, and at the same time, reflects the separate evolutionary history of the two clades. If Paraplectanoides, nephilids, and phonognathids are all lumped in Araneidae s.l., the family contains a huge diversity of taxonomic concepts and forms, about which fewer generalizations can be made. As a ranked family, it has lower information content.
We reexamined Paraplectanoides and confirmed that it does not share the key nephilid synapomorphies (see Taxonomy), and thus the first alternative (to recognize two families for Paraplectanoides + Nephilidae) better reflects the evolutionary history and diagnosability of each group and maximizes the information content of ranked clades. This avoids the paraphyly of Araneidae and we formally propose this nomenclature in the Taxonomy section below.
Logical Considerations
Rather than redefine Araneidae s.l., Kallal et al. (2020) allege its paraphyly if defined s.s. This logic is subject to modal scope fallacy. Modal scope fallacy means that a proposition is taken as necessarily true or false even when the necessity is unwarranted (Schurz 1994; Swartz 2022). Kallal et al.’s (2020) logic seems to be that Paraplectanoides, because it is currently catalogued as araneid, will always be an araneid. Therefore, anything related to it must also be an araneid, a modal scope fallacy.
If every genus that is placed as sister to another family retained its prior family affiliation, the family classification of spiders, representing distinct patterns of morphological diversity, would be severely diminished. For example, if another araneid genus is placed phylogenetically as sister to another family, their logic would lump every intervening group within an ever-growing Araneidae s.l. At the extreme, all spiders, Araneae, could become araneids, because new sister-group relationships will continue to appear as phylogenetic systematics advances.
Clade Ages and Higher-Level Classification
Kallal et al. (2020) criticize our use of temporal banding demonstrating what a strict application would mean for araneoid spider family circumscription. We agree that strict uniform temporal banding is an impractical and unfeasible goal across the Tree of Life. We nevertheless think that comparable clade ages, if feasible, can add meaning to Linnean ranks. Equally ranked taxa always invite comparison, albeit sometimes unwarranted. Some modern phylogenetic studies have started to use ages to align classifications within well-defined clades, for example, in birds and mammals (Chen et al. 2019; Delsuc et al. 2019; Presslee et al. 2019; Harvey et al. 2020). Clade age can thus be an informative piece of the evolutionarily informed classification, however, the spider systematics community has not adopted clade age as a criterion to judge alternative classifications. Spider families are typically older than many other animal families. The tendency to lump more sister clades into families (like Araneidae s.l.) will likely further increase their age and is in our view an unnecessary step in the wrong direction.
CONCLUSIONS
Most authors agree that monophyly is paramount and that the assignment of ranks in biological classification is in part subjective. Kuntner et al. (2019) proposed that a suite of criteria be decisive in classification decisions to minimize subjectivity. Because our prior call for enhanced repeatability in higher classification was met with criticism (Kallal et al. 2020), herein we provide a rebuttal of that critique. In our view, the key weaknesses of these criticisms are:
Kallal et al. (2020) argue that because Paraplectanoides is traditionally classified as an araneid, and because phylogenies suggest that it groups as sister to nephilids, nephilids should be classified as araneids. This is a modal scope fallacy.
Discovering a sister taxon to a well-defined clade does not, in itself, invalidate that clade’s validity or rank as Kallal et al. (2020) suggest for Nephilidae. Classifications sometimes must account for valid but depauperate evolutionary lineages. Hence, Paraplectanoides + Nephilidae does not invalidate Nephilidae as a family group, particularly because Paraplectanoides lacks key nephilid synapomorphies. The Caerostris placement in Kallal et al. (2020) was simply incorrect, and that genus, likewise, does not affect nephilid nomenclature. Caerostris is consistently recovered as sister to the remainder of Araneidae and our classification treats the genus accordingly as an araneid.
The Araneidae s.l. diagnosis in Kallal et al. (2020) is vague and circumscribes a ranked clade of less information content than our proposal because it does not acknowledge the key diagnostic differences that define Nephilidae, Phonognathidae, and Araneidae, which, if recognized, increase the information content of the classification in general (see Taxonomy for diagnosis of Nephilidae, Phonognathidae, and Araneidae). Although Kallal et al. (2020) agree that Nephilidae and Phonognathidae are diagnosable clades, ironically, they neither named nor diagnosed the far larger Araneidae s.s. Scharff et al. (2020) recognized the problem and called Araneidae s.s. the ARA clade. In other words, one only gets to use the formal family name Araneidae once, and it is better, more conservative, historically more consistent, and more practical to use it to mean what it has historically meant—core Araneidae (see Taxonomy for diagnosis for Araneidae).
Kallal et al. (2020) criticized Kuntner et al. (2019) for using clade age as a criterion (temporal banding). Ideally, rank should correlate with age (Chen et al. 2019; Delsuc et al. 2019; Presslee et al. 2019; Harvey et al. 2020), but there should be additional criteria: monophyly, classification information content, and diagnosability. As one way to add objective meaning to ranks, taking clade ages into account can increase the utility of classifications to comparative biology. Araneidae s.l. increases the age of Araneidae, and thus the variance in age of families within Araneae and as compared to other animal families.
Our analysis confirms Orbipurae topology and places Paraplectanoididae as sister to the well-defined and diagnosable Nephilidae (Fig. 1). Therefore, in the Taxonomy, we emend Orbipurae to include the monotypic family Paraplectanoididae fam. nov., and revalidate Nephilidae, Phonognathidae, and Araneidae sensu Kuntner et al. (2019).
Our amended classification of Orbipurae eliminates ambiguity in family classification and presents a utilitarian solution with clearly diagnosable, monophyletic families whose biological meaning is well defined. To account for diagnosability and the needs of biodiversity conservation, decisions about lumping or splitting should strongly favor information content. Consequently, classifications must be able to accommodate depauperate evolutionary lineages; discovering them does not necessarily invalidate (i.e., dictate lumping) the rank classification of sister clades. Finally, clade age should not be entirely dismissed in determining rank because, ceteris paribus, comparable ages of similarly ranked taxa will continue to benefit comparative biology. Even if often seen as arbitrary, decisions whether to lump or split have pragmatic consequences that need careful consideration.
TAXONOMY
Orbipurae is a clade that contains the families Nephilidae, Phonognathidae, and Araneidae (Kuntner et al. 2019); Paraplectanoides is currently classified as an araneid (WSC 2023), but our examination revealed this classification is not based on shared diagnostic features. Based on our results, we propose Paraplectanoididae as a new family in Orbipurae (Araneoidea). Here, we circumscribe all these families, something previously not offered in any one publication. Phylogenetic definitions follow the PhyloCode (Cantino and De Queiroz 2020).
Prior diagnosis of only one of the following clades, Nephilidae, meets modern standards. Paraplectanoididae fam. nov., naming a depauperate lineage, is equal to the diagnosis of the genus Paraplectanoides provided here. Phonognathidae is a relatively small family and is easy to diagnose. Araneidae, on the other hand, is vast and has never been well-diagnosed. Below we offer hypotheses for formal diagnoses of these taxa, which will certainly be improved in the future as more taxa and diagnostic characters emerge, and perhaps as disparate and inconsilient groups are removed from Araneidae.
Clade Orbipurae Kuntner et al. 2019
We define Orbipurae at the node sometimes discussed as “Araneidae” sensu lato (Dimitrov et al. 2017; Kallal et al. 2020; Scharff et al. 2020; WSC 2023). Because Araneidae sensu lato has at times contained all the recognized families within Araneoidea, our definition carefully excludes non-Orbipurae araneoid families.
Definition.
Orbipurae is the most inclusive crown clade that contains the common ancestor of Araneus, Phonognatha, Paraplectanoides, and Nephila, but not of Theridiosoma, Synotaxus, Tetragnatha, Anapis, Theridion, Nesticus, Linyphia, Arkys, Cyatholipus, Malkara, Mimetus, and Physoglenes. This is a maximum-crown-clade definition (Cantino and De Queiroz 2020).
Reference phylogeny.
Diagnosis.
Spiders that construct two- or three-dimensional webs that usually contain orb web elements, albeit with numerous modifications or losses. Based on available data, Orbipurae can be diagnosed by a combination of morphological features, for example, the presence of a cheliceral chilum, book lung covers usually grooved, lateral eyes usually on tubercles, and the male palpal median apophysis sharing a hematodocha with the embolic division.
Composition.
The global clade currently contains 188 genera that are catalogued as “araneids” (WSC 2023).
Family Phonognathidae Simon, 1894 New Rank
Definition.
Phonognathidae is the most inclusive crown clade that contains the common ancestor of Phonognatha, but not of Araneus and not of Paraplectanoides and not of Nephila. This is a maximum-crown-clade definition (Cantino and De Queiroz 2020).
Reference phylogeny.
Diagnosis.
Spiders of the family Phonognathidae construct two-dimensional orb webs whose hub is modified into a retreat, either constructed with silk (Leviellus) or by using a rolled leaf (Phonognatha, Artiphex, Deliochus, and Zygiella). Unlike in nephilids, the cheliceral condyle (boss) in Phonognathidae is smooth and is not striated. Further diagnostic features include distal grouping of setae on palpal tibia and elongated male palpal femur.
Composition.
The family currently contains the genera ArtiphexKallal and Hormiga 2022 (a replacement name for ArtifexKallal and Hormiga 2018), Deliochus Simon 1894, Leviellus Wunderlich 2004, Phonognatha Simon 1894, and Zygiella F. O. Pickard-Cambridge 1902.
Family Araneidae Clerck, 1757
Comments.
We define Araneidae at the node discussed as Araneidae s.s. (Kuntner et al. 2019) and as “the ARA clade” (Scharff et al. 2020). Araneidae will probably continue to be redefined, as advances in comparative morphology, taxonomy, and phylogenetics occur.
Definition.
Araneidae is the most inclusive crown clade that contains the common ancestor of Araneus, but not of Phonognatha and not of Paraplectanoides and not of Nephila. This is a maximum-crown-clade definition (Cantino and De Queiroz 2020).
Reference phylogeny.
Diagnosis.
Spiders of the family Araneidae usually construct two- or three-dimensional orb webs albeit with numerous modifications or losses. Unlike in nephilids, the cheliceral condyle (boss) in Araneidae is smooth, and is not striated. Araneidae may also be diagnosed by a combination of the squat shape of the male palpal tibia, the relatively globular tegulum, eye pattern with lateral eyes widely separated from the medians, and possibly, the presence of an epigynal scape or its homologs.
Composition.
The global family currently contains 175 genera (WSC 2023).
Family Paraplectanoididae Kuntner, Coddington, Agnarsson & Bond fam. nov.
Definition.
Paraplectanoididae is the most inclusive crown clade that contains the common ancestor of Paraplectanoides, but not of Araneus and not of Phonognatha and not of Nephila. This is a maximum-crown-clade definition (Cantino and De Queiroz 2020).
Reference phylogeny.
Diagnosis.
Spiders of the family Paraplectanoididae construct a three-dimensional nest (Hickman 1975) whose architecture is unlike the archetypal orb web built by most representatives of Orbipurae. Paraplectanoides crassipes builds an ovoid sheet that lacks sticky lines; the only potentially homologous features with the classical orb web are the radii and a horizontal hub (Eberhard 2020). Paraplectanoididae males and females uniquely possess a flange on the cheliceral fang (Supplementary Fig. S6 available on Dryad). Females have an additional flange modification of the cheliceral paturon and a highly elevated head region of the carapace (Davies 1988). Unlike in nephilids, the cheliceral condyle (boss) in Paraplectanoides is smooth, and is not striated (Supplementary Fig. S6 available on Dryad).
Composition.
The family currently only contains the genus Paraplectanoides Keyserling, 1886 with the type species P. crassipes Keyserling, 1886 and P. kochi O. Pickard-Cambridge, 1877. Paraplectanoides is the type genus for the family, by monotypy. Although more species and genera can plausibly be discovered in Australasia or elsewhere on Gondwanan terrains (see Turk et al. 2020), current knowledge suggests that the family and the genus may in fact be monotypic with P. crassipes as the only valid species (V. Framenau in litt.), and with P. kochi being misplaced in Paraplectanoides.
Comments.
Simon (1895) placed Paraplectanoides in the group Anepsieae within Argiopidae, but Anepsion is a derived araneid not proximal to Paraplectanoides (Scharff et al. 2020). Hickman (1975) described the male and provided observations on life history of P. crassipes. Although Davies (1988) considered Paraplectanoides to be an “araneine” due to its transverse furrows on the epigastric plates and a male palp with a paramedian apophysis and a radix, the key treated the genus as incertae sedis.
Family Nephilidae Simon, 1894 Family Rank Resurrected
Definition.
Nephilidae is the most inclusive crown clade that contains the common ancestor of Nephila, but not of Araneus and not of Phonognatha and not of Paraplectanoides. This is a maximum-crown-clade definition (Cantino and De Queiroz 2020).
Reference phylogeny.
Diagnosis.
An unreversed synapomorphy for Nephilidae is also its defining morphological feature: the striated cheliceral boss in both sexes (Kuntner 2005: Figs 6B, 20A–C). In addition, Kuntner et al. (2008) hypothesized 15 unambiguously optimized synapomorphies, as well as additional ambiguously optimized ones that defined Nephilidae. Nephilid lateral eyes are separate in both sexes and male abdomen is sclerotized. Among behavioral characters are those pertaining to web building behavior (the sticky spiral location using the fourth leg tap, double radius construction, double radius attachment on the frame). Nephilid web architectures are either classically orbiculate and aerial (Nephila, Trichonephila) or modified into elongate, almost rectangular ladder webs against substrate (Clitaetra, Indoetra, and Herennia), or hybrid that are partially aerial and partially substrate anchored, and are always planar, vertical and sticky (Kuntner et al. 2019). However, unlike in Paraplectanoididae, nephilid web modifications are still recognizable through the orb’s classical, homologous elements: frames, hubs, radii, non-sticky and sticky (gluey) spirals (Hormiga et al. 1995; Kuntner et al. 2008; Blackledge et al. 2011; Eberhard 2020), but with sometimes added signal lines (Nephilengys, Nephilingis;Kuntner 2007), pseudoradii (Herennia;Kuntner 2005), or modified hub reinforcements (Clitaetra;Kuntner 2006).
Composition.
Nephilidae contains the genera Clitaetra Simon, 1889; Herennia Thorell, 1877; Indoetra Kuntner 2006; Nephila Leach, 1815; Nephilengys L. Koch, 1872; Nephilingis Kuntner 2013; and Trichonephila Dahl, 1911. For currently deemed valid species, see Table 1 in Kuntner et al. (2019); other catalogued species names are likely to be proposed as synonyms of these names.
Comments.
Kuntner (2006) proposed Nephilidae Simon, 1894 at the family rank and defined it as the least inclusive clade containing Clitaetra, Herennia, Nephila, and Nephilengys. Nephilid monophyly and exclusivity have subsequently been confirmed using molecular and total evidence phylogenetics (Kuntner et al. 2013) and phylogenomics (Kuntner et al. 2019). However, since the above nephilid genera have been further split to reflect their evolutionary history, age and diagnosability, the family Nephilidae now contains species of the seven genera listed above.
SUPPLEMENTARY MATERIAL
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.t1g1jwt5c.
ACKNOWLEDGMENTS
We thank M. Hedin, V. Framenau, and an anonymous reviewer for constructive comments on the manuscript.
FUNDING
The project was supported by the Slovenian Research Agency grants P1-0255 and J1-9163 to MK, the Evert and Marion Schlinger Foundation and National Science Foundation grant DEB 1937604 to JEB, and Global Genome Initiative (Smithsonian Institution) funding to JAC.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.



