-
PDF
- Split View
-
Views
-
Cite
Cite
Nishitha S Hosamane, Adam M Didouchevski, Ayse Malci, Jeffrey P Gavornik, Michael S Sidorov, Sleep is necessary for experience-dependent sequence plasticity in mouse primary visual cortex, Sleep, Volume 48, Issue 3, March 2025, zsae262, https://doi.org/10.1093/sleep/zsae262
Close - Share Icon Share
Abstract
Repeated exposure to familiar visual sequences drives experience-dependent and sequence-specific plasticity in mouse primary visual cortex (V1). Prior work demonstrated a critical role for sleep in consolidating a related but mechanistically distinct form of experience-dependent plasticity in V1. Here, we assessed the role of sleep in consolidation of spatiotemporal sequence learning (sequence plasticity) in mouse V1.
Visually evoked potentials were recorded in awake, head-fixed mice viewing sequences of four visual stimuli. Each sequence was presented 200 times per session, across multiple sessions, to drive plasticity. The effects of sleep consolidation time and sleep deprivation on plasticity were assessed.
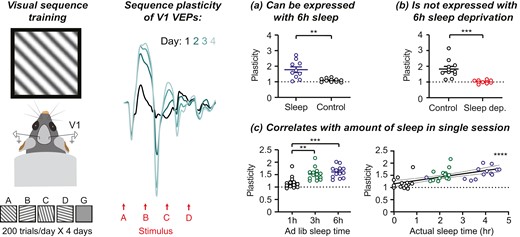
Sequence plasticity occurred in V1 following as little as 1 hour of ad libitum sleep and increased with longer periods of sleep. Sleep deprivation blocked sequence plasticity consolidation, which recovered following subsequent sleep.
Sleep is required for the consolidation of sequence plasticity in mouse V1.
A major function of sleep is to consolidate plasticity and learning. In mice, repeated exposure to visual sequences drives “sequence plasticity” in visual cortex that reflects familiarity and expectation. We report that sleep is required for consolidation of sequence plasticity in mouse visual cortex. Our findings support prior work demonstrating a critical role for sleep in consolidating related, but mechanistically distinct, forms of experience-dependent plasticity. Our results suggest that the requirement for sleep in consolidation generalizes across multiple forms of plasticity in visual cortex.
Sleep is a highly conserved phenomenon that subserves multiple functions, including to promote learning and memory [1–8]. The visual system provides an attractive model system that has been used to study the role of sleep in consolidating multiple forms of experience-dependent plasticity in vivo. For example, work in cats demonstrated a critical role for sleep in consolidating ocular dominance plasticity [9, 10]. In mice, repeated exposure to a familiar visual stimulus drives a form of plasticity in V1 termed stimulus-specific (or orientation-specific) response potentiation (SRP) [11, 12]. SRP reflects behavioral habituation to a familiar stimulus and is expressed via a day-over-day increase in the magnitude of visually evoked potentials (VEPs) and single-unit activity driven by this stimulus [13]. Sleep between sessions is critical for consolidating SRP: six hours of ad libitum sleep following stimulus presentation is sufficient to consolidate SRP and 6 hours sleep deprivation blocks SRP [14].
A related but distinct form of experience-dependent potentiation in V1 is induced by the presentation of familiar visual sequences. Spatiotemporal sequence learning, or “sequence plasticity,” can be induced by presenting mice with sequences of four short visual elements in a consistent order [15]. Like SRP, sequence plasticity is expressed by a day-over-day increase in VEP magnitude to elements in the familiar sequence. In addition, both SRP and sequence plasticity do not have a strict critical period and can be induced in juvenile and adult mice [11, 15]. Despite the superficial similarities, converging evidence suggests that SRP and sequence plasticity are mechanistically distinct phenomena. For example, SRP induction requires NMDA receptor activation but sequence plasticity does not, and sequence plasticity requires cholinergic activity but SRP does not [11, 13, 15, 16]. Prior work has shown that sleep promotes SRP in V1 [14]; however, the requirement for sleep in consolidating sequence plasticity is unknown. As ongoing work seeks to characterize both SRP and sequence plasticity at the mechanistic level, it is critical to understand how generalized the requirement for sleep is in consolidating these related forms of experience-dependent plasticity in V1.
Here, we tested the hypothesis that sleep is necessary for the induction of sequence plasticity in mouse V1. We demonstrate that: (1) a single 6-hour session of ad libitum sleep following sequence exposure is sufficient to induce sequence plasticity, (2) sleep deprivation for 6 hours blocks sequence plasticity, and (3) sequence plasticity is correlated with the amount of actual sleep between recording sessions. Overall, our findings highlight the critical role sleep plays in the consolidation of experience-dependent sequence plasticity in the visual cortex.
Methods
Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Children’s National Research Institute. All mice (congenic C57BL6/J) were group housed on a 12-hour light/dark cycle with lights on at 06:00 am with access to food and water ad libitum. All experiments were conducted during the light phase. Adult (P60–P100) male and female mice were used in equal ratios for all experiments. All studies used separate cohorts of mice; no mice were used for multiple experiments.
In vivo local field potential recordings
Mice underwent survival surgeries to chronically implant recording electrodes in V1 using methods similar to prior work [15, 17]. Mice were anesthetized using intraperitoneal injection of ketamine (40 mg/kg) and xylazine (10 mg/kg), and bupivacaine (0.25%) was administered as a local analgesic under the scalp. Following small craniotomies, tungsten microelectrodes (FHC: #30070) were implanted bilaterally in binocular V1 layer 4 (from bregma, in mm: A/P −3.28, M/L +/− 3.0–3.1, D/V −0.47), and a silver ground wire was placed in cerebellum. A steel headpost was attached to the skull anterior to bregma to enable head fixation during experiments. Dental cement (Parkell: C&B Metabond) was used to secure the electrodes and headpost and create a protective head cap. After surgeries, mice recovered in their home cage for at least 48 hours with ad libitum access to food (placed on the floor) and water. Mice in all studies were habituated to the recording apparatus twice for 15 minutes per session on the day before experiments. During habituation and all experiments, mice were awake, head-fixed, and body restrained in a dark, quiet environment and oriented towards a computer monitor 20 cm away (Figure 1A). During habituation, the monitor displayed a gray screen. During experiments, visual sequences were presented as described below. Local field potentials (LFPs) were recorded using a Plexon OmniPlex Neural Recording Data Acquisition System that amplified and digitized data with a 40 kHz acquisition rate and 0.1 Hz high-pass and 200 Hz low-pass filtration. Raw data were then downsampled to 1 kHz for analysis.
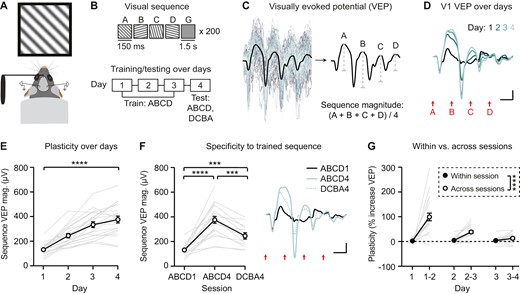
Presentation of familiar visual sequences drives plasticity in V1 VEP magnitude across days. (A) Awake, habituated, head-fixed mice viewed sequences of visual stimuli on a monitor. (B) Top: A single sequence consisted of four oriented grating stimuli, presented for 150 ms, always in the same order (ABCD). Sequences were separated by a 1.5 seconds gray screen (G). Bottom: Mice were trained using 200 presentations of familiar sequence ABCD across 3 days. On day 4, mice were presented with both familiar sequence ABCD and novel sequence DCBA. (C) Quantification of the magnitude of sequence VEPs, which represent the average of 200 trials per session. (D) Group average VEPs to sequence ABCD across 4 days of training (n = 21 mice). (E) VEPs driven by sequence ABCD increase in magnitude across days (n = 21). (F) Sequence plasticity was partially specific to the trained sequence (ABCD1: n = 21, ABCD4: n = 21, DCBA4: n = 13). (G) Comparing plasticity within recording sessions versus plasticity between sessions (across days). Plasticity within a session reflects a comparison between the first 100 trials and the second 100 trials. Plasticity across sessions reflects a comparison of all 200 trials. Scale bars: 100 µV, 100 ms. Error bars indicate ± SEM, ****p < .0001.
Visual sequence presentation
Psychtoolbox and custom MATLAB scripts (https://github.com/jeffgavornik/VEPStimulusSuite) were used to generate visual sequences similar to as previously described [15–17]. Prior to sequence presentations, mice were habituated to a gray screen for 5 minutes. During experiments, sequences of four visual stimuli (“ABCD”) were presented with a stimulus duration of 150 ms for each element. Each stimulus was a full-field oriented sinusoidal grating with 100% contrast and 0.05 cycles/deg spatial frequency (A: 45° orientation, B: 105°, C: 15°, D: 75°). Each 600 ms ABCD trial was separated by a 1.5 seconds gray screen. Mice viewed 200 total trials in four blocks of 50, with each block separated by a 30 seconds gray screen period (Figure 1B). On test days, 200 trials of the novel DCBA or CABD sequence, as noted, were presented in four blocks of 50 immediately following ABCD presentation.
Visually evoked potential analysis
VEPs [18] were calculated by averaging evoked responses to 200 trials. Custom MATLAB software (https://github.com/jeffgavornik/VEPAnalysisSuite) was used to quantify VEP magnitude, defined as the difference between the minimum negative potential and maximum positive potential following stimulus presentation. Sequence magnitude is defined as the average VEP magnitude elicited by each stimulus in the sequence [(A + B + C + D)/4] (Figure 1C, Supplementary Figure S1) [15]. To assess plasticity to familiar sequences, evoked ABCD VEP magnitudes were quantified across multiple recording sessions (Figures 1E and F, 2D, 3C and E, 4C and E and 5C, Supplementary Figure S3A and B). To assess whether plasticity was specific to the trained sequence, ABCD VEPs were compared with DCBA or CABD VEPs within sessions, as noted (Figures 1F, 2D, 3C and E, 4E and 5C). In addition, in order to compare the amount of plasticity between experimental groups, sequence VEPs were normalized to a within-animal baseline (defined as the sequence VEP magnitude during the first recording session; Figures 1G, 2E, 3D, 4D and 5D, Supplementary Figure S3C). In Figure 1G, plasticity within a session reflects a comparison between VEPs driven by the first 100 sequences presentations and VEPs driven by the second 200 sequences.
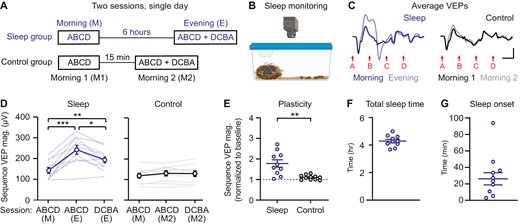
Plasticity occurs after six hours of ad libitum sleep. (A) Mice viewed sequences twice in 1 day with 6 hours (experimental/sleep) or 15 minutes (control) between sessions. (B) Home cages were videotaped to quantify sleep. (C) Group average VEPs in sleep (n = 10) and control (n = 10) groups. (D) Sequence VEP magnitude increases following 6 hours of ad libitum sleep (n = 10) and remains unchanged in the control group (n = 10). (E) A direct comparison of plasticity between sleep and control groups, with sequence VEP magnitude normalized to baseline. (F) Total sleep time and (G) sleep onset in experimental mice during the 6-hour ad libitum sleep period. Scale bars: 100 µV, 100 ms. Error bars indicate ± SEM, *p < .05, **p < .01, ***p < .001.
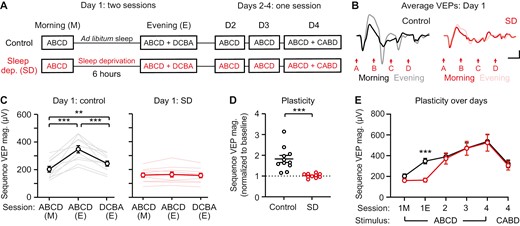
Six hours sleep deprivation blocks sequence plasticity. (A) Sequence VEPs were recorded before and after 6 hours of sleep deprivation (SD; experimental) or ad libitum sleep (control). VEPs were recorded once a day for the subsequent 3 days. (B) Group average VEP traces on day 1 (control: n = 10, SD: n = 11). (C) Sequence VEP magnitude increases following 6 hours of ad libitum sleep (n = 11) and remains unchanged after 6 hours of sleep deprivation (n = 10). (D) A direct comparison of plasticity between sleep deprivation and control groups, with sequence VEP magnitude normalized to baseline. (E) Plasticity recovers in sleep-deprived mice after day 1. Scale bars: 100 µV, 100 ms. Error bars indicate ± SEM, ***p < .001.
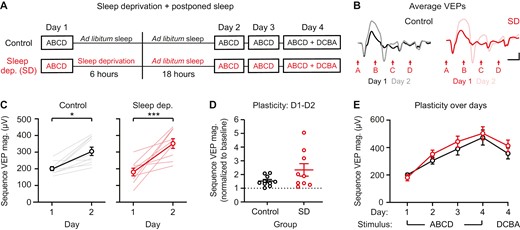
The first 6 hours following stimulus presentation are not essential for consolidation of sequence plasticity. (A) Sequence VEPs were recorded once before 6 hours of ad libitum sleep (control) or sleep deprivation (SD; experimental), and then mice in both groups were returned to the animal facility for 18 hours of ad libitum sleep. (B) Group average VEPs on day 1 and day 2 (control: n = 10, SD: n = 9). (C) Sequence VEP magnitude is increased on day 2 in the control group (n = 10) and SD group (n = 9). (D) A direct comparison of plasticity between control and SD groups, with sequence VEP magnitude normalized to baseline. (E) Sequence VEP magnitude potentiates similarly between groups. Scale bars: 100 µV, 100 ms. Error bars indicate ± SEM, *p < .05, ***p < .001.
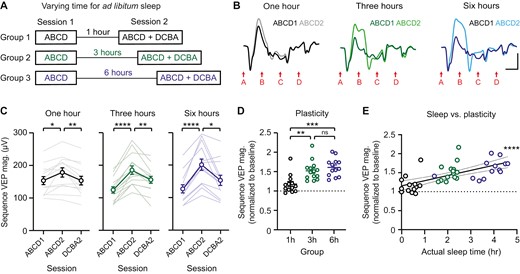
Sequence plasticity is correlated with the amount of sleep between recording sessions. (A) Sequences were presented twice in 1 day with 1, 3, or 6 hours between sessions. During this time, mice were returned to their home cage for ad libitum sleep and video recorded. (B) Group average VEPs for both sessions in 1 hour (n = 13), 3 hour (n = 14), and 6 hour (n = 13) groups. (C) Sequence-specific plasticity is expressed in each group. (D) A direct comparison of plasticity between groups, with sequence VEP magnitude normalized to baseline. (E) Plasticity is positively correlated with amount of sleep, as measured by video. Within-group correlations are shown in Figure S5. Scale bars: 100 µV, 100 ms. Error bars indicate ± SEM, *p < .05, **p < .01, ***p < .001, ****p < .0001.
VEPs were recorded bilaterally in V1, with results averaged across hemispheres to yield a single sequence magnitude for each mouse. Thus, in all experiments, “n” denotes the total number of mice. In instances where the LFP signal was noisy or electrode placement was inaccurate in one hemisphere, only data from the unaffected hemisphere were analyzed. If a poor signal was present in both hemispheres, the mouse was excluded from analysis (12 mice excluded across all experiments).
Experimental design
For experiment 1 (Figure 1), mice (n = 21) were trained using sequence ABCD for 4 consecutive days (Figure 1B). On day 4, a subset of mice (n = 13) were also presented with novel sequence DCBA. For all 4 days, trials 1–100 and 101–200 were then independently analyzed and sequence magnitudes were compared to assess plasticity in a single recording session compared to plasticity across sessions (Figure 1G). For experiment 2 (Figure 2), VEPs were recorded twice in a single day. During the first session (“morning”), mice were presented with sequence ABCD. During the second session, mice were presented with both familiar sequence ABCD and novel sequence DCBA. Mice were randomly assigned to spend either 6 hours (n = 10) or 15 minutes (n = 10) in their home cage between recording sessions (Figure 2A) while sleep was monitored via video camera and scored offline (Figure 2B). Thus, the second session was either labeled “evening” (in the 6 hour/sleep group) or “morning 2” in the control group. For experiment 3 (Figure 3), mice were randomly assigned to either sleep (n = 11) or sleep deprivation (n = 10) groups. On day 1, VEPs were recorded twice in a single day (morning and evening), separated by 6 hours of ad libitum sleep or sleep deprivation. Sequence ABCD was presented in the morning session, and both ABCD and DCBA were presented in the evening session. VEPs were then recorded once a day, in the morning, to sequence ABCD, for the following 3 days. On day 4, mice also were presented sequence CABD (Figure 3A). In this study, following the initial period of either ad libitum sleep or sleep deprivation on day 1, all mice were allowed to sleep normally throughout the remainder of the study. For experiment 4 (Figure 4), mice were randomly assigned to either sleep (n = 10) or sleep deprivation (n = 9) groups. On day 1, mice in both groups were presented sequence ABCD in the morning, followed by 6 hours of ad libitum sleep or sleep deprivation. There was no evening recording session on day 1. Mice in both groups were then returned to their home cage to sleep ad libitum overnight. VEPs were then recorded once a day, in the morning, to sequence ABCD, for the following 3 days. On day 4, mice also were presented sequence DCBA (Figure 4A). In experiments 2–4, morning recordings were performed between 7 and 10 am (ZT 1—ZT 4) and evening recordings were performed between 3 and 6 pm (ZT 8—ZT 11). For experiment 5 (Figure 5), VEPs were recorded twice in a single day. Mice were randomly assigned to 1 hour (n = 13), 3 hours (n = 14), or 6 hours (n = 13) in the home cage between recording sessions. Mice were trained using sequence ABCD during the first recording session and tested using sequences ABCD and DCBA during the second recording session (Figure 5A). Sleep was monitored via video camera during the period between recordings and scored offline.
Sleep monitoring, analysis, and deprivation
For sleep monitoring experiments (Figures 2 and 5), mice were returned to their home cage for a period of ad libitum sleep in a quiet, well-lit room immediately following the first recording session. Cages were passively videotaped for the duration of this period to enable post hoc quantification of sleep. Sleep behavior was visually scored using a non-invasive, immobility-defined approach with 40 seconds of activity/inactivity as a minimum threshold for defining wake/sleep states. Each mouse was tracked individually. This method has been previously validated under similar group housing conditions and strongly correlates with EEG/EMG-defined sleep [19–21]. We quantified total sleep, sleep onset, sleep efficiency, and number of wakings (Figures 2F-G and 5E, Supplementary Figures S4 and S5B-D). Sleep efficiency was defined as the percentage of time spent asleep after initial sleep onset. For sleep deprivation experiments (Figures 3 and 4), mice in the experimental sleep deprivation group were sleep deprived for 6 hours using gentle handling techniques, such as cage tapping, prodding, and inversion. This method of sleep deprivation has previously been shown to effectively disrupt sleep while inducing a minimal stress response compared to other methods [22–24].
Statistics
A one-way repeated measures (RM) ANOVA was used for most comparisons of VEP magnitudes over time (Figures 1E, 2D, 3C and 5C, Supplementary Figures S2A-D and S3A and B). A mixed-effects analysis was used in Figure 1F when a subset of mice were not presented sequence DCBA on day 4. A one-way ANOVA was used to compare plasticity (Figure 5D), VEP magnitude (Supplementary Figure S5A), and total sleep time (Supplementary Figure S5C) between three groups. For one-way ANOVAs, RM one-way ANOVAs, and mixed effects analyses, Tukey–Kramer multiple comparison post hoc tests were used to compare individual groups when there was a statistically significant main effect. Student’s t-tests were used to directly compare normalized plasticity between two groups (Figures 2E, 3D and 4D, Supplementary Figure S3C). A two-way RM ANOVA with days and within session/across session as factors was used to compare plasticity within sessions and across sessions (Figure 1G). A two-way RM ANOVA, with time and sleep/sleep deprivation as factors, was used to assess how plasticity changes across days (Figures 3E, 4C and 4E). For two-way RM ANOVAs, Sidak’s multiple comparison post hoc test was used to compare VEP magnitudes on individual days when a statistically significant main effect was present. A simple linear regression was used to assess the relationship between sleep time and plasticity (Figure 5E, Supplementary Figure S5D and E). Statistical analysis was performed using Prism 10. All data are represented as mean ± SEM, where *p < .05, **p < .01, ***p < .001, ****p < .0001.
Results
Plasticity in V1 occurs to familiar visual sequences
We recorded VEPs in V1 layer 4 as awake, head-fixed mice viewed visual sequences once daily across 4 consecutive days (Figure 1A-C; Supplementary Figure S1). Consistent with previous work, sequence VEP magnitude increased across four days of training to the familiar sequence ABCD (Figure 1D and E; one-way RM ANOVA, F(1.657,33.14) = 61.68, p < .0001). As expected, plasticity was observed to each individual stimulus (Supplementary Figure S2) and to a similar degree in males and females (Supplementary Figure S3) [25]. We confirmed that after training, day 4 VEPs were greater to the trained sequence ABCD than the novel sequence DCBA (Figure 1F; mixed-effects analysis, p < .0001; post hoc ABCD1—ABCD4: p < .0001, ABCD4—DCBA4: p = .0001). Day 4 VEPs to novel sequence DCBA were larger than day 1 VEPs to sequence ABCD (post hoc ABCD1—DCBA4: p = .0001), demonstrating that as expected, there is some residual plasticity to familiar stimuli presented out of order [15]. The amount of plasticity expressed across days was greater than the amount of plasticity expressed within sessions (Figure 1G; two-way RM ANOVA, main effect of within/across session: p < .0001). We therefore hypothesized that sleep between sessions is required for consolidation of sequence plasticity in V1.
Sequence plasticity occurs after 6 hours of ad libitum sleep
To test the role of sleep in consolidation of sequence plasticity, we first asked whether a single 6-hour period of ad libitum sleep following exposure to visual sequences would be sufficient to enable plasticity. On day 1, mice viewed sequence ABCD in the morning, followed by either 6 hours (experimental/sleep group) or 15 minutes (control group) in their home cage with video monitoring (Figure 2A and B). Experimental mice showed significant plasticity between morning (M) and evening (E) sessions (Figure 2C and D; one-way RM ANOVA, F(1.333,12) = 25.53, p = .0001; post hoc ABCD (M)—ABCD (E): p = .0010; ABCD (M)—DCBA (E): p = .0013; ABCD (E)—DCBA (E): p = .0105), while control mice showed a trend towards slightly increased VEP magnitude (Figure 2C and D; one-way RM ANOVA, F(1.936,17.42) = 3.690, p = .0473, post hoc ABCD (M1)—ABCD (M2): p = .0611; ABCD (M)—DCBA (E): p = .1161; ABCD (E)—DCBA (E): p = .9622). A direct comparison of plasticity between experimental and control groups confirmed that plasticity is greater in experimental mice allowed 6 hours ad libitum sleep (Figure 2E; Student’s t-test, t(19) = 3.876, p = .0010). We confirmed that experimental mice slept during the 6-hour period, on average for 4.3 ± 0.1 hours with a sleep onset of 26.1 ± 7.4 minutes, sleep efficiency of 77.9 ± 1.6%, and 7.9 ± 0.6 wakings (Figure 2F and G, Supplementary Figure S4). Together, these results suggest that sleep may play a role in the induction of sequence plasticity. However, an alternative hypothesis is that 6 hours of time is critical for sequence plasticity, regardless of whether mice sleep during this period.
Six hours of sleep deprivation blocks sequence plasticity
To test the hypothesis that sleep (and not time) is required for the induction of sequence plasticity, we assessed the effect of 6 hours of sleep deprivation between morning and evening presentations of ABCD sequences. In this cohort, mice were either sleep deprived (experimental) or allowed to sleep ad libitum (control) during the six-hour period following morning sequence presentations (Figure 3A). Control mice showed significantly increased VEPs to sequence ABCD in evening sessions relative to morning sessions (Figure 3B and C; one-way RM ANOVA, F(1.227,12.27) = 33.39, p < .0001; post hoc ABCD (M)—ABCD (E): p = .0002; ABCD (M)—DCBA (E): p = .0030; ABCD (E)—DCBA (E): p = .0009). Sleep-deprived mice showed no change in VEPs between morning and evening sessions (Figure 3B and C; F(1.478,13.30) = 1.155, p = .3275). A direct comparison of plasticity between experimental and control groups confirmed impaired plasticity in the sleep-deprived group (Figure 3D; Student’s t-test, t(19) = 4.442, p = .0003). Following day 1 evening recordings, mice in both groups were returned to normal housing, enabling sleep prior to subsequent morning recordings on days 2–4 (Figure 3A). This design allowed us to test whether plasticity would recover in the sleep deprived group following subsequent sleep. On day 2 and beyond, VEP magnitudes to sequence ABCD in the sleep deprived group were comparable to control levels (Figure 3E; two-way RM ANOVA, day/genotype interaction: F(5,95) = 2.881, p = .0182, post hoc on day 1E: p = .0001, day 2: p > .9999, day 3: p > .9999, day 4: p > .9999). Sequence plasticity on day 4 was significantly greater in both groups compared to the novel sequence CABD (Figure 3E; two-way RM ANOVA, post hoc on day 4: ABCD4 (sleep)—CABD4 (sleep): p = .0057; ABCD4 (SD)—CABD4 (SD): p = .0258). Together, these results demonstrate that 6 hours of sleep deprivation blocks sequence plasticity in V1, but that this plasticity can recover with subsequent sleep.
We next asked whether the 6 hours immediately following sequence presentation are critical for consolidation. To address this question, a new cohort of mice viewed sequence ABCD in the morning, followed by either 6 hours of sleep deprivation (experimental) or 6 hours of ad libitum sleep (control). Following this six-hour period, all mice were allowed to sleep ad libitum in their home cage overnight prior to the second recording session on day 2 (Figure 4A). The key difference between this study and the prior study is that here, experimental mice were not presented the visual stimulus for a second time before overnight ad libitum sleep. This design allowed us to ask whether postponed sleep, beyond the 6-hour window but prior to re-exposure to the familiar sequence, would be sufficient to consolidate plasticity. Both control and experimental mice showed significantly increased sequence VEPs from day 1 to day 2 (Figure 4B and C; two-way RM ANOVA, main effect of time: F(4,68) = 40.17, p < .0001, post hoc control ABCD1—ABCD2: p = .0356, post hoc experimental ABCD1—ABCD2: p = .0001). A direct comparison of plasticity between control and experimental groups on day 2 confirmed that there was no difference in the magnitude of plasticity between groups (Figure 4D; t(17) = 1.806, p = .0886). Plasticity was similar between control and experimental groups across all 4 days of this study (Figure 4E; two-way RM ANOVA, main effect of sleep/sleep deprivation: F(1,17) = 0.5769, p = .4579). These results suggest that the initial six hours after stimulus presentation may not be essential for plasticity, provided mice are able to sleep afterwards.
Sequence plasticity is correlated with the amount of sleep between recording sessions
Our findings suggest that sleep between recording sessions is essential for sequence plasticity (Figure 2-4); however, the amount of sleep required for plasticity to be expressed remains unclear. To examine the duration of sleep required to consolidate plasticity, we recorded VEPs twice in 1 day, with sessions separated by 1 hour, 3 hours, or 6 hours of ad libitum sleep (Figure 5A). Baseline (Session 1) VEPs were comparable between groups (Supplementary Figure S5A). In all three experimental conditions, mice showed a statistically significant increase in sequence VEP magnitude from Session 1 to Session 2 (Figure 5B and C; one-way RM ANOVA, 1 hour: F(1.774,21.29) = 7.113, p = .0055, post hoc ABCD1—ABCD2: p = .0159, 3 hour: F(1.863,24.22) = 43.80, p < .0001, post hoc ABCD1—ABCD2: p < .0001, 6 hour: F(1.677,20.13) = 20.28, p < .0001, post hoc ABCD1—ABCD2: p < .0001). However, the amount of plasticity observed was significantly increased in the three hour and six hour groups relative to the 1 hour group (Figure 5D; one-way ANOVA: F(2,37) = 11.32, p = .0001, post hoc 1 hour vs 3 hour: p = .0024, 1 hour vs 6 hour: p = .0002, 3 hour vs 6 hour: = 0.6247). As reported in Figure 2, mice do not sleep for the entire duration of the ad libitum sleep period. Here, mice in the 1 hour, 3 hour, and 6 hour groups on average slept 0.4 ± 0.1 hours, 2.2 ± 0.1 hours, and 3.7 ± 0.2 hours, respectively (Supplementary Figure S5B and C). Given that sleep duration was significantly different across experimental groups, we then examined the relationship between sleep and plasticity within individual mice. When data were pooled across groups, the magnitude of plasticity was positively correlated with observed time asleep within animals (Figure 5E; R2 = 0.4333, p = .0001). Within individual groups, there was a trend towards a correlation in the 3 hour group (p = .0503) and the 6 hour group (p = .0731), but not in the 1 hour group (p = .4059) (Supplementary Figure S5D). In 6 hour ad libitum sleep groups pooled from Figures 2 and 3, sleep duration was positively correlated with the magnitude of sequence plasticity (Supplementary Figure S5E; p = .0175). Together, these data suggest that significant sequence plasticity can occur with less than 1 hour of actual sleep, but that 2–3 + hours is ideal to enable consolidation.
Discussion
This study tested the hypothesis that sleep is required to consolidate sequence plasticity in V1. We found that 6 hours of ad libitum sleep following visual sequence presentation was sufficient to induce plasticity (Figure 2), and that sleep deprivation during this 6-hour window blocked plasticity (Figure 3). In addition, the magnitude of plasticity was positively correlated with the amount of sleep following stimulus presentation (Figure 5). Together, this work demonstrates that sleep is critical for consolidating sequence plasticity in V1.
While sleep deprivation prevented sequence plasticity in V1, plasticity was able to recover in sleep-deprived mice following subsequent sleep (Figure 3). Interestingly, plasticity was normal in mice that were sleep deprived for 6 hours followed by 18 hours of typical housing and ad libitum sleep (Figure 4). This result suggests that sleep in the first 6 hours after stimulus presentation is not critical for consolidation. Understanding the time course and duration of sleep required for consolidation will be critical if future studies aim to manipulate sleep itself or sleep-related circuits in vivo. Surprisingly, as little as 1 hour of ad libitum sleep (~25 ± 5 minutes actual observed sleep) was sufficient to induce statistically significant plasticity (Figure 5). However, plasticity was substantially greater with 3–6 hours ad libitum sleep (~2–4 hours actual observed sleep). Typical sleep onset in this study averaged ~20 minutes (Figure 2G), but did range as high as 60+ minutes in some animals (Supplementary Figure S5). Thus, at least a 3-hour sleep period is likely ideal for assessing plasticity in future studies.
Prior work demonstrated that sleep is required for the induction of V1 SRP and has provided insights into the circuit mechanisms by which sleep consolidates SRP [14, 26]. During a non-rapid eye movement (NREM) sleep period of consolidation, coherence increases between spiking in the thalamic lateral geniculate nucleus (LGN) and spindle-frequency (7–15 Hz) LFPs in V1. Critically, inhibition of corticothalamic inputs to LGN during NREM sleep, but not REM sleep, blocks this SRP consolidation [26]. Here, we have not yet assessed the role of REM versus NREM sleep in consolidating sequence plasticity; this is an inherent limit to video-based sleep scoring. While both SRP and sequence plasticity are expressed through increased firing rates in V1 and increased VEP magnitudes [11, 15], there is substantial evidence suggesting that SRP and sequence plasticity are mechanistically distinct. First, SRP is NMDAR-dependent but sequence plasticity persists in the presence of systemic NMDAR antagonists at a dose that blocks SRP [11, 13, 15]. Second, hippocampal lesions block sequence plasticity in V1, while V1 SRP persists in mice with hippocampal lesions [27]. Third, experience-dependent plasticity associated with SRP has been observed in multiple layers and cell types (including inhibitory PV + and SOM + cells) in V1 [28, 29], while less is currently known about the microcircuit loci of V1 sequence plasticity. Finally, sequence plasticity requires cholinergic input, and specifically, activation of M2 mAChRs, in V1, whereas SRP does not [15, 16]. This difference between SRP and sequence plasticity is of particular interest because while SRP is consolidated during NREM sleep, increased cholinergic activity is a well-known feature of REM sleep [30–32]. Future work is needed to understand the mechanisms by which sleep consolidates sequence plasticity and to test the hypothesis that cholinergic activity during REM sleep is important for sequence plasticity but not SRP.
Neural sequence reactivation is thought to play an important role in memory consolidation and recall, though the functional details and specific role of sleep remain elusive [33]. Previous works linking sleep-dependent memory formation with various forms of replay have focused primarily on episodic memory in hippocampus [34] which is not generally thought to be functionally involved with low-level vision. Recent works, however, have established that navigational signals in the medial temporal lobe can modulate mouse V1 [35], that coordinated replay occurs between hippocampus and V1 during sleep [36, 37], and that hippocampal lesions prevent sequence plasticity despite having no impact on V1 VEPs [27]. Together, the evidence suggests that some form of coordinated sequence replay during sleep is required for visual sequence consolidation, likely involving a cortico-hippocampal-cortical loop similar to that associated with memory consolidation in the auditory cortex [38]. Another possibility is that sleep reveals sequence coding plasticity established during visual exposure by renormalizing net synaptic strengths. In this scenario, plasticity events that encode spatiotemporal expectations occur regardless of whether the animals sleep but are effectively masked by stochastic plasticity events. By homeostatically rebalancing cortical synapses [8], sleep increases the signal-to-noise of sequence-specific VEPs and reveals an otherwise obscured time-dependent plasticity process. Given the magnitude of plasticity (100 of µV) it is unlikely that a lack of renormalization could completely mask the VEP potentiation as seen here, and the requirement for some form of coordinated activity replay seems the most likely explanation for our results. It is interesting to note that SRP is not disrupted by hippocampal lesioning, implying that the mechanistic role of sleep in visual plasticity consolidation may differ based on the type of memory or temporal complexity of visual information. This distinction could explain why ad libitum sleep following deprivation completely restores sequence plasticity, an observation seemingly at odds with previous studies showing that episodic memory does not recover following sleep loss [39].
In this study, we quantified sequence plasticity by measuring changes in V1 layer 4 VEPs as a function of experience. The VEP, by definition, is a measure of evoked activity in the LFP [18], representing population-level synaptic activity and not single-unit spiking [40]. In contrast, much of the prior work on the role of sleep in SRP assessed plasticity by quantifying the orientation specificity of single units [14, 26]. While these methods are not interchangeable, we are confident that they ultimately are measuring the same underlying phenomena: both SRP and sequence plasticity have been validated using VEPs and single-unit spiking in V1 [11, 13, 15]. An advantage of directly measuring single-unit activity is that it provides the resolution to compare excitatory and inhibitory cells, whereas an advantage of the VEP is its translatability to humans. For example, VEPs may also be quantified from surface EEGs, and a human study using a similar task design reported changes in VEP timing to familiar visual sequences [41].
Overall, this work demonstrated that a period of sleep is necessary to consolidate experience-dependent plasticity in V1 to visual sequences. The requirement for sleep in consolidating sequence plasticity is similar to the known requirement for sleep in consolidating SRP, but it is not yet known if the mechanisms underlying this consolidation are shared.
Supplementary material
Supplementary material is available at SLEEP online.
Acknowledgments
This work was supported by NIH grants R00EY028964 (to MSS) and R01EY030200 (to JPG).
Disclosure Statement
Financial Disclosure: None. Non-financial Disclosure: None.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.





Comments