-
PDF
- Split View
-
Views
-
Cite
Cite
Afsara B Zaheed, Ronald D Chervin, Adam P Spira, Laura B Zahodne, Mental and physical health pathways linking insomnia symptoms to cognitive performance 14 years later, Sleep, Volume 46, Issue 3, March 2023, zsac262, https://doi.org/10.1093/sleep/zsac262
Close - Share Icon Share
Abstract
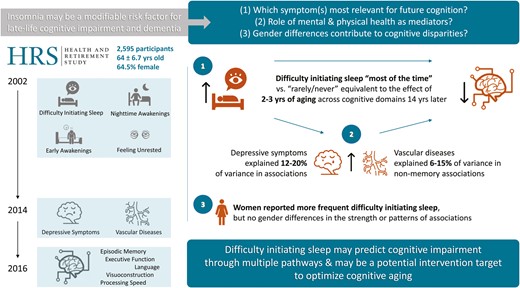
Insomnia may be a modifiable risk factor for later-life cognitive impairment. We investigated: (1) which insomnia symptoms are associated with subsequent cognitive functioning across domains; (2) whether insomnia–cognition associations are mediated by mental and physical health; and (3) whether these associations are modified by gender.
Participants included 2595 adults ages 51–88 at baseline (Mage=64.00 ± 6.66, 64.5% women) in the Health and Retirement Study. The frequency of insomnia symptoms (difficulty initiating sleep, night time awakenings, early awakenings, and feeling unrested upon awakening) at baseline (2002) were quantified using a modified Jenkins Sleep Questionnaire. Cognition was assessed in 2016 via the Harmonized Cognitive Assessment Protocol and operationalized with factor scores corresponding to five domains. Depressive symptoms and vascular conditions in 2014 were assessed via self-report. Structural equation models estimated total, indirect, and direct effects of insomnia symptoms on subsequent cognition through depressive symptoms and vascular diseases, controlling for baseline sociodemographic and global cognition.
Frequent difficulty initiating sleep was associated with poorer episodic memory, executive function, language, visuoconstruction, and processing speed 14 years later (−0.06 ≤ β ≤ −0.04; equivalent to 2.2–3.4 years of aging). Depressive symptoms explained 12.3%–19.5% of these associations and vascular disease explained 6.3%–14.6% of non-memory associations. No other insomnia symptoms were associated with cognition, and no associations were modified by gender.
Difficulty initiating sleep in later life may predict future cognitive impairment through multiple pathways. Future research with longitudinal assessments of insomnia, insomnia treatments, and cognition is needed to evaluate insomnia as a potential intervention target to optimize cognitive aging.
Sleep problems are common among older adults, and growing evidence points to insomnia as a potentially modifiable risk factor for later-life cognitive impairment and dementia. However, the clinical presentation of insomnia is heterogenous, and prior research on the effects of insomnia on subsequent cognition has rarely employed comprehensive neuropsychological measures. Moreover, investigating potential biopsychosocial mechanisms underlying prospective insomnia–cognition associations may help to identify targets for interventions aimed at promoting healthy cognitive aging and reducing disparities. Thus, we investigated which insomnia symptoms are associated with subsequent cognitive function across five domains. We also investigated whether insomnia–cognition associations are explained by depressive symptoms or vascular disease burden and whether they differ between men and women.
Introduction
Preservation of cognitive functioning is an important component of healthy aging [1], and progressive cognitive impairment is a core clinical feature of Alzheimer’s disease and related dementias. Prior research suggests that approximately one-third of dementia risk may be modifiable [2]. Given the global public health burden of dementia and present lack of disease-modifying treatments [3], identification of biopsychosocial factors that can be modified to prevent or slow the progression of cognitive impairment in later life is crucial. Growing evidence points to sleep as one such factor [4]. A meta-analysis suggests that approximately 15% of late-life cognitive impairment and Alzheimer’s disease may be partially attributable to sleep problems [5], and insomnia, specifically, may be a risk factor for later life cognitive impairment, decline, and dementia (see [6–9] for a review).
Investigating the relationship between insomnia and cognitive impairment in later life using finer-grained measurements of both insomnia and cognition may help reveal mechanisms underlying their associations, and consequently, identify targets for interventions aimed at reducing late life cognitive disparities and overall dementia risk. However, mixed evidence has been published on the strength and directionality of potential influence [10], which may reflect methodological issues such as cross-sectional vs. longitudinal study designs or differences in the measurement of insomnia and cognition across studies. Though clinically meaningful, the use of sum scores of self-reported insomnia symptoms (such as those provided by the Insomnia Severity Index [11]) in prior research has made it difficult to discern whether certain symptoms (e.g. night time awakenings) are more strongly related to or detrimental for cognitive outcomes than others. Moreover, the heterogeneity of insomnia presentation and treatment across individuals has made it difficult to interpret the meaning of sum scores.
Objective cognitive assessments are used clinically to identify domain-specific deficits and determine the severity of cognitive impairment, both of which can inform diagnosis [12] and treatment recommendations. A meta-analysis investigating concurrent associations between insomnia and various cognitive outcomes found that individuals with insomnia generally perform worse on tasks of episodic memory, working memory, and aspects of executive functions [13]. While separate studies have shown insomnia to be associated with declines in memory [14] and executive functioning [15] among older adults, none have investigated the prospective effects of insomnia on multiple domains of cognition within a single sample.
Previous research on insomnia—cognition associations across different domains within the same sample has included concurrent measures of sleep and cognition [16–19]. This methodology may obfuscate the nature of insomnia–cognition associations, given evidence of bidirectional associations between sleep and cognitive disorders [20]. Moreover, methodological differences in the study samples (e.g. small, clinic-based [17] vs. large, epidemiological [16]; midlife vs. older adult) and measurement of cognition (e.g. computer-based assessments [19] vs. traditional oral or paper and pencil assessments; using different tests to measure the same construct) in prior research likely contribute to their inconsistent findings regarding the effects of insomnia on late-life cognitive functioning across domains. A review of the extant literature suggests that composite scores representing performances in cognitive domains may be more sensitive than individual cognitive tests for detecting group differences (i.e. between insomnia vs. control groups) [21]. Thus, investigating the specificity of insomnia–cognition associations (i.e. which symptoms matter, and whether their associations are similar or differ across domains) may aid in discerning what mechanisms underlie their associations.
The relationship between insomnia and cognitive impairment likely reflects the effects of multifactorial underlying biopsychosocial mechanisms. Insomnia symptoms have been associated with various adverse mental and physical health outcomes throughout the lifespan [22, 23], including depression, diabetes, hypertension, and coronary artery diseases [24–27]. Notably, depression and vascular diseases are risk factors for cognitive impairment in later life [2, 28, 29], and may be modifiable intermediaries driving insomnia—cognition associations. There is also growing empirical evidence for gender disparities in insomnia [30–32] and late-life cognitive disorders such as Alzheimer’s disease [33–35], and these disparities may reflect both biological sex differences between males and females as well as differences in the lived social experiences of cis-gendered men and women throughout the life course. Differential risk of exposure or vulnerability to the impacts of insomnia symptoms may be one mechanism underlying gender inequalities in cognitive aging. Indeed, prevailing estimates suggest that women are approximately 1.5-times more likely to have insomnia, and this estimate increases to 1.73-times greater risk for women ages 65 and older [36]. A deeper understanding of these potential mechanisms is needed to inform the development of interventions that can reduce late-life cognitive morbidity and disparities in cognitive aging.
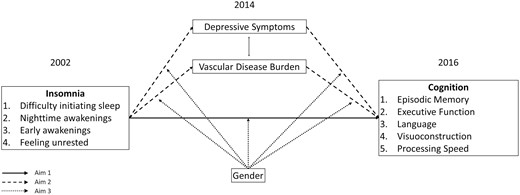
In the present time-lagged mediation study, we examined the association between specific insomnia symptoms and subsequent performance across five cognitive domains in a population-based sample of middle-aged and older adults in the United States (Aim 1). We also investigated the role of depressive symptoms and vascular diseases as potential mediators of these associations (Aim 2), and gender as a potential moderator (Aim 3). A conceptual model of these aims is depicted in Figure 1. We hypothesized that greater frequency of each insomnia symptom type in 2002 would be associated with lower cognitive performances across all domains in 2016, and these associations would be partially mediated through higher incident depressive symptoms and vascular disease burden in 2014. Finally, we explored whether gender interacted with insomnia, vascular disease, or depression to differentially influence cognitive outcomes between men and women.
Methods
Data source and participants
Publicly available data were drawn from three waves of the Health and Retirement Study (HRS), a US representative survey of adults age 51 and older followed biennially since 1992 with refresher samples added every six years to account for attrition and maintain the study design [37]. Detailed information regarding the HRS longitudinal panel design, sampling, and all variables are available on the HRS website (https://hrs.isr.umich.edu/). All participants provided written informed consent at the time of participation, and all study procedures were approved by the University of Michigan Institutional Review Board.
The core HRS interview began assessing self-reported insomnia symptoms at each wave in 2002. In 2016, the HRS conducted the Harmonized Cognitive Assessment Protocol (HCAP), a substudy focused on dementia. The HCAP protocol was developed to obtain comprehensive data on neuropsychological functioning using established measures that would harmonize with other longitudinal studies of aging [38, 39]. A random selection of 5500 participants age 65+ from the 2016 core HRS panel was invited to participate in the face-to-face substudy, and final data were available for 3496 individuals. Participants from the HCAP sample were included in the current study if they also had available data on insomnia symptoms and relevant covariates in 2002.
Exposures: insomnia symptoms
Insomnia symptoms were measured using a modified version of the Jenkins Sleep Questionnaire [40], which included the following four items: How often do you have trouble falling asleep? How often do you have trouble with waking up during the night? How often do you have trouble with waking up too early and not being able to fall asleep again? How often do you feel really rested when you wake up in the morning? Prior research in the HRS has found the prevalence rates of sleep problems measured using the modified Jenkins Sleep Questionnaire to be similar to other studies conducted in older adults [41]. Participants rated the frequency of these symptoms on the following scale: (1) most of the time, (2) sometimes, or (3) rarely or never. Responses for difficulty initiating sleep, night time awakenings, and early awakenings were reverse coded such that higher scores reflected poorer sleep across all four insomnia variables.
Cognitive outcomes
Cognition in 2016 was operationalized using a factor score for each of the five distinct cognitive domains: episodic memory (EM), executive functioning (EF), language (Lang), processing speed (PS), and visuospatial and construction abilities (i.e. visuoconstruction, VC). The factors were obtained through a prior confirmatory factor analysis [42] on the administered HCAP cognitive battery based on theoretical groupings [38]. A list of each cognitive test, grouped by domain, is summarized in Table 1. For each domain, higher scores reflected better cognitive performance.
| Cognitive domain . | Neuropsychological test (sub-test) . |
|---|---|
| Episodic Memory (EM) | CERAD Word List (immediate, delayed recall, and recognition trials) |
| CERAD Constructional Praxis (delayed recall trial) | |
| WMS-IV Logical Memory (immediate and delayed recall trials) | |
| Brave Man story task (immediate and delayed recall trials) | |
| MMSE Word List delayed recall | |
| Executive Function (EF) | Number Series |
| Raven’s Standard Progressive Matrices | |
| Trail-Making Test Part B (time) | |
| Language (Lang) | Animal fluency |
| TICS Verbal Description Naming | |
| MMSE Visual Confrontation Naming | |
| MMSE Sentence Writing | |
| Visuoconstruction (VC) | CERAD Constructional Praxis (copy trial) |
| MMSE Polygons Copy | |
| Processing Speed (PS) | Symbol Digits Modalities Test |
| Trail-Making Test Part A (time) | |
| Backwards Counting | |
| Letter Cancellation |
| Cognitive domain . | Neuropsychological test (sub-test) . |
|---|---|
| Episodic Memory (EM) | CERAD Word List (immediate, delayed recall, and recognition trials) |
| CERAD Constructional Praxis (delayed recall trial) | |
| WMS-IV Logical Memory (immediate and delayed recall trials) | |
| Brave Man story task (immediate and delayed recall trials) | |
| MMSE Word List delayed recall | |
| Executive Function (EF) | Number Series |
| Raven’s Standard Progressive Matrices | |
| Trail-Making Test Part B (time) | |
| Language (Lang) | Animal fluency |
| TICS Verbal Description Naming | |
| MMSE Visual Confrontation Naming | |
| MMSE Sentence Writing | |
| Visuoconstruction (VC) | CERAD Constructional Praxis (copy trial) |
| MMSE Polygons Copy | |
| Processing Speed (PS) | Symbol Digits Modalities Test |
| Trail-Making Test Part A (time) | |
| Backwards Counting | |
| Letter Cancellation |
CERAD = Consortium to Establish a Registry for Alzheimer’s Disease, WMS-IV = Wechsler Memory Scale-IV, MMSE = Mini-Mental State Exam, TICS = Telephone Interview for Cognitive Status.
| Cognitive domain . | Neuropsychological test (sub-test) . |
|---|---|
| Episodic Memory (EM) | CERAD Word List (immediate, delayed recall, and recognition trials) |
| CERAD Constructional Praxis (delayed recall trial) | |
| WMS-IV Logical Memory (immediate and delayed recall trials) | |
| Brave Man story task (immediate and delayed recall trials) | |
| MMSE Word List delayed recall | |
| Executive Function (EF) | Number Series |
| Raven’s Standard Progressive Matrices | |
| Trail-Making Test Part B (time) | |
| Language (Lang) | Animal fluency |
| TICS Verbal Description Naming | |
| MMSE Visual Confrontation Naming | |
| MMSE Sentence Writing | |
| Visuoconstruction (VC) | CERAD Constructional Praxis (copy trial) |
| MMSE Polygons Copy | |
| Processing Speed (PS) | Symbol Digits Modalities Test |
| Trail-Making Test Part A (time) | |
| Backwards Counting | |
| Letter Cancellation |
| Cognitive domain . | Neuropsychological test (sub-test) . |
|---|---|
| Episodic Memory (EM) | CERAD Word List (immediate, delayed recall, and recognition trials) |
| CERAD Constructional Praxis (delayed recall trial) | |
| WMS-IV Logical Memory (immediate and delayed recall trials) | |
| Brave Man story task (immediate and delayed recall trials) | |
| MMSE Word List delayed recall | |
| Executive Function (EF) | Number Series |
| Raven’s Standard Progressive Matrices | |
| Trail-Making Test Part B (time) | |
| Language (Lang) | Animal fluency |
| TICS Verbal Description Naming | |
| MMSE Visual Confrontation Naming | |
| MMSE Sentence Writing | |
| Visuoconstruction (VC) | CERAD Constructional Praxis (copy trial) |
| MMSE Polygons Copy | |
| Processing Speed (PS) | Symbol Digits Modalities Test |
| Trail-Making Test Part A (time) | |
| Backwards Counting | |
| Letter Cancellation |
CERAD = Consortium to Establish a Registry for Alzheimer’s Disease, WMS-IV = Wechsler Memory Scale-IV, MMSE = Mini-Mental State Exam, TICS = Telephone Interview for Cognitive Status.
Mediators: depressive symptoms and vascular disease burden
Depressive symptoms in 2014 were assessed with eight items from the Center for Epidemiologic Studies Depression scale (CES-D) [43]. Items were modified into a yes/no format and summed such that higher scores corresponded to more depressive symptoms. Prior research has demonstrated the dichotomized short-form CES-D to have good internal consistency (α = .72) and factor structure in line with the original 20-item version [44] in adults over age 50. Emerging research suggests that the risk for cognitive decline and progression of Alzheimer’s disease increases as the number of cumulative cardiovascular and cerebrovascular diseases increases [45]. Thus, in line with prior research [46, 47], vascular disease burden in 2014 was quantified as the sum of the self-reported presence of the following conditions: hypertension, heart disease, diabetes, and stroke. Responses ranged from 0 to 4, with higher values indicating greater vascular disease burden.
Covariates
Age, gender (Aims 1 and 2), race and ethnicity, level of education, household wealth (a marker of socioeconomic status), and marital status at study baseline (2002) were included as sociodemographic covariates given their known associations with both insomnia [48] and late life cognition [49]. Age was participants’ age in years at study baseline. Participants self-reported their sex and/or gender (hereafter referred to as gender) on a binary scale (men = 0, women = 1). Race and ethnicity were self-reported, and participants were coded into the following mutually exclusive groups: non-Hispanic White (NHW), non-Hispanic Black (NHB), Hispanic of any race, and non-Hispanic Others (NHO). The largest group, NHW, served as the reference category. Education was participants’ self-reported years of education, a continuous variable (0–17). Net household wealth was a continuous variable calculated as the sum of assets minus debts. Marital status was self-reported and coded as a binary variable (0 = never married, divorced/separated, or widowed; 1 = currently married/partnered).
Baseline (2002) measures of both hypothesized mediators were also included as covariates to strengthen causal inferences [50] between insomnia and changes in depressive symptoms and vascular diseases between 2002 and 2014. In other words, variance in depressive symptoms and vascular diseases in 2014 reflect changes from 2002 to 2014. As the comprehensive HCAP neuropsychological battery was not administered in the HRS until 2016, we also covaried for an available 2002 measure of global cognition given that cognitive functioning earlier in life is one of the strongest predictors of cognitive functioning in older adulthood [51]. Thus, cognitive outcomes as modeled for 2016 reflect only variance that is not explained by global cognition in 2002 and approximates cognitive changes from 2002 to 2016. Global cognition in 2002 was measured using the modified Telephone Interview for Cognitive Status (TICS) [52], which includes subtests assessing immediate and delayed memory, attention, processing speed, and working memory. The TICS was a continuous variable (0–27) with higher scores indicating better baseline global cognition.
Analytic strategy
Descriptive statistics and gender differences in the variables of interest were analyzed in SPSS version 27, with statistical significance set at p < .05. Structural equation models in MPlus version 8.2 [53] estimated the effects of baseline (2002) insomnia symptoms on subsequent (2016) cognitive performances independently and via physical and psychological health mediators (2014). Sampling weights were not available for the HCAP sample at the time of this study; thus, analyses were conducted on unweighted data. Missing data were managed using Full Information Maximum Likelihood (FIML), the default approach in MPlus, which accounts for non-random missingness related to any variables included in the models; model estimates are produced using all available data [54]. Model fit was evaluated with the following commonly used indices: comparative fit index (CFI), Tucker-Lewis index (TLI), root-mean-square error of approximation (RMSEA), and standardized root-mean square residual (SRMR). CFI > 0.95, TLI > 0.95, RMSEA < 0.05, and SRMR < 0.06 were used as criteria for adequate model fit [55].
In the Aim 1 models, CFA-derived factor scores of the five cognitive domains (EM, EF, Lang, PS, and VC) in 2016 were each regressed onto the four insomnia exposure variables (difficulty initiating sleep, night time awakenings, early awakenings, and feeling unrested) and all covariates measured in 2002. Separate models were conducted for each cognitive domain. The four insomnia variables were each regressed onto the covariates. Correlations were allowed among the four insomnia variables and between the two covariates due to their well-established relationships in the literature [56, 57] and to more accurately reproduce the covariance matrix and achieve adequate model fit, which is required for interpreting parameter estimates from a structural equation model.
In the Aim 2 (i.e. mediation) models, each of the cognitive domains measured in 2016 was separately regressed onto depressive symptoms and vascular disease burden measured in 2014, as well as on the insomnia exposures and covariates measured in 2002. Mediation was only examined for the insomnia exposures that were associated with the cognitive outcomes in the Aim 1 analyses [58]. The 2014 mediators were modeled simultaneously, allowed to covary, and also regressed onto the 2002 insomnia exposures and covariates. Indirect effects in the mediation models refer to the product of the association between (a) an insomnia symptom and a single mediator and (b) the association between that mediator and a cognitive outcome, independent of all covariates. Direct effects refer to the association between an insomnia symptom and a cognitive outcome, independent of both mediators and all covariates. Total effects, which are equivalent to the effects estimated in Aim 1, refer to the sum of direct and indirect effects and correspond to the association between an insomnia symptom and a cognitive outcome, independent of all covariates.
In the Aim 3 (i.e. moderated mediation) models, multiplicative interaction terms between binary gender and each of the insomnia exposures, as well as between gender and each mediator were computed. All continuous variables were mean-centered prior to the creation of interaction terms. Models included a single insomnia-by-gender interaction at a time alongside both mediator-by-gender terms to facilitate interpretations, and all interaction terms were regressed onto their constituent parts.
As the models included multiple exposures, mediators, and outcomes, all analyses were adjusted using the Benjamini–Hochberg method to control for multiple comparisons and reduce the chance of making type I errors [59, 60]. The overall false discovery rate (FDR) was set at 0.05 for all SEM models. The clinical relevance of the primary results was determined by comparing the magnitude of unstandardized effects of insomnia symptoms to that of age, the greatest known risk factor for dementia. Unstandardized effects quantify the difference in the cognitive outcome corresponding to a one-unit increase in the predictor. Specifically, the effect of reporting an insomnia symptom “most of the time” compared to “rarely or never” represents a two-unit increase in that predictor, and the unstandardized effect of age corresponds to an age increase of one year. For any insomnia symptom found to be significantly associated with cognition, the equivalent years of aging were computed [61] with the following equation: [2*unstandardized effect of insomnia symptom on cognitive domain]/unstandardized effect of age on that cognitive domain.
Results
Of the 3496 individuals in the HCAP substudy, n = 688 were excluded from the current analyses due to HRS entry after 2002 resulting in non-availability of data on all study exposures and covariates. An additional n = 213 individuals who entered the HRS in or before 2002 were excluded due to missing data on exposures or covariates in 2002. Descriptive characteristics of the 2595 participants included in the final analyses are provided in Table 2. Compared to the final analytic sample, individuals who were excluded due to missing data on exposures and covariates in 2002 were more likely to be married/partnered, male, Hispanic, and report race other than Black or White. These excluded participants also had fewer years of education, less wealth, reported fewer night time awakenings, and had lower scores across all cognitive outcomes. Bivariate correlations between study variables of interest are provided in Supplementary Table S1
| . | Full sample (N = 2595) . | Men (n = 920) . | Women (n = 1675) . | Effect size† . | |||
|---|---|---|---|---|---|---|---|
| Variable [sample range] . | Mean . | SD . | Mean . | SD . | Mean . | SD . | |
| Covariates | |||||||
| Age [51–88] | 64.00 | 6.76 | 64.22 | 6.27 | 63.88 | 7.02 | 0.05 |
| Gender (% women) | 64.5 | – | – | – | – | – | – |
| Race/ethnicity (%) | |||||||
| Non-Hispanic White | 76.0 | – | 81.7 | – | 72.9 | – | .10 |
| Non-Hispanic Black | 13.5 | – | 9.8 | – | 15.5 | – | .08*** |
| Non-Hispanic Other | 1.8 | – | 1.3 | – | 2.1 | – | .03 |
| Hispanic, any race | 8.7 | – | 7.2 | – | 9.5 | – | .04* |
| Education [0–17] | 12.66 | 3.13 | 13.02 | 3.17 | 12.46 | 3.10 | 0.18*** |
| Wealth‡ [−2.66 to 331.95] | 4.25 | 9.50 | 4.88 | 12.81 | 3.90 | 7.04 | 0.10* |
| Marital status (% married/partnered) | 73.8 | – | 85.9 | – | 67.2 | – | .20*** |
| Depressive symptoms [0–8] | 1.26 | 1.85 | 0.95 | 1.60 | 1.43 | 1.95 | 0.26*** |
| Vascular burden [0–4] | 0.73 | 0.82 | 0.79 | 0.82 | 0.70 | 0.82 | 0.10* |
| Global cognition [2–27] | 16.72 | 4.10 | 16.20 | 3.84 | 17.01 | 4.22 | 0.20*** |
| Exposures | |||||||
| Difficulty initiating sleep [1–3] | 1.49 | 0.67 | 1.33 | 0.57 | 1.58 | 0.71 | 0.38*** |
| 1. Rarely or never (%) | 60.9 | – | 72.4 | – | 54.6 | – | – |
| 2. Sometimes (%) | 29.0 | – | 22.3 | – | 32.7 | – | – |
| 3. Most of the Time (%) | 10.1 | – | 5.3 | – | 12.7 | – | – |
| Night time awakenings [1–3] | 1.85 | 0.77 | 1.77 | 0.75 | 1.89 | 0.78 | 0.15*** |
| 1. Rarely or Never (%) | 38.3 | – | 42.0 | – | 36.3 | – | – |
| 2. Sometimes (%) | 38.5 | – | 38.8 | – | 38.3 | – | – |
| 3. Most of the time (%) | 23.2 | – | 19.2 | – | 25.4 | – | – |
| Early morning awakening [1–3] | 1.52 | 0.68 | 1.46 | 0.65 | 1.55 | 0.69 | 0.13** |
| 1. Rarely or never (%) | 58.5 | – | 62.8 | – | 56.1 | – | – |
| 2. Sometimes (%) | 31.1 | – | 28.3 | – | 32.7 | – | – |
| 3. Most of the time (%) | 10.4 | – | 8.9 | – | 11.2 | – | – |
| Feeling rested [1–3] | 1.48 | 0.70 | 1.42 | 0.66 | 1.52 | 0.71 | 0.14*** |
| 1. Most of the time (%) | 63.6 | – | 67.9 | – | 61.2 | – | – |
| 2. Sometimes (%) | 24.6 | – | 22.2 | – | 26.0 | – | – |
| 3. Rarely or never (%) | 11.8 | – | 9.9 | – | 12.8 | – | – |
| Mediators | |||||||
| Depressive symptoms [0–8] | 1.29 | 1.87 | 0.99 | 1.66 | 1.46 | 1.96 | 0.25*** |
| Vascular burden [0–4] | 1.40 | 1.00 | 1.48 | 1.00 | 1.36 | 1.00 | 0.13** |
| Outcomes | |||||||
| Episodic memory [−15.66 to 9.10] | −0.32 | 4.12 | −0.82 | 3.72 | −0.05 | 4.30 | 0.19*** |
| Executive function [−11.54 to 5.52] | −0.41 | 3.11 | −0.16 | 2.97 | −0.55 | 3.18 | 0.13** |
| Language [−17.89 to 12.28] | −0.41 | 4.66 | −0.51 | 4.33 | −0.36 | 4.83 | 0.03 |
| Visuoconstruction [−5.89 to 2.78] | −0.14 | 1.57 | 0.00 | 1.48 | −0.21 | 1.60 | 0.14*** |
| Processing speed [−1.88 to 1.02] | −0.06 | 0.44 | −0.06 | 0.41 | −0.06 | 0.46 | 0.00 |
| . | Full sample (N = 2595) . | Men (n = 920) . | Women (n = 1675) . | Effect size† . | |||
|---|---|---|---|---|---|---|---|
| Variable [sample range] . | Mean . | SD . | Mean . | SD . | Mean . | SD . | |
| Covariates | |||||||
| Age [51–88] | 64.00 | 6.76 | 64.22 | 6.27 | 63.88 | 7.02 | 0.05 |
| Gender (% women) | 64.5 | – | – | – | – | – | – |
| Race/ethnicity (%) | |||||||
| Non-Hispanic White | 76.0 | – | 81.7 | – | 72.9 | – | .10 |
| Non-Hispanic Black | 13.5 | – | 9.8 | – | 15.5 | – | .08*** |
| Non-Hispanic Other | 1.8 | – | 1.3 | – | 2.1 | – | .03 |
| Hispanic, any race | 8.7 | – | 7.2 | – | 9.5 | – | .04* |
| Education [0–17] | 12.66 | 3.13 | 13.02 | 3.17 | 12.46 | 3.10 | 0.18*** |
| Wealth‡ [−2.66 to 331.95] | 4.25 | 9.50 | 4.88 | 12.81 | 3.90 | 7.04 | 0.10* |
| Marital status (% married/partnered) | 73.8 | – | 85.9 | – | 67.2 | – | .20*** |
| Depressive symptoms [0–8] | 1.26 | 1.85 | 0.95 | 1.60 | 1.43 | 1.95 | 0.26*** |
| Vascular burden [0–4] | 0.73 | 0.82 | 0.79 | 0.82 | 0.70 | 0.82 | 0.10* |
| Global cognition [2–27] | 16.72 | 4.10 | 16.20 | 3.84 | 17.01 | 4.22 | 0.20*** |
| Exposures | |||||||
| Difficulty initiating sleep [1–3] | 1.49 | 0.67 | 1.33 | 0.57 | 1.58 | 0.71 | 0.38*** |
| 1. Rarely or never (%) | 60.9 | – | 72.4 | – | 54.6 | – | – |
| 2. Sometimes (%) | 29.0 | – | 22.3 | – | 32.7 | – | – |
| 3. Most of the Time (%) | 10.1 | – | 5.3 | – | 12.7 | – | – |
| Night time awakenings [1–3] | 1.85 | 0.77 | 1.77 | 0.75 | 1.89 | 0.78 | 0.15*** |
| 1. Rarely or Never (%) | 38.3 | – | 42.0 | – | 36.3 | – | – |
| 2. Sometimes (%) | 38.5 | – | 38.8 | – | 38.3 | – | – |
| 3. Most of the time (%) | 23.2 | – | 19.2 | – | 25.4 | – | – |
| Early morning awakening [1–3] | 1.52 | 0.68 | 1.46 | 0.65 | 1.55 | 0.69 | 0.13** |
| 1. Rarely or never (%) | 58.5 | – | 62.8 | – | 56.1 | – | – |
| 2. Sometimes (%) | 31.1 | – | 28.3 | – | 32.7 | – | – |
| 3. Most of the time (%) | 10.4 | – | 8.9 | – | 11.2 | – | – |
| Feeling rested [1–3] | 1.48 | 0.70 | 1.42 | 0.66 | 1.52 | 0.71 | 0.14*** |
| 1. Most of the time (%) | 63.6 | – | 67.9 | – | 61.2 | – | – |
| 2. Sometimes (%) | 24.6 | – | 22.2 | – | 26.0 | – | – |
| 3. Rarely or never (%) | 11.8 | – | 9.9 | – | 12.8 | – | – |
| Mediators | |||||||
| Depressive symptoms [0–8] | 1.29 | 1.87 | 0.99 | 1.66 | 1.46 | 1.96 | 0.25*** |
| Vascular burden [0–4] | 1.40 | 1.00 | 1.48 | 1.00 | 1.36 | 1.00 | 0.13** |
| Outcomes | |||||||
| Episodic memory [−15.66 to 9.10] | −0.32 | 4.12 | −0.82 | 3.72 | −0.05 | 4.30 | 0.19*** |
| Executive function [−11.54 to 5.52] | −0.41 | 3.11 | −0.16 | 2.97 | −0.55 | 3.18 | 0.13** |
| Language [−17.89 to 12.28] | −0.41 | 4.66 | −0.51 | 4.33 | −0.36 | 4.83 | 0.03 |
| Visuoconstruction [−5.89 to 2.78] | −0.14 | 1.57 | 0.00 | 1.48 | −0.21 | 1.60 | 0.14*** |
| Processing speed [−1.88 to 1.02] | −0.06 | 0.44 | −0.06 | 0.41 | −0.06 | 0.46 | 0.00 |
Covariates and exposures were measured in 2002, mediators in 2014, and outcomes in 2016.
†Cohen’s d (range 0–∞) reported for continuous variables and Phi coefficient (range 0–1) reported for categorical variables.
‡Reported in hundred-thousands.
*p < .05, **p < .01, ***p < .001.
| . | Full sample (N = 2595) . | Men (n = 920) . | Women (n = 1675) . | Effect size† . | |||
|---|---|---|---|---|---|---|---|
| Variable [sample range] . | Mean . | SD . | Mean . | SD . | Mean . | SD . | |
| Covariates | |||||||
| Age [51–88] | 64.00 | 6.76 | 64.22 | 6.27 | 63.88 | 7.02 | 0.05 |
| Gender (% women) | 64.5 | – | – | – | – | – | – |
| Race/ethnicity (%) | |||||||
| Non-Hispanic White | 76.0 | – | 81.7 | – | 72.9 | – | .10 |
| Non-Hispanic Black | 13.5 | – | 9.8 | – | 15.5 | – | .08*** |
| Non-Hispanic Other | 1.8 | – | 1.3 | – | 2.1 | – | .03 |
| Hispanic, any race | 8.7 | – | 7.2 | – | 9.5 | – | .04* |
| Education [0–17] | 12.66 | 3.13 | 13.02 | 3.17 | 12.46 | 3.10 | 0.18*** |
| Wealth‡ [−2.66 to 331.95] | 4.25 | 9.50 | 4.88 | 12.81 | 3.90 | 7.04 | 0.10* |
| Marital status (% married/partnered) | 73.8 | – | 85.9 | – | 67.2 | – | .20*** |
| Depressive symptoms [0–8] | 1.26 | 1.85 | 0.95 | 1.60 | 1.43 | 1.95 | 0.26*** |
| Vascular burden [0–4] | 0.73 | 0.82 | 0.79 | 0.82 | 0.70 | 0.82 | 0.10* |
| Global cognition [2–27] | 16.72 | 4.10 | 16.20 | 3.84 | 17.01 | 4.22 | 0.20*** |
| Exposures | |||||||
| Difficulty initiating sleep [1–3] | 1.49 | 0.67 | 1.33 | 0.57 | 1.58 | 0.71 | 0.38*** |
| 1. Rarely or never (%) | 60.9 | – | 72.4 | – | 54.6 | – | – |
| 2. Sometimes (%) | 29.0 | – | 22.3 | – | 32.7 | – | – |
| 3. Most of the Time (%) | 10.1 | – | 5.3 | – | 12.7 | – | – |
| Night time awakenings [1–3] | 1.85 | 0.77 | 1.77 | 0.75 | 1.89 | 0.78 | 0.15*** |
| 1. Rarely or Never (%) | 38.3 | – | 42.0 | – | 36.3 | – | – |
| 2. Sometimes (%) | 38.5 | – | 38.8 | – | 38.3 | – | – |
| 3. Most of the time (%) | 23.2 | – | 19.2 | – | 25.4 | – | – |
| Early morning awakening [1–3] | 1.52 | 0.68 | 1.46 | 0.65 | 1.55 | 0.69 | 0.13** |
| 1. Rarely or never (%) | 58.5 | – | 62.8 | – | 56.1 | – | – |
| 2. Sometimes (%) | 31.1 | – | 28.3 | – | 32.7 | – | – |
| 3. Most of the time (%) | 10.4 | – | 8.9 | – | 11.2 | – | – |
| Feeling rested [1–3] | 1.48 | 0.70 | 1.42 | 0.66 | 1.52 | 0.71 | 0.14*** |
| 1. Most of the time (%) | 63.6 | – | 67.9 | – | 61.2 | – | – |
| 2. Sometimes (%) | 24.6 | – | 22.2 | – | 26.0 | – | – |
| 3. Rarely or never (%) | 11.8 | – | 9.9 | – | 12.8 | – | – |
| Mediators | |||||||
| Depressive symptoms [0–8] | 1.29 | 1.87 | 0.99 | 1.66 | 1.46 | 1.96 | 0.25*** |
| Vascular burden [0–4] | 1.40 | 1.00 | 1.48 | 1.00 | 1.36 | 1.00 | 0.13** |
| Outcomes | |||||||
| Episodic memory [−15.66 to 9.10] | −0.32 | 4.12 | −0.82 | 3.72 | −0.05 | 4.30 | 0.19*** |
| Executive function [−11.54 to 5.52] | −0.41 | 3.11 | −0.16 | 2.97 | −0.55 | 3.18 | 0.13** |
| Language [−17.89 to 12.28] | −0.41 | 4.66 | −0.51 | 4.33 | −0.36 | 4.83 | 0.03 |
| Visuoconstruction [−5.89 to 2.78] | −0.14 | 1.57 | 0.00 | 1.48 | −0.21 | 1.60 | 0.14*** |
| Processing speed [−1.88 to 1.02] | −0.06 | 0.44 | −0.06 | 0.41 | −0.06 | 0.46 | 0.00 |
| . | Full sample (N = 2595) . | Men (n = 920) . | Women (n = 1675) . | Effect size† . | |||
|---|---|---|---|---|---|---|---|
| Variable [sample range] . | Mean . | SD . | Mean . | SD . | Mean . | SD . | |
| Covariates | |||||||
| Age [51–88] | 64.00 | 6.76 | 64.22 | 6.27 | 63.88 | 7.02 | 0.05 |
| Gender (% women) | 64.5 | – | – | – | – | – | – |
| Race/ethnicity (%) | |||||||
| Non-Hispanic White | 76.0 | – | 81.7 | – | 72.9 | – | .10 |
| Non-Hispanic Black | 13.5 | – | 9.8 | – | 15.5 | – | .08*** |
| Non-Hispanic Other | 1.8 | – | 1.3 | – | 2.1 | – | .03 |
| Hispanic, any race | 8.7 | – | 7.2 | – | 9.5 | – | .04* |
| Education [0–17] | 12.66 | 3.13 | 13.02 | 3.17 | 12.46 | 3.10 | 0.18*** |
| Wealth‡ [−2.66 to 331.95] | 4.25 | 9.50 | 4.88 | 12.81 | 3.90 | 7.04 | 0.10* |
| Marital status (% married/partnered) | 73.8 | – | 85.9 | – | 67.2 | – | .20*** |
| Depressive symptoms [0–8] | 1.26 | 1.85 | 0.95 | 1.60 | 1.43 | 1.95 | 0.26*** |
| Vascular burden [0–4] | 0.73 | 0.82 | 0.79 | 0.82 | 0.70 | 0.82 | 0.10* |
| Global cognition [2–27] | 16.72 | 4.10 | 16.20 | 3.84 | 17.01 | 4.22 | 0.20*** |
| Exposures | |||||||
| Difficulty initiating sleep [1–3] | 1.49 | 0.67 | 1.33 | 0.57 | 1.58 | 0.71 | 0.38*** |
| 1. Rarely or never (%) | 60.9 | – | 72.4 | – | 54.6 | – | – |
| 2. Sometimes (%) | 29.0 | – | 22.3 | – | 32.7 | – | – |
| 3. Most of the Time (%) | 10.1 | – | 5.3 | – | 12.7 | – | – |
| Night time awakenings [1–3] | 1.85 | 0.77 | 1.77 | 0.75 | 1.89 | 0.78 | 0.15*** |
| 1. Rarely or Never (%) | 38.3 | – | 42.0 | – | 36.3 | – | – |
| 2. Sometimes (%) | 38.5 | – | 38.8 | – | 38.3 | – | – |
| 3. Most of the time (%) | 23.2 | – | 19.2 | – | 25.4 | – | – |
| Early morning awakening [1–3] | 1.52 | 0.68 | 1.46 | 0.65 | 1.55 | 0.69 | 0.13** |
| 1. Rarely or never (%) | 58.5 | – | 62.8 | – | 56.1 | – | – |
| 2. Sometimes (%) | 31.1 | – | 28.3 | – | 32.7 | – | – |
| 3. Most of the time (%) | 10.4 | – | 8.9 | – | 11.2 | – | – |
| Feeling rested [1–3] | 1.48 | 0.70 | 1.42 | 0.66 | 1.52 | 0.71 | 0.14*** |
| 1. Most of the time (%) | 63.6 | – | 67.9 | – | 61.2 | – | – |
| 2. Sometimes (%) | 24.6 | – | 22.2 | – | 26.0 | – | – |
| 3. Rarely or never (%) | 11.8 | – | 9.9 | – | 12.8 | – | – |
| Mediators | |||||||
| Depressive symptoms [0–8] | 1.29 | 1.87 | 0.99 | 1.66 | 1.46 | 1.96 | 0.25*** |
| Vascular burden [0–4] | 1.40 | 1.00 | 1.48 | 1.00 | 1.36 | 1.00 | 0.13** |
| Outcomes | |||||||
| Episodic memory [−15.66 to 9.10] | −0.32 | 4.12 | −0.82 | 3.72 | −0.05 | 4.30 | 0.19*** |
| Executive function [−11.54 to 5.52] | −0.41 | 3.11 | −0.16 | 2.97 | −0.55 | 3.18 | 0.13** |
| Language [−17.89 to 12.28] | −0.41 | 4.66 | −0.51 | 4.33 | −0.36 | 4.83 | 0.03 |
| Visuoconstruction [−5.89 to 2.78] | −0.14 | 1.57 | 0.00 | 1.48 | −0.21 | 1.60 | 0.14*** |
| Processing speed [−1.88 to 1.02] | −0.06 | 0.44 | −0.06 | 0.41 | −0.06 | 0.46 | 0.00 |
Covariates and exposures were measured in 2002, mediators in 2014, and outcomes in 2016.
†Cohen’s d (range 0–∞) reported for continuous variables and Phi coefficient (range 0–1) reported for categorical variables.
‡Reported in hundred-thousands.
*p < .05, **p < .01, ***p < .001.
Differential associations between individual insomnia symptoms and cognitive domains
Standardized estimates from the Aim 1 models are reported in Table 3 and depicted in Figure 2. More frequent difficulty initiating sleep in 2002 was associated with poorer performance in EM (β = −0.0= 57, SE 0.018, p = .002), EF (β = −0.046, SE = 0.017, p = .005), Lang (β = −0.063, SE = 0.017, p < .001), VC (β = −0.040, SE = 0.018, p = .024), and PS (β = −0.040, SE = 0.017, p = .016) in 2016. To evaluate the clinical relevance of these effects, the magnitudes of the unstandardized effects of difficulty initiating sleep on EM (−.350), EF (−.217), Lang (−.437), VC (−.095), and PS (−.027) were compared to those of baseline age (−.257 to −.025) in the corresponding Aim 1 model. Reports of difficulty initiating sleep “most of the time” compared to “rarely/never” were equivalent to the effect of 3.06 years of aging on EM, 2.63 years on EF, 3.40 years on Lang, 2.68 years on VC, and 2.16 years on PS. After adjustment for difficulty initiating sleep, the other insomnia symptoms (night time awakenings, early awakenings, and feeling unrested in the morning) were not independently associated with any of the cognitive outcomes.
Multivariable-adjusted standardized regression path estimates between insomnia symptoms (and covariates) in 2002 and cognition in 2016
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Age | −0.372* | [−0.404, −0.341] | −0.355* | [−0.384, −0.325] | −0.370* | [−0.400, −0.339] | −0.303* | [−0.335, −0.271] | −0.389* | [−0.418, −0.360] |
| Woman | 0.093* | [0.060, 0.125] | −0.030 | [−0.060, 0.000] | 0.031 | [0.000. 0.062] | −0.039* | [−0.071, −0.007] | 0.019 | [−0.010, 0.049] |
| Non-Hispanic Black | −0.087* | [−0.120, −0.055] | −0.225* | [−0.256, −0.195] | −0.153* | [−0.184, −0.121] | −0.203* | [−0.236, −0.171] | −0.182* | [−0.212, −0.151] |
| Non-Hispanic other | −0.034 | [−0.065, −0.004] | −0.026 | [−0.054, 0.002] | −0.040* | [−0.070, −0.011] | −0.012 | [−0.043, 0.018] | −0.026 | [−0.054, 0.003] |
| Hispanic | −0.055* | [−0.090, −0.020] | −0.081* | [−0.113, −0.050] | −0.043* | [−0.076, −0.009] | −0.053* | [−0.087, −0.018] | −0.093* | [−0.125, −0.061] |
| Education | 0.174* | [0.136, 0.211] | 0.284* | [0.249, 0.318] | 0.232* | [0.196, 0.268] | 0.275* | [0.238, 0.312] | 0.253* | [0.218, 0.288] |
| Wealth | 0.012 | [−0.019, 0.043] | 0.018 | [−0.011, 0.046] | 0.027 | [−0.003, 0.057] | 0.007 | [−0.024, 0.038] | 0.007 | [−0.022, 0.036] |
| Marital status | 0.020 | [−0.013, 0.052] | 0.007 | [−0.023, 0.037] | 0.011 | [−0.020, 0.042] | 0.002 | [−0.031, 0.034] | 0.013 | [−0.017, 0.043] |
| Depressive symptoms | −0.014 | [−0.051, 0.022] | −0.037 | [−0.071, −0.003] | −0.022 | [−0.057, 0.013] | −0.037 | [−0.074, −0.001] | −0.049* | [−0.082, −0.015] |
| Vascular impacts | −0.032 | [−0.064, 0.000] | −0.070* | [−0.099, −0.040] | −0.046* | [−0.077, 0.016] | −0.067* | [−0.099, −0.035] | −0.077* | [−0.107, −0.048] |
| Global cognition | 0.287* | [0.253, 0.322] | 0.218* | [0.186, 0.250] | 0.268* | [0.235, 0.302] | 0.204* | [0.169, 0.239] | 0.231* | [0.199, 0.263] |
| Difficulty initiating sleep | −0.057* | [−0.092, −0.021] | −0.046* | [−0.079, −0.014] | −0.063* | [−0.096, −0.029] | −0.040* | [−0.076, −0.005] | −0.040* | [−0.073, −0.008] |
| Night time awakenings | 0.031 | [−0.003, 0.065] | 0.015 | [−0.016, 0.047] | 0.036 | [0.003, 0.069] | 0.019 | [−0.015, 0.053] | 0.013 | [−0.019, 0.044] |
| Early morning awakening | −0.003 | [−0.038, 0.032] | 0.011 | [−0.021, 0.043] | −0.003 | [−0.037, 0.030] | 0.004 | [−0.031, 0.039] | 0.018 | [−0.014, 0.050] |
| Feeling unrested | −0.002 | [−0.036, 0.033] | 0.000 | [−0.031, 0.032] | 0.002 | [−0.031, 0.035] | 0.008 | [−0.027, 0.042] | 0.010 | [−0.022, 0.042] |
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Age | −0.372* | [−0.404, −0.341] | −0.355* | [−0.384, −0.325] | −0.370* | [−0.400, −0.339] | −0.303* | [−0.335, −0.271] | −0.389* | [−0.418, −0.360] |
| Woman | 0.093* | [0.060, 0.125] | −0.030 | [−0.060, 0.000] | 0.031 | [0.000. 0.062] | −0.039* | [−0.071, −0.007] | 0.019 | [−0.010, 0.049] |
| Non-Hispanic Black | −0.087* | [−0.120, −0.055] | −0.225* | [−0.256, −0.195] | −0.153* | [−0.184, −0.121] | −0.203* | [−0.236, −0.171] | −0.182* | [−0.212, −0.151] |
| Non-Hispanic other | −0.034 | [−0.065, −0.004] | −0.026 | [−0.054, 0.002] | −0.040* | [−0.070, −0.011] | −0.012 | [−0.043, 0.018] | −0.026 | [−0.054, 0.003] |
| Hispanic | −0.055* | [−0.090, −0.020] | −0.081* | [−0.113, −0.050] | −0.043* | [−0.076, −0.009] | −0.053* | [−0.087, −0.018] | −0.093* | [−0.125, −0.061] |
| Education | 0.174* | [0.136, 0.211] | 0.284* | [0.249, 0.318] | 0.232* | [0.196, 0.268] | 0.275* | [0.238, 0.312] | 0.253* | [0.218, 0.288] |
| Wealth | 0.012 | [−0.019, 0.043] | 0.018 | [−0.011, 0.046] | 0.027 | [−0.003, 0.057] | 0.007 | [−0.024, 0.038] | 0.007 | [−0.022, 0.036] |
| Marital status | 0.020 | [−0.013, 0.052] | 0.007 | [−0.023, 0.037] | 0.011 | [−0.020, 0.042] | 0.002 | [−0.031, 0.034] | 0.013 | [−0.017, 0.043] |
| Depressive symptoms | −0.014 | [−0.051, 0.022] | −0.037 | [−0.071, −0.003] | −0.022 | [−0.057, 0.013] | −0.037 | [−0.074, −0.001] | −0.049* | [−0.082, −0.015] |
| Vascular impacts | −0.032 | [−0.064, 0.000] | −0.070* | [−0.099, −0.040] | −0.046* | [−0.077, 0.016] | −0.067* | [−0.099, −0.035] | −0.077* | [−0.107, −0.048] |
| Global cognition | 0.287* | [0.253, 0.322] | 0.218* | [0.186, 0.250] | 0.268* | [0.235, 0.302] | 0.204* | [0.169, 0.239] | 0.231* | [0.199, 0.263] |
| Difficulty initiating sleep | −0.057* | [−0.092, −0.021] | −0.046* | [−0.079, −0.014] | −0.063* | [−0.096, −0.029] | −0.040* | [−0.076, −0.005] | −0.040* | [−0.073, −0.008] |
| Night time awakenings | 0.031 | [−0.003, 0.065] | 0.015 | [−0.016, 0.047] | 0.036 | [0.003, 0.069] | 0.019 | [−0.015, 0.053] | 0.013 | [−0.019, 0.044] |
| Early morning awakening | −0.003 | [−0.038, 0.032] | 0.011 | [−0.021, 0.043] | −0.003 | [−0.037, 0.030] | 0.004 | [−0.031, 0.039] | 0.018 | [−0.014, 0.050] |
| Feeling unrested | −0.002 | [−0.036, 0.033] | 0.000 | [−0.031, 0.032] | 0.002 | [−0.031, 0.035] | 0.008 | [−0.027, 0.042] | 0.010 | [−0.022, 0.042] |
*Significant after adjustment for multiple comparisons using the Benjamini−Hochberg method.
CI= confidence interval.
Models were fully saturated, resulting in perfect model fit: CFI = 1.000; TLI = 1.000; RMSEA = 0.000 (90% CI [0.000, 0.000]); SRMR = 0.000.
Multivariable-adjusted standardized regression path estimates between insomnia symptoms (and covariates) in 2002 and cognition in 2016
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Age | −0.372* | [−0.404, −0.341] | −0.355* | [−0.384, −0.325] | −0.370* | [−0.400, −0.339] | −0.303* | [−0.335, −0.271] | −0.389* | [−0.418, −0.360] |
| Woman | 0.093* | [0.060, 0.125] | −0.030 | [−0.060, 0.000] | 0.031 | [0.000. 0.062] | −0.039* | [−0.071, −0.007] | 0.019 | [−0.010, 0.049] |
| Non-Hispanic Black | −0.087* | [−0.120, −0.055] | −0.225* | [−0.256, −0.195] | −0.153* | [−0.184, −0.121] | −0.203* | [−0.236, −0.171] | −0.182* | [−0.212, −0.151] |
| Non-Hispanic other | −0.034 | [−0.065, −0.004] | −0.026 | [−0.054, 0.002] | −0.040* | [−0.070, −0.011] | −0.012 | [−0.043, 0.018] | −0.026 | [−0.054, 0.003] |
| Hispanic | −0.055* | [−0.090, −0.020] | −0.081* | [−0.113, −0.050] | −0.043* | [−0.076, −0.009] | −0.053* | [−0.087, −0.018] | −0.093* | [−0.125, −0.061] |
| Education | 0.174* | [0.136, 0.211] | 0.284* | [0.249, 0.318] | 0.232* | [0.196, 0.268] | 0.275* | [0.238, 0.312] | 0.253* | [0.218, 0.288] |
| Wealth | 0.012 | [−0.019, 0.043] | 0.018 | [−0.011, 0.046] | 0.027 | [−0.003, 0.057] | 0.007 | [−0.024, 0.038] | 0.007 | [−0.022, 0.036] |
| Marital status | 0.020 | [−0.013, 0.052] | 0.007 | [−0.023, 0.037] | 0.011 | [−0.020, 0.042] | 0.002 | [−0.031, 0.034] | 0.013 | [−0.017, 0.043] |
| Depressive symptoms | −0.014 | [−0.051, 0.022] | −0.037 | [−0.071, −0.003] | −0.022 | [−0.057, 0.013] | −0.037 | [−0.074, −0.001] | −0.049* | [−0.082, −0.015] |
| Vascular impacts | −0.032 | [−0.064, 0.000] | −0.070* | [−0.099, −0.040] | −0.046* | [−0.077, 0.016] | −0.067* | [−0.099, −0.035] | −0.077* | [−0.107, −0.048] |
| Global cognition | 0.287* | [0.253, 0.322] | 0.218* | [0.186, 0.250] | 0.268* | [0.235, 0.302] | 0.204* | [0.169, 0.239] | 0.231* | [0.199, 0.263] |
| Difficulty initiating sleep | −0.057* | [−0.092, −0.021] | −0.046* | [−0.079, −0.014] | −0.063* | [−0.096, −0.029] | −0.040* | [−0.076, −0.005] | −0.040* | [−0.073, −0.008] |
| Night time awakenings | 0.031 | [−0.003, 0.065] | 0.015 | [−0.016, 0.047] | 0.036 | [0.003, 0.069] | 0.019 | [−0.015, 0.053] | 0.013 | [−0.019, 0.044] |
| Early morning awakening | −0.003 | [−0.038, 0.032] | 0.011 | [−0.021, 0.043] | −0.003 | [−0.037, 0.030] | 0.004 | [−0.031, 0.039] | 0.018 | [−0.014, 0.050] |
| Feeling unrested | −0.002 | [−0.036, 0.033] | 0.000 | [−0.031, 0.032] | 0.002 | [−0.031, 0.035] | 0.008 | [−0.027, 0.042] | 0.010 | [−0.022, 0.042] |
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Age | −0.372* | [−0.404, −0.341] | −0.355* | [−0.384, −0.325] | −0.370* | [−0.400, −0.339] | −0.303* | [−0.335, −0.271] | −0.389* | [−0.418, −0.360] |
| Woman | 0.093* | [0.060, 0.125] | −0.030 | [−0.060, 0.000] | 0.031 | [0.000. 0.062] | −0.039* | [−0.071, −0.007] | 0.019 | [−0.010, 0.049] |
| Non-Hispanic Black | −0.087* | [−0.120, −0.055] | −0.225* | [−0.256, −0.195] | −0.153* | [−0.184, −0.121] | −0.203* | [−0.236, −0.171] | −0.182* | [−0.212, −0.151] |
| Non-Hispanic other | −0.034 | [−0.065, −0.004] | −0.026 | [−0.054, 0.002] | −0.040* | [−0.070, −0.011] | −0.012 | [−0.043, 0.018] | −0.026 | [−0.054, 0.003] |
| Hispanic | −0.055* | [−0.090, −0.020] | −0.081* | [−0.113, −0.050] | −0.043* | [−0.076, −0.009] | −0.053* | [−0.087, −0.018] | −0.093* | [−0.125, −0.061] |
| Education | 0.174* | [0.136, 0.211] | 0.284* | [0.249, 0.318] | 0.232* | [0.196, 0.268] | 0.275* | [0.238, 0.312] | 0.253* | [0.218, 0.288] |
| Wealth | 0.012 | [−0.019, 0.043] | 0.018 | [−0.011, 0.046] | 0.027 | [−0.003, 0.057] | 0.007 | [−0.024, 0.038] | 0.007 | [−0.022, 0.036] |
| Marital status | 0.020 | [−0.013, 0.052] | 0.007 | [−0.023, 0.037] | 0.011 | [−0.020, 0.042] | 0.002 | [−0.031, 0.034] | 0.013 | [−0.017, 0.043] |
| Depressive symptoms | −0.014 | [−0.051, 0.022] | −0.037 | [−0.071, −0.003] | −0.022 | [−0.057, 0.013] | −0.037 | [−0.074, −0.001] | −0.049* | [−0.082, −0.015] |
| Vascular impacts | −0.032 | [−0.064, 0.000] | −0.070* | [−0.099, −0.040] | −0.046* | [−0.077, 0.016] | −0.067* | [−0.099, −0.035] | −0.077* | [−0.107, −0.048] |
| Global cognition | 0.287* | [0.253, 0.322] | 0.218* | [0.186, 0.250] | 0.268* | [0.235, 0.302] | 0.204* | [0.169, 0.239] | 0.231* | [0.199, 0.263] |
| Difficulty initiating sleep | −0.057* | [−0.092, −0.021] | −0.046* | [−0.079, −0.014] | −0.063* | [−0.096, −0.029] | −0.040* | [−0.076, −0.005] | −0.040* | [−0.073, −0.008] |
| Night time awakenings | 0.031 | [−0.003, 0.065] | 0.015 | [−0.016, 0.047] | 0.036 | [0.003, 0.069] | 0.019 | [−0.015, 0.053] | 0.013 | [−0.019, 0.044] |
| Early morning awakening | −0.003 | [−0.038, 0.032] | 0.011 | [−0.021, 0.043] | −0.003 | [−0.037, 0.030] | 0.004 | [−0.031, 0.039] | 0.018 | [−0.014, 0.050] |
| Feeling unrested | −0.002 | [−0.036, 0.033] | 0.000 | [−0.031, 0.032] | 0.002 | [−0.031, 0.035] | 0.008 | [−0.027, 0.042] | 0.010 | [−0.022, 0.042] |
*Significant after adjustment for multiple comparisons using the Benjamini−Hochberg method.
CI= confidence interval.
Models were fully saturated, resulting in perfect model fit: CFI = 1.000; TLI = 1.000; RMSEA = 0.000 (90% CI [0.000, 0.000]); SRMR = 0.000.
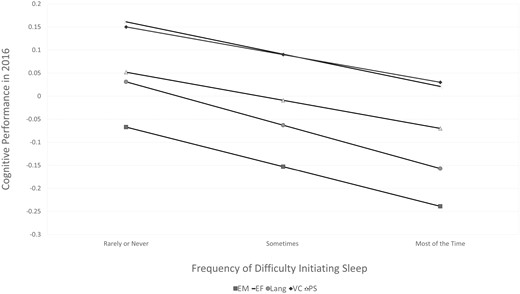
Standardized regression estimates of associations between difficulty initiating sleep in 2002 and cognition in 2016, adjusted for covariates. EM = episodic memory, EF = executive function, Lang = language, VC = visuoconstruction, PS = processing speed.
The robustness of these findings was investigated with the following sensitivity analyses: (1) running alternate models that included only one insomnia symptom at a time, (2) including body mass index (BMI) as an additional covariate, and (3) removing 2002 global cognition as a covariate. Including all four of the insomnia symptoms in the models concurrently may have reduced our ability to detect or accurately estimate the effects of any given symptom. However, difficulty initiating sleep at baseline remained the only insomnia symptom related to cognition in these individual models. The inclusion of baseline BMI as an additional covariate to account for unmeasured risk for comorbid sleep apnea also did not alter the primary findings, and BMI in 2002 was not significantly associated with any cognitive outcome. Finally, covarying for global cognition at baseline may have led to an overly conservative estimation of the effects of insomnia symptoms on subsequent cognitive functioning. However, results from models excluding global cognition in 2002 did not substantially differ from the primary findings, and difficulty initiating sleep remained the only insomnia symptom prospectively associated with all five domains of cognition.
Indirect effects of insomnia symptoms on cognition through mental and physical health
Given the Aim 1 results, potential mediation through depressive symptoms and vascular disease burden was only investigated for the effects of difficulty initiating sleep on cognition. Aim 2 results are summarized in Table 4 and depicted in Figure 3. There was an indirect effect of difficulty initiating sleep on all five cognitive domains through depressive symptoms. Specifically, more frequent difficulty initiating sleep in 2002 was associated with more depressive symptoms in 2014 (β = 0.095, SE = 0.020, p < .001), which was subsequently associated with poorer performance in EM (β = −0.070, SE = 0.018, p < .001), EF (β = −0.078, SE = 0.017, p < .001), Lang (β = −0.080, SE = 0.017, p < .001), VC (β = −0.072, SE = 0.018, p < .001), and PS (β = −0.086, SE = 0.017, p < .001) in 2016. Depressive symptoms accounted for 12.3% of the total effect of difficulty initiating sleep on EM, 14.8% of the effect on EF, 12.7% of the effect on Lang, 17.1% of the effect on VC, and 19.5% of the effect on PS.
Standardized specific indirect, total indirect, and direct path estimates between difficulty initiating sleep in 2002 and cognition in 2016 via the mediators
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Path . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Indirect via vascular diseases | −0.002 | [−0.004, −0.001] | −0.005* | [−0.009, −0.001] | −0.004* | [−0.008, −0.001] | −0.006* | [−0.011, −0.002] | −0.005* | [−0.009, −0.001] |
| Indirect via Depressive symptoms | −0.007* | [−0.011, −0.002] | −0.007* | [−0.012, −0.003] | −0.008* | [−0.012, −0.003] | −0.007* | [−0.011, −0.002] | −0.008* | [−0.013, −0.004] |
| Total indirect | −0.008* | [−0.013, −0.003] | −0.012* | [−0.018, −0.007] | −0.012* | [−0.018, −0.006] | −0.013* | [−0.019, −0.007] | −0.013* | [−0.019, −0.007] |
| Direct | −0.049* | [−0.084, −0.014] | −0.035 | [−0.067, −0.002] | −0.051* | [−0.085, −0.018] | −0.028 | [−0.063, 0.007] | −0.028 | [−0.060, 0.005] |
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Path . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Indirect via vascular diseases | −0.002 | [−0.004, −0.001] | −0.005* | [−0.009, −0.001] | −0.004* | [−0.008, −0.001] | −0.006* | [−0.011, −0.002] | −0.005* | [−0.009, −0.001] |
| Indirect via Depressive symptoms | −0.007* | [−0.011, −0.002] | −0.007* | [−0.012, −0.003] | −0.008* | [−0.012, −0.003] | −0.007* | [−0.011, −0.002] | −0.008* | [−0.013, −0.004] |
| Total indirect | −0.008* | [−0.013, −0.003] | −0.012* | [−0.018, −0.007] | −0.012* | [−0.018, −0.006] | −0.013* | [−0.019, −0.007] | −0.013* | [−0.019, −0.007] |
| Direct | −0.049* | [−0.084, −0.014] | −0.035 | [−0.067, −0.002] | −0.051* | [−0.085, −0.018] | −0.028 | [−0.063, 0.007] | −0.028 | [−0.060, 0.005] |
*Significant after adjustment for multiple comparisons using the Benjamini–Hochberg method.
CI= confidence interval.
Standardized specific indirect, total indirect, and direct path estimates between difficulty initiating sleep in 2002 and cognition in 2016 via the mediators
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Path . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Indirect via vascular diseases | −0.002 | [−0.004, −0.001] | −0.005* | [−0.009, −0.001] | −0.004* | [−0.008, −0.001] | −0.006* | [−0.011, −0.002] | −0.005* | [−0.009, −0.001] |
| Indirect via Depressive symptoms | −0.007* | [−0.011, −0.002] | −0.007* | [−0.012, −0.003] | −0.008* | [−0.012, −0.003] | −0.007* | [−0.011, −0.002] | −0.008* | [−0.013, −0.004] |
| Total indirect | −0.008* | [−0.013, −0.003] | −0.012* | [−0.018, −0.007] | −0.012* | [−0.018, −0.006] | −0.013* | [−0.019, −0.007] | −0.013* | [−0.019, −0.007] |
| Direct | −0.049* | [−0.084, −0.014] | −0.035 | [−0.067, −0.002] | −0.051* | [−0.085, −0.018] | −0.028 | [−0.063, 0.007] | −0.028 | [−0.060, 0.005] |
| . | Episodic memory . | Executive function . | Language . | Visuoconstruction . | Processing speed . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Path . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Indirect via vascular diseases | −0.002 | [−0.004, −0.001] | −0.005* | [−0.009, −0.001] | −0.004* | [−0.008, −0.001] | −0.006* | [−0.011, −0.002] | −0.005* | [−0.009, −0.001] |
| Indirect via Depressive symptoms | −0.007* | [−0.011, −0.002] | −0.007* | [−0.012, −0.003] | −0.008* | [−0.012, −0.003] | −0.007* | [−0.011, −0.002] | −0.008* | [−0.013, −0.004] |
| Total indirect | −0.008* | [−0.013, −0.003] | −0.012* | [−0.018, −0.007] | −0.012* | [−0.018, −0.006] | −0.013* | [−0.019, −0.007] | −0.013* | [−0.019, −0.007] |
| Direct | −0.049* | [−0.084, −0.014] | −0.035 | [−0.067, −0.002] | −0.051* | [−0.085, −0.018] | −0.028 | [−0.063, 0.007] | −0.028 | [−0.060, 0.005] |
*Significant after adjustment for multiple comparisons using the Benjamini–Hochberg method.
CI= confidence interval.
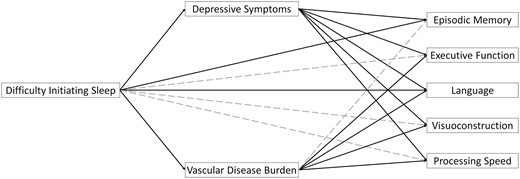
Direct and indirect path estimates between difficulty initiating sleep in 2002 on cognition in 2016 via physical and mental health mediators in 2014. Solid lines depict significant paths after adjustment for multiple comparisons using the Benjamini–Hochberg method. Covariates (i.e. baseline measures of depressive symptoms, vascular disease burden, global cognition, and sociodemographic characteristics) are not depicted for simplicity.
There was also an indirect effect of difficulty initiating sleep on all cognitive domains, except EM, through vascular disease burden. Specifically, more frequent difficulty initiating sleep in 2002 was associated with higher vascular disease burden in 2014 (β = 0.057, SE = 0.017, p = .001), which was subsequently associated with worse performances in EF (β = −0.088, SE = 0.019, p < .001), Lang (β = −0.075, SE = 0.020, p < .001), VC (β = −0.110, SE = 0.020, p < .001), and PS (β = −0.089, SE = 0.019, p < .001), but not EM (β = −0.031, SE = 0.021, p =.133), in 2016. Vascular disease burden accounted for 10.6% of the total effect of difficulty initiating sleep on EF, 6.3% of the effect on Lang, 14.6% of the effect on VC, and 12.2% of the effect on PS.
After accounting for indirect effects through depressive symptoms and vascular disease burden, direct effects of difficulty initiating sleep remained for EM and Lang only, such that more frequent difficulty initiating sleep at the study baseline was associated with worse performances in EM (β = −0.049, SE = 0.018, p = .007) and Lang (β = −0.051, SE = 0.017, p = .003) 14 years later above and beyond the impacts of depressive symptoms and vascular disease burden in 2014.
Cerebrovascular events, such as stroke, may have stronger and more acute effects on cognitive function in comparison to longer-term risk factors for dementia, such as hypertension. Thus, the vascular disease burden variable was separated into stroke (dichotomous) and other vascular disease burden (presence of hypertension, heart disease, and diabetes; range: 0–3) in a sensitivity analysis. The revised mediation model included baseline measures of stroke and other vascular disease burden as covariates in place of the singular vascular disease burden covariate used in the original analyses. Descriptive statistics from 2002 revealed that 3.4% of the sample reported a prior history of stroke and the average number of other vascular conditions reported was less than one (M = 0.70, SE = 0.77). These values increased in 2014, with 11.8% of the sample reporting a history of stroke and the average number of other vascular diseases nearly doubled (M = 1.28, SE = 0.90). After controlling for multiple comparisons, there were no indirect effects of difficulty initiating sleep in 2002 on any of the five cognitive domains in 2016 via either stroke or other vascular disease burden in 2014 (Supplementary Table S2). The association between difficulty initiating sleep and subsequent vascular diseases was stronger and more reliable (β = 0.050, SE = 0.017, p = .004) than the association between difficulty initiating sleep and subsequent stroke (β = 0.038, SE = 0.019, p = .052). Conversely, the associations between stroke and poorer subsequent cognitive function were much stronger [−0.146 ≤ β ≤ −0.099] than the associations between vascular diseases and subsequent cognitive function [−0.042 ≤ β ≤ 0.017]. An additional sensitivity analysis was conducted using recomputed measures of depressive symptoms in 2002 and 2014 (range: 0–7) to exclude the item pertaining to sleep in the CES-D scale [43]; the original results did not change.
Gender differences in associations between insomnia and cognition
Gender differences in key variables of interest are reported in Table 2. Compared to men, women reported more frequent difficulty initiating sleep [t(2593) = −9.27, p < .001], night time awakenings [t(2593) = −3.74, p < .001], early awakenings [t(2593) = −3.25, p = .001], and feeling unrested [t(2593) = −3390, p = .001] at study baseline, and these were small-to-medium effects. While men reported more vascular diseases in 2014 [t(2572) = 3.05, p = .002], women reported more depressive symptoms [t(2510) = −6.07, p < .001], and both were small effects. In terms of cognition, women had better performance in EM [t(2494)= −4.52, p < .001] while men had better performances in EF [t(2494)= 3.04, p = .002] and VC [t(2494)= 3.25, p = .001]; these were all small effects. Gender differences were not observed for Lang (p =. 42) or PS (p = .93). Bivariate correlations revealed negative associations between difficulty initiating sleep and all cognitive domains for women, but only associations with EM and Lang for men (Supplement).
All moderated mediation models fit well (Table 5). Models revealed no significant interactions between gender and any of the four insomnia symptoms nor the two mediators. In other words, the strength and pattern of associations among the individual insomnia symptoms, mental and physical health mediators, and cognitive outcomes did not differ between men and women.
| . | Mediator: depressive symptoms . | Mediator: vascular burden . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | A path interaction† . | B path interaction‡ . | A path interaction . | B path interaction . | C path interaction§ . | |||||
| Cognitive domain model . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Difficulty initiating sleep | ||||||||||
| Episodic memory | 0.060 | [−0.007, 0.127] | −0.026 | [−0.086, 0.034] | 0.028 | [−0.029, 0.085] | 0.000 | [−0.053, 0.052] | 0.050 | [−0.011, 0.110] |
| Executive function | −0.004 | [−0.060, 0.051] | −0.017 | [−0.065, 0.031] | 0.027 | [−0.028, 0.083] | ||||
| Language | −0.024 | [−0.082, 0.033] | −0.005 | [−0.055, 0.045] | 0.032 | [−0.025, 0.090] | ||||
| Visuoconstruction | −0.010 | [−0.070, 0.049] | −0.003 | [−0.054, 0.049] | 0.032 | [−0.028, 0.092] | ||||
| Processing Speed | −0.008 | [−0.063, 0.048] | −0.021 | [−0.069, 0.027] | 0.029 | [−0.027, 0.084] | ||||
| Night time awakenings | ||||||||||
| Episodic memory | −0.032 | [−0.091, 0.028] | −0.021 | [−0.080, 0.038] | −0.017 | [−0.067, 0.034] | −0.002 | [−0.054, 0.050] | 0.053 | [0.000. 0.106] |
| Executive function | −0.001 | [−0.056, 0.053] | −0.018 | [−0.066, 0.030] | 0.031 | [−0.017, 0.079] | ||||
| Language | −0.022 | [−0.079, 0.035] | −0.007 | [−0.057, 0.043] | 0.047 | [−0.003, 0.097] | ||||
| Visuoconstruction | −0.007 | [−0.066, 0.052] | −0.004 | [−0.056, 0.048] | 0.039 | [−0.013, 0.091] | ||||
| Processing speed | −0.005 | [−0.060, 0.050] | −0.022 | [−0.071, 0.026] | 0.035 | [−0.014, 0.083] | ||||
| Early morning awakening | ||||||||||
| Episodic memory | 0.026 | [−0.034, 0.086] | −0.021 | [−0.081, 0.040] | 0.017 | [−0.033, 0.068] | 0.001 | [−0.052, 0.053] | 0.019 | [−0.035, 0.072] |
| Executive function | 0.000 | [−0.055, 0.055] | −0.016 | [−0.064, 0.032] | 0.004 | [−0.045, 0.053] | ||||
| Language | −0.020 | [−0.077, 0.038] | −0.004 | [−0.054, 0.046] | 0.006 | [−0.045, 0.058] | ||||
| Visuoconstruction | −0.005 | [−0.064, 0.055] | −0.001 | [−0.053, 0.050] | 0.004 | [−0.049, 0.058] | ||||
| Processing speed | −0.006 | [−0.061, 0.049] | −0.021 | [−0.069, 0.027] | 0.019 | [−0.031, 0.068] | ||||
| Feeling unrested | ||||||||||
| Episodic memory | 0.033 | [−0.027, 0.094] | −0.023 | [−0.083, 0.038] | 0.031 | [−0.021, 0.082] | 0.001 | [−0.051, 0.053] | 0.025 | [−0.030, 0.080] |
| Executive function | −0.003 | [−0.058, 0.053] | −0.016 | [−0.064, 0.032] | 0.016 | [−0.034, 0.067] | ||||
| Language | −0.021 | [−0.079, 0.037] | −0.004 | [−0.054, 0.046] | 0.010 | [−0.042, 0.063] | ||||
| Visuoconstruction | −0.009 | [−0.069, 0.051] | −0.002 | [−0.054, 0.050] | 0.022 | [−0.033, 0.076] | ||||
| Processing speed | 0.000 | [−0.056, 0.056] | −0.019 | [−0.067, 0.029] | −0.012 | [−0.063, 0.039] | ||||
| . | Mediator: depressive symptoms . | Mediator: vascular burden . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | A path interaction† . | B path interaction‡ . | A path interaction . | B path interaction . | C path interaction§ . | |||||
| Cognitive domain model . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Difficulty initiating sleep | ||||||||||
| Episodic memory | 0.060 | [−0.007, 0.127] | −0.026 | [−0.086, 0.034] | 0.028 | [−0.029, 0.085] | 0.000 | [−0.053, 0.052] | 0.050 | [−0.011, 0.110] |
| Executive function | −0.004 | [−0.060, 0.051] | −0.017 | [−0.065, 0.031] | 0.027 | [−0.028, 0.083] | ||||
| Language | −0.024 | [−0.082, 0.033] | −0.005 | [−0.055, 0.045] | 0.032 | [−0.025, 0.090] | ||||
| Visuoconstruction | −0.010 | [−0.070, 0.049] | −0.003 | [−0.054, 0.049] | 0.032 | [−0.028, 0.092] | ||||
| Processing Speed | −0.008 | [−0.063, 0.048] | −0.021 | [−0.069, 0.027] | 0.029 | [−0.027, 0.084] | ||||
| Night time awakenings | ||||||||||
| Episodic memory | −0.032 | [−0.091, 0.028] | −0.021 | [−0.080, 0.038] | −0.017 | [−0.067, 0.034] | −0.002 | [−0.054, 0.050] | 0.053 | [0.000. 0.106] |
| Executive function | −0.001 | [−0.056, 0.053] | −0.018 | [−0.066, 0.030] | 0.031 | [−0.017, 0.079] | ||||
| Language | −0.022 | [−0.079, 0.035] | −0.007 | [−0.057, 0.043] | 0.047 | [−0.003, 0.097] | ||||
| Visuoconstruction | −0.007 | [−0.066, 0.052] | −0.004 | [−0.056, 0.048] | 0.039 | [−0.013, 0.091] | ||||
| Processing speed | −0.005 | [−0.060, 0.050] | −0.022 | [−0.071, 0.026] | 0.035 | [−0.014, 0.083] | ||||
| Early morning awakening | ||||||||||
| Episodic memory | 0.026 | [−0.034, 0.086] | −0.021 | [−0.081, 0.040] | 0.017 | [−0.033, 0.068] | 0.001 | [−0.052, 0.053] | 0.019 | [−0.035, 0.072] |
| Executive function | 0.000 | [−0.055, 0.055] | −0.016 | [−0.064, 0.032] | 0.004 | [−0.045, 0.053] | ||||
| Language | −0.020 | [−0.077, 0.038] | −0.004 | [−0.054, 0.046] | 0.006 | [−0.045, 0.058] | ||||
| Visuoconstruction | −0.005 | [−0.064, 0.055] | −0.001 | [−0.053, 0.050] | 0.004 | [−0.049, 0.058] | ||||
| Processing speed | −0.006 | [−0.061, 0.049] | −0.021 | [−0.069, 0.027] | 0.019 | [−0.031, 0.068] | ||||
| Feeling unrested | ||||||||||
| Episodic memory | 0.033 | [−0.027, 0.094] | −0.023 | [−0.083, 0.038] | 0.031 | [−0.021, 0.082] | 0.001 | [−0.051, 0.053] | 0.025 | [−0.030, 0.080] |
| Executive function | −0.003 | [−0.058, 0.053] | −0.016 | [−0.064, 0.032] | 0.016 | [−0.034, 0.067] | ||||
| Language | −0.021 | [−0.079, 0.037] | −0.004 | [−0.054, 0.046] | 0.010 | [−0.042, 0.063] | ||||
| Visuoconstruction | −0.009 | [−0.069, 0.051] | −0.002 | [−0.054, 0.050] | 0.022 | [−0.033, 0.076] | ||||
| Processing speed | 0.000 | [−0.056, 0.056] | −0.019 | [−0.067, 0.029] | −0.012 | [−0.063, 0.039] | ||||
Estimates for the A paths are identical across all five cognitive domain models for each mediator.
†Insomnia symptom × gender → mediator.
‡mediator × gender → cognition.
§Insomnia symptom × gender → cognition.
CI = confidence interval.
Difficulty initiating sleep model fit: CFI = 0.995; TLI = 0.972; RMSEA = 0.040 (90% CI [0.035, 0.045]); SRMR = 0.010.
Night time awakenings model fit: CFI = 0.996; TLI = 0.981; RMSEA = 0.032 (90% CI [0.027, 0.038]); SRMR = 0.009.
Early morning awakenings model fit: CFI = 0.995; TLI = 0.973; RMSEA = 0.039 (90% CI [0.034, 0.044]); SRMR = 0.009.
Feeling unrested model fit: CFI = 0.994; TLI = 0.967; RMSEA = 0.043 (90% CI [0.038, 0.048]); SRMR = 0.010.
| . | Mediator: depressive symptoms . | Mediator: vascular burden . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | A path interaction† . | B path interaction‡ . | A path interaction . | B path interaction . | C path interaction§ . | |||||
| Cognitive domain model . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Difficulty initiating sleep | ||||||||||
| Episodic memory | 0.060 | [−0.007, 0.127] | −0.026 | [−0.086, 0.034] | 0.028 | [−0.029, 0.085] | 0.000 | [−0.053, 0.052] | 0.050 | [−0.011, 0.110] |
| Executive function | −0.004 | [−0.060, 0.051] | −0.017 | [−0.065, 0.031] | 0.027 | [−0.028, 0.083] | ||||
| Language | −0.024 | [−0.082, 0.033] | −0.005 | [−0.055, 0.045] | 0.032 | [−0.025, 0.090] | ||||
| Visuoconstruction | −0.010 | [−0.070, 0.049] | −0.003 | [−0.054, 0.049] | 0.032 | [−0.028, 0.092] | ||||
| Processing Speed | −0.008 | [−0.063, 0.048] | −0.021 | [−0.069, 0.027] | 0.029 | [−0.027, 0.084] | ||||
| Night time awakenings | ||||||||||
| Episodic memory | −0.032 | [−0.091, 0.028] | −0.021 | [−0.080, 0.038] | −0.017 | [−0.067, 0.034] | −0.002 | [−0.054, 0.050] | 0.053 | [0.000. 0.106] |
| Executive function | −0.001 | [−0.056, 0.053] | −0.018 | [−0.066, 0.030] | 0.031 | [−0.017, 0.079] | ||||
| Language | −0.022 | [−0.079, 0.035] | −0.007 | [−0.057, 0.043] | 0.047 | [−0.003, 0.097] | ||||
| Visuoconstruction | −0.007 | [−0.066, 0.052] | −0.004 | [−0.056, 0.048] | 0.039 | [−0.013, 0.091] | ||||
| Processing speed | −0.005 | [−0.060, 0.050] | −0.022 | [−0.071, 0.026] | 0.035 | [−0.014, 0.083] | ||||
| Early morning awakening | ||||||||||
| Episodic memory | 0.026 | [−0.034, 0.086] | −0.021 | [−0.081, 0.040] | 0.017 | [−0.033, 0.068] | 0.001 | [−0.052, 0.053] | 0.019 | [−0.035, 0.072] |
| Executive function | 0.000 | [−0.055, 0.055] | −0.016 | [−0.064, 0.032] | 0.004 | [−0.045, 0.053] | ||||
| Language | −0.020 | [−0.077, 0.038] | −0.004 | [−0.054, 0.046] | 0.006 | [−0.045, 0.058] | ||||
| Visuoconstruction | −0.005 | [−0.064, 0.055] | −0.001 | [−0.053, 0.050] | 0.004 | [−0.049, 0.058] | ||||
| Processing speed | −0.006 | [−0.061, 0.049] | −0.021 | [−0.069, 0.027] | 0.019 | [−0.031, 0.068] | ||||
| Feeling unrested | ||||||||||
| Episodic memory | 0.033 | [−0.027, 0.094] | −0.023 | [−0.083, 0.038] | 0.031 | [−0.021, 0.082] | 0.001 | [−0.051, 0.053] | 0.025 | [−0.030, 0.080] |
| Executive function | −0.003 | [−0.058, 0.053] | −0.016 | [−0.064, 0.032] | 0.016 | [−0.034, 0.067] | ||||
| Language | −0.021 | [−0.079, 0.037] | −0.004 | [−0.054, 0.046] | 0.010 | [−0.042, 0.063] | ||||
| Visuoconstruction | −0.009 | [−0.069, 0.051] | −0.002 | [−0.054, 0.050] | 0.022 | [−0.033, 0.076] | ||||
| Processing speed | 0.000 | [−0.056, 0.056] | −0.019 | [−0.067, 0.029] | −0.012 | [−0.063, 0.039] | ||||
| . | Mediator: depressive symptoms . | Mediator: vascular burden . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | A path interaction† . | B path interaction‡ . | A path interaction . | B path interaction . | C path interaction§ . | |||||
| Cognitive domain model . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . | β . | 95% CI . |
| Difficulty initiating sleep | ||||||||||
| Episodic memory | 0.060 | [−0.007, 0.127] | −0.026 | [−0.086, 0.034] | 0.028 | [−0.029, 0.085] | 0.000 | [−0.053, 0.052] | 0.050 | [−0.011, 0.110] |
| Executive function | −0.004 | [−0.060, 0.051] | −0.017 | [−0.065, 0.031] | 0.027 | [−0.028, 0.083] | ||||
| Language | −0.024 | [−0.082, 0.033] | −0.005 | [−0.055, 0.045] | 0.032 | [−0.025, 0.090] | ||||
| Visuoconstruction | −0.010 | [−0.070, 0.049] | −0.003 | [−0.054, 0.049] | 0.032 | [−0.028, 0.092] | ||||
| Processing Speed | −0.008 | [−0.063, 0.048] | −0.021 | [−0.069, 0.027] | 0.029 | [−0.027, 0.084] | ||||
| Night time awakenings | ||||||||||
| Episodic memory | −0.032 | [−0.091, 0.028] | −0.021 | [−0.080, 0.038] | −0.017 | [−0.067, 0.034] | −0.002 | [−0.054, 0.050] | 0.053 | [0.000. 0.106] |
| Executive function | −0.001 | [−0.056, 0.053] | −0.018 | [−0.066, 0.030] | 0.031 | [−0.017, 0.079] | ||||
| Language | −0.022 | [−0.079, 0.035] | −0.007 | [−0.057, 0.043] | 0.047 | [−0.003, 0.097] | ||||
| Visuoconstruction | −0.007 | [−0.066, 0.052] | −0.004 | [−0.056, 0.048] | 0.039 | [−0.013, 0.091] | ||||
| Processing speed | −0.005 | [−0.060, 0.050] | −0.022 | [−0.071, 0.026] | 0.035 | [−0.014, 0.083] | ||||
| Early morning awakening | ||||||||||
| Episodic memory | 0.026 | [−0.034, 0.086] | −0.021 | [−0.081, 0.040] | 0.017 | [−0.033, 0.068] | 0.001 | [−0.052, 0.053] | 0.019 | [−0.035, 0.072] |
| Executive function | 0.000 | [−0.055, 0.055] | −0.016 | [−0.064, 0.032] | 0.004 | [−0.045, 0.053] | ||||
| Language | −0.020 | [−0.077, 0.038] | −0.004 | [−0.054, 0.046] | 0.006 | [−0.045, 0.058] | ||||
| Visuoconstruction | −0.005 | [−0.064, 0.055] | −0.001 | [−0.053, 0.050] | 0.004 | [−0.049, 0.058] | ||||
| Processing speed | −0.006 | [−0.061, 0.049] | −0.021 | [−0.069, 0.027] | 0.019 | [−0.031, 0.068] | ||||
| Feeling unrested | ||||||||||
| Episodic memory | 0.033 | [−0.027, 0.094] | −0.023 | [−0.083, 0.038] | 0.031 | [−0.021, 0.082] | 0.001 | [−0.051, 0.053] | 0.025 | [−0.030, 0.080] |
| Executive function | −0.003 | [−0.058, 0.053] | −0.016 | [−0.064, 0.032] | 0.016 | [−0.034, 0.067] | ||||
| Language | −0.021 | [−0.079, 0.037] | −0.004 | [−0.054, 0.046] | 0.010 | [−0.042, 0.063] | ||||
| Visuoconstruction | −0.009 | [−0.069, 0.051] | −0.002 | [−0.054, 0.050] | 0.022 | [−0.033, 0.076] | ||||
| Processing speed | 0.000 | [−0.056, 0.056] | −0.019 | [−0.067, 0.029] | −0.012 | [−0.063, 0.039] | ||||
Estimates for the A paths are identical across all five cognitive domain models for each mediator.
†Insomnia symptom × gender → mediator.
‡mediator × gender → cognition.
§Insomnia symptom × gender → cognition.
CI = confidence interval.
Difficulty initiating sleep model fit: CFI = 0.995; TLI = 0.972; RMSEA = 0.040 (90% CI [0.035, 0.045]); SRMR = 0.010.
Night time awakenings model fit: CFI = 0.996; TLI = 0.981; RMSEA = 0.032 (90% CI [0.027, 0.038]); SRMR = 0.009.
Early morning awakenings model fit: CFI = 0.995; TLI = 0.973; RMSEA = 0.039 (90% CI [0.034, 0.044]); SRMR = 0.009.
Feeling unrested model fit: CFI = 0.994; TLI = 0.967; RMSEA = 0.043 (90% CI [0.038, 0.048]); SRMR = 0.010.
Discussion
This study adds to the growing literature on insomnia as a contributor to cognitive impairment among older adults and suggests that difficulty initiating sleep in mid-to-late life, compared to other insomnia symptoms, may be especially indicative of future cognitive impairment across multiple domains. Further, these associations are at least partially mediated through greater depressive symptoms and cumulative vascular disease burden. Residual direct effects of sleep initiation problems on episodic memory and language functioning suggest that additional mechanisms underlie associations between sleep initiation and those cognitive domains. Although gender differences in the levels of insomnia symptoms, depressive symptoms, vascular disease burden, and cognition observed in the present study were in line with established research and prevalence rates, the pattern and strength of associations among these variables did not differ between men and women.
Difficulty initiating sleep and cognitive functioning in later life
Associations between poor sleep and diminished cognition were only observed for difficulty initiating sleep in the present study. This is consistent with prior research showing that cross-sectional and longitudinal cognitive impairment in older adults is more strongly associated with difficulty initiating sleep rather than difficulty maintaining sleep [62–64]. In a cross-sectional study of older adults, longer self-reported sleep onset latency (SOL; perhaps related to difficulty initiating sleep in the present study), but not sleep quality (perhaps similar to feeling unrested), was associated with worse visuospatial reasoning, memory, and processing speed [65]. There is also support from a recent longitudinal study for subjective long SOL as a unique risk factor for incident cognitive impairment and decline in older adults as determined by a comprehensive neuropsychological battery and consensus diagnosis [66]. Notably, the effect of reporting difficulty initiating sleep “most of the time” compared to “rarely/never” on cognition in the current study was equivalent to 2.2–3.4 years of aging. Prior research in a US population-representative cohort suggests that a medical or pharmaceutical intervention that delays Alzheimer’s disease by 3 years could result in a 21% reduction in overall Alzheimer’s disease prevalence compared to the status quo of no such intervention [67]. Delaying Alzheimer’s disease not only impacts longevity and quality of life among older individuals but also promises economic relief against the public health impact of the disease.
Sleep problems are reported by as many as half of the older adult population, with primary complaints of disrupted sleep due to frequent night time and early morning awakenings [68]. Although problems with sleep initiation are more common among younger adults [69], the present finding that only difficulty initiating sleep was associated with subsequently poorer cognition may help with the differentiation of sleep changes in later life that are age-normative vs. suggestive of pathological processes. Indeed, a recent cross-sectional analysis from an epidemiological sample of Canadians ages 45–85 found that sleep onset insomnia, but not sleep maintenance insomnia, was associated with prodromal signs of neurodegeneration, including cognitive, motor, and autonomic symptoms [70]. Another study of the relationship between multiple self-reported sleep quality factors (i.e. sleep duration, latency, disturbances, efficiency, and day time dysfunction) and levels of beta-amyloid deposition in the brain measured using PET found that longer objective SOL was the only factor associated with greater beta-amyloid impact [71]. Although cross-sectional, this finding suggests that chronic SOL extended by 30 min more than average could be associated with the equivalent of approximately 2 years in beta-amyloid accumulation, which is a hallmark neuropathological indicator of Alzheimer’s disease. However, cross-sectional findings on associations between difficulty initiating sleep/SOL and beta-amyloid impact are mixed [72, 73], warranting additional prospective research in this area.
Links between difficulty initiating sleep and later cognition observed in the present study are also in line with growing evidence for a relationship between circadian dysfunction and neurodegenerative diseases [74]. For example, delayed circadian rhythms, which can manifest as difficulty initiating sleep or chronic sleep-onset insomnia, were prospectively associated with a 1.83-fold greater risk of mild cognitive impairment or dementia over a 5-year period in a community-dwelling sample of older women [75]. Mechanistically, circadian disruption may influence neurodegeneration via a positive feedback loop involving increased oxidative stress, disruption of metabolic processes, and/or reduced clearance of metabolic waste [76]. Lucey and Bateman’s proposed model of the role of deficient sleep in Alzheimer’s disease pathogenesis posits that sleep problems in later life may increase the risk for amyloid deposition and ultimately affect cognitive functioning via disruption of synaptic integrity in cortical networks [77]. Beta-amyloid concentrations in cerebrospinal fluid have been observed to fluctuate on a diurnal pattern, with greater concentrations during wake than sleep [76]. Both sleep deprivation and increased wakefulness are associated with greater short- and long-term accumulation of beta-amyloid and tau [20]. Sleep has also been hypothesized to serve its restorative function through greater clearance of extracellular waste via the glymphatic system in the brain [78] as well as through synaptic plasticity and reorganization processes [79]; however, research on the associations between these processes and their influence on cognitive aging is still in its infancy [80]. Alternatively, it may be that sleep problems first presenting later in life serve as an indicator of reduced brain integrity [81] or a preclinical indicator of neurodegenerative processes already in place in older adults [82], rather than a causal or risk factor, per sé. It may also be that the present association reflects unmeasured effects of pharmacological sleep aids on cognition among those reporting insomnia symptoms [83].
Irrespective of any causal mechanisms that may underlie associations between insomnia and cognitive impairment in later life, the current study suggests that difficulty initiating sleep rather than other insomnia symptoms in mid-to-late life has greater predictive utility for identifying future cognitive impairment. While night time awakenings, early morning awakenings, and non-restorative sleep are common in later life and often co-occur with dementia, they may not be indicative of future risk for incident cognitive impairment. The current findings suggest that interventions to reduce insomnia, with a focus on difficulty initiating sleep, may have additional benefits for cognitive functioning. Accordingly, a clinical trial was proposed in 2020 to explore the efficacy of Cognitive Behavioral Therapy for Insomnia as a strategy to reduce the risk for dementia (e.g. by reducing beta-amyloid accumulation) in at-risk older adults [84]. However, additional prospective and experimental research is needed to corroborate our preliminary findings and determine how better sleep can be harnessed throughout the lifespan to facilitate healthy cognitive aging.
Mechanisms underlying sleep—cognition associations
Indirect effects
Insomnia and mental/physical health
Consistent with prior research, in the present study frequent difficulty initiating sleep was associated with greater depressive symptoms [85] and vascular disease burden [86] 12 years later. Indeed, difficulty initiating sleep in comparison to other insomnia phenotypes appears to be more strongly associated with concurrent and subsequent depression [18, 87, 88] and the development of metabolic syndrome [89]. Among middle-aged men, difficulty initiating sleep also increases the risk for incident diabetes [90] and heart disease [91]. A sensitivity analysis from the present study suggests that difficulty initiating sleep may be more proximally related to incident systemic vascular diseases, such as hypertension, diabetes, and heart disease, than to cerebrovascular events. Both cognitive and somatic hyperarousal processes associated with difficulty initiating sleep [92–94] may underlie the associations between sleep onset insomnia and subsequent depression and vascular disease. Individuals with insomnia symptoms who also report persistent and pervasive intrusive thoughts (i.e. perseverations) in response to stressors, as compared to those who do not report perseverations, are more likely to develop depression 1–2 years later [95]. Moreover, individuals with higher perseverations in the context of sleep initiation, but not maintenance difficulties, report more severe subsequent depression. Somatic hyperarousal is characterized by increased the hypothalamic-pituitary-adrenal axis and autonomic nervous system activity. Emerging research suggests that difficulty with sleep initiation is associated with cardiovascular dysregulation both prior to sleep onset and upon waking in the morning [96, 97].
Mental/physical health and cognitive aging
Depressive symptoms partially mediated the effect of difficulty initiating sleep on all five measured domains of cognition in the present study. This indirect pathway may reflect important mechanisms by which depression can facilitate cognitive impairment, for example through impaired cellular signaling and neuronal plasticity that may ultimately lead to global losses in brain volume [98]. In particular, depression in later life is associated with hippocampal volume loss [99], which may help to explain diminished episodic memory functioning associated with insomnia in the present study.
Independent of depression, vascular disease burden partially mediated the effect of sleep initiation problems for all cognitive domains, except episodic memory. Subcortical ischemic vascular disease has been associated with subsequent declines in psychomotor speed, speeded tasks of language, executive functions, and global cognition measured with a brief screener, but not with memory in prior research [100]. Vascular disease may have variable effects on cognitive functioning, as prior research suggests that greater burden is associated with linear declines in attention, language, speed, and executive functions, but curvilinear trends for memory and global cognition [101]. A sensitivity analysis from the present study suggests that different types of vascular diseases (e.g. cerebrovascular vs. systemic cardiometabolic diseases) may have differential effects on subsequent cognitive function. In general, prior research in a longitudinal cohort sample suggests that cumulative effects of cardiovascular and cerebrovascular diseases result in the greatest risk for cognitive decline and progression of Alzheimer’s disease [45]. Considered together, the present findings raise the possibility that treatment of difficulty initiating sleep among older adults may help reduce the risk for cognitive impairment indirectly, through decreased risk for both depression and vascular disease burden.
Direct effects
In the present study, difficulty initiating sleep was associated with worse subsequent memory and language performance even after adjusting for the effects of depressive symptoms and vascular disease burden. This domain specificity may reflect underlying changes to sleep architecture [102] or involvement of temporal lobe pathology [103]. Older adults with insomnia, in comparison to normal sleepers, show less slow-wave activity [104] which predicts a higher rate of amyloid accumulation over time [105]. Moreover, neuroimaging research in middle-aged and older adults with chronic primary insomnia, as compared to demographically-matched controls, revealed poorer subjective and objective sleep to be associated with greater hippocampal atrophy and worse neuropsychological performance across measures of language and memory [106]. Indeed, loss of hippocampal structural integrity is associated with worse episodic memory ability among older adults [107]. However, these studies have primarily explored concurrent associations among insomnia, neural architecture or physiology, and cognition; additional prospective research is needed to clarify the directionality of these associations.
Gender considerations
Associations among insomnia symptoms, physical and mental health mediators and cognition did not differ between men and women in the present study, arguing against differential sex- or gender-based impacts of these risk factors on late life cognitive impairment. However, we did observe differences in the levels of these key study variables, in line with prior research [31], which may reflect differential risk of exposure to these factors. Indeed, women in comparison to men reported greater frequency of all four insomnia symptoms measured in the present study, but this difference was disproportionately large for difficulty initiating sleep.
Prior research on the natural course of chronic insomnia in mid-to-late life suggests that women may be particularly susceptible to developing difficulty initiating sleep over time, compared to men [108]. This vulnerability may partially reflect biological differences, as sleep disturbances among women in mid-late life have been linked to changing hormones during the menopausal transition [109]. Differential exposure to other risk factors throughout the life course may also contribute to more frequent difficulty initiating sleep among women. Risk factors such as anxiety, environmental noise at night time, and relationship problems have all been associated with insomnia symptoms among women, but less strongly or not at all among men [109]. Indeed, women tend to present with greater sleep reactivity in response to psychosocial stress compared to men [110]. Together, these results suggest that women may be at greater risk for sleep disturbances most relevant for cognitive aging, which in turn could contribute to documented gender differences in dementia risk.
Limitations, future directions, and strengths
The measurement of insomnia symptoms at a single time-point is a limitation of the present study, precluding us from quantifying the relative stability, remission, or progression of the reported symptoms over time. Future research should investigate the role of insomnia chronicity on normal and pathological cognitive aging over time. Additionally, treatment status was not assessed in the present study because the HRS did not inquire about participants’ use of pharmacological sleep aids as part of the core interview until 2006. Thus, the possibility that variability in insomnia treatment (e.g. sedative-hypnotic use) among participants accounted for the observed associations between more frequent difficulty initiating sleep and lower subsequent cognition cannot be ruled out. Future longitudinal research should incorporate information on insomnia treatments to clarify what intervention targets might minimize cognitive impairment. The presence of an association between difficulty initiating sleep and cognitive performance 14 years later despite multiple unavoidable sources of noise (e.g. time span between exposures and outcomes, availability of only one time-point of comprehensive cognitive data, single-item measures of insomnia symptoms) supports the criticality of future research on insomnia and cognitive aging.
The absence of objective indicators of sleep and vascular disease burden is an additional limitation. Although self-reported vascular disease burden has been widely used in prior epidemiological research, differences in healthcare access and health literacy may contribute to inaccurate assessment or awareness of vascular diseases among participants, as well as disparities in disease management. Future research using objective measures of vascular disease burden is needed to corroborate our preliminary findings. Moreover, prior research has shown differences in cognitive outcomes associated with subjectively and objectively (e.g. via actigraphy or polysomnography) measured sleep problems [111]. However, insomnia is clinically defined by an individual’s perceived dissatisfaction with sleep quantity or quality, and self-reported questionnaires are quick and cost-effective measures commonly employed in primary care settings to screen for insomnia, unlike polysomnography. Thus, the present findings reiterate the practical utility of these measures, at minimum, as a way to screen for individuals at-risk for future cognitive decline who may not yet present with cognitive impairment.
In addition, although insomnia can occur in the context of other sleep disorders, participants were not evaluated for other sleep diagnoses. However, associations between insomnia and depression, at least, do not appear to depend on the presence or absence of a comorbid sleep disorder [94]. Further, our sensitivity analysis including BMI (a proxy for sleep apnea) did not suggest a strong influence. Our results also suggest that mechanisms other than vascular diseases and depressive symptoms may link insomnia to subsequent cognitive function. Future research inquiry on whether anxiety may be another mediator of insomnia—cognition associations is needed given known correlations between anxiety and both difficulty initiating sleep and cognitive performance.
The primary strength of this study was the use of a comprehensive neuropsychological battery, to assess cognitive functioning across multiple domains in older adults including validated objective measures that harmonize with several longitudinal studies of aging around the world. Identification of cognitive dysfunction in particular domains provides clinical utility for determining the probable etiology and severity of late-life cognitive impairment. The use of a harmonized cognitive battery will also allow for direct cross-national comparisons of the present findings with related future research. This study was also strengthened by the inclusion of relevant covariates, such as established predictors of cognitive function in later life (e.g. prior cognitive functioning, markers of socioeconomic status), as well as the inclusion of baseline measurements of the physical and mental health mediators. Existing literature also points to the bidirectionality of associations between insomnia and depression and between insomnia and vascular health. Accordingly, the present study design employed a lagged mediation model over 14 years. Measuring the exposures, mediators, and outcomes over three-time points rather than concurrently and accounting for autoregressive associations (e.g. by controlling for baseline measurements of the mediators) helped to strengthen causal inferences. Finally, although the present results are not fully generalizable to the complete US older adult population (due to the unavailability of HCAP sampling weights at the time of this study), our large, community-based sample is more representative of older adults in the United States than clinic-based studies.
Conclusions
Individuals who experience frequent difficulty initiating sleep in mid-late life may be at heightened risk for cognitive impairment later in life, although prospective research with the longitudinal assessment of insomnia and insomnia treatment, as well as repeated administration of identical cognitive measures will be necessary to clarify potential causal mechanisms. Investigation of the specificity and generality of relationships between insomnia and cognition revealed depressive symptoms and vascular health as important mediators in driving these associations. However, residual direct associations indicate the presence of additional underlying mechanisms for episodic memory and language functioning. While the strength and pattern of associations among insomnia, mental and physical health mediators, and subsequent cognition did not differ between men and women, women appear to be at greater risk for difficulty initiating sleep in later life. These findings have relevant implications for clinical practice, as sleep initiation difficulties may alert providers to patients at heightened risk for subsequent cognitive impairment. Future intervention research is needed to better understand the mechanisms and modifiability of difficulty initiating sleep—cognition associations in later life, and which interventions reduce late-life cognitive impairment and gender disparities in cognitive aging.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number F31AG067717 (PI: Afsara B. Zaheed). The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740, PI: David R. Weir) and is conducted by the University of Michigan. The sponsor had no role in the current analyses or the preparation of this paper.
Disclosure Statements
Financial Disclosures: AS has received grant support from the NIH. He has received honoraria for serving as a consultant to Merck and from Springer Nature Switzerland AG for guest editing special issues of Current Sleep Medicine Reports. RC has received grant support from the NIH. He has received royalties as an editor and author for UpToDate and as an editor for Cambridge University Press. He has received royalties from Zansors for a licensed questionnaire. The other authors have no financial disclosures to report.
Non-Financial Disclosures: RC is a member of the board of directors for the International Pediatric Sleep Association and the advisory board for the non-profit Pajama Program. He is named in patents, patents pending, and copyrighted material owned by the University of Michigan and focused on the assessment and treatment of sleep disorders. The other authors have no disclosures or conflicts of interest to report.





Comments