-
PDF
- Split View
-
Views
-
Cite
Cite
Kang Min Park, Keun Tae Kim, Dong Ah Lee, Gholam K Motamedi, Yong Won Cho, Glymphatic system dysfunction in restless legs syndrome: evidenced by diffusion tensor imaging along the perivascular space, Sleep, Volume 46, Issue 11, November 2023, zsad239, https://doi.org/10.1093/sleep/zsad239
Close - Share Icon Share
Abstract
There is growing evidence pointing at glymphatic system dysfunction in diseases with circadian disruption, such as sleep disorders. Lower diffusivity in the direction of perivascular space has been shown in several neurological and sleep-related disorders; however, its role in restless legs syndrome (RLS) is unclear. We hypothesized that similarly, in RLS the diffusivity in glymphatic system is decreased. Here, we aimed to evaluate glymphatic system functionality in patients with RLS, compare it to healthy controls, and analyze the correlation between its function and clinical characteristics.
Sixty-nine patients with primary RLS and 51 healthy controls were recruited at a tertiary hospital. All participants underwent diffusion tensor imaging (DTI) and magnetic resonance imaging (MRI) using a 3T MRI scanner, and the DTI along the perivascular space (DTI-ALPS) index was calculated using DTI data. We compared the DTI-ALPS index between the patients with RLS and healthy controls. We also conducted the correlation analysis between the DTI-ALPS index and clinical characteristics, including age, age of onset, symptom duration, and RLS severity.
DTI-ALPS index differed significantly between the patients with RLS and healthy controls; the DTI-ALPS index in the patients with RLS was lower than that in the healthy controls (1.48 vs. 0.60, p = 0.008). There was no significant correlation between the DTI-ALPS index and clinical characteristics.
A significantly lower DTI-ALPS index in patients with RLS suggests that the glymphatic system function is impaired in patients with RLS.
This research evaluated the glymphatic system’s functionality in Restless Legs Syndrome (RLS) patients using diffusion tensor imaging along the perivascular space (DTI-ALPS) index, derived from Diffusion Tensor Imaging. The index was markedly lower in RLS patients, indicating glymphatic dysfunction, but no significant correlation was observed with their clinical characteristics. The study proposes DTI-ALPS as a potential biomarker for assessing glymphatic function in RLS patients, emphasizing the need for further research on its diagnostic and therapeutic implications.
Introduction
Restless legs syndrome (RLS) is a sleep-related sensorimotor disorder with a prominent circadian pattern and diurnal variations. RLS is characterized by an urge to move, usually in the evening or night, and is exacerbated by rest and resolved by movement [1]. Several factors are thought to contribute to the nocturnal nature of RLS symptoms: sleep deprivation, immobility, and intrinsic circadian rhythm [2]. Furthermore, the circadian oscillation of RLS is also attributed to several other important circadian factors such as cerebrospinal fluid (CSF) iron levels, variations in adenosine function, and hypocretin function [3, 4], and disturbance in diurnal variation of the default mode network [5]. Recently, there has been growing evidence of glymphatic system dysfunction in diseases with circadian disruption, such as sleep disorders and neurodegenerative disorders [6–8].
The glymphatic system network is a highly organized fluid transport system that promotes the movement of CSF into the brain through periarterial spaces and the clearance of metabolic waste from the interstitial fluid (ISF) through the perivenous spaces [9]. It is presumed that impairment of glymphatic drainage system (inflow and outflow parameters) reflects excessive perfusion accompanied by insufficient drainage [10]. There is enough evidence to support the crucial role of sleep in the glymphatic system [11]. The high magnitude of slow-wave activity and the decrease in neurotransmitter concentrations such as norepinephrine, enhance glymphatic system function. Consequently, poor sleep quality directly impedes glymphatic clearance and promotes depolarization of aquaporin-4 (AQP4) water channel, thereby affecting glymphatic system circulation [11]. Therefore, considering poor sleep quality in patients with RLS, we could expect the glymphatic system dysfunction in patients with RLS. However, there have not yet been any studies investigating glymphatic system function in patients with RLS.
Recently, various magnetic resonance imaging (MRI) techniques have been devised to evaluate the function of the glymphatic system [12, 13]. Quantitative evaluation of the enlarged perivascular spaces was proposed as an indirect method of estimating glymphatic system dysfunction [14]. As high-resolution and high signal-to-noise imaging is now possible with the latest advanced imaging devices and acquisition methods, more perivascular spaces can be observed in young and healthy participants [15]. Diffusion tensor image analysis along the perivascular space (DTI-ALPS) method is based on the concept that perivascular ISF movement in the white matter near the lateral ventricles is prominent along the parallel medullary veins [16]. The DTI-ALPS index is measured based on the value obtained from the diffusion image in each corresponding direction by specifying the region of interest (ROI) in the area where the lateral medullary veins and white matter fibers travel perpendicularly, i.e. the projection and association fibers. The index reflects the ratio of diffusivity in the direction of perivascular space against diffusivity in the direction perpendicular to both fiber tract and perivascular space. The DTI-ALPS method has good reproducibility, and the glymphatic system function can be analyzed with a single scan, and offers advantages such as being noninvasive with no exposure to contrast agents. Hence, the DTI-ALPS index has been widely used to evaluate glymphatic system functions in various neurological disorders, such as sleep disorders and epilepsy [17–21]. In Alzheimer’s disease, this method has shown decreased diffusivity in the direction of perivascular space [22]. Similarly, in patients with idiopathic REM sleep disorder (RBD), the DTI-ALPS index has been shown to be significantly lower than in healthy controls [18].
We hypothesized that similar to other neurological and sleep disorders, fluid diffusivity is decreased in RLS, and the severity of dysfunction is associated with the clinical presentations. This study aimed to evaluate glymphatic system function in patients with RLS using the DTI-ALPS index, and to analyze the correlation between glymphatic system function and clinical characteristics of patients with RLS.
Methods
Participants: patients with RLS and healthy controls
Patients with primary RLS and healthy controls were enrolled at a tertiary hospital. The study was approved by the Institutional Review Board. All participants signed an informed consent form. Patients who met the inclusion criteria were selected from “RLS MRI dataset” collected at our center. The inclusion criteria were (1) diagnosis of primary RLS by a board-certified sleep medicine specialist using the International RLS/WED Study Group (IRLSSG) criteria [23], (2) absence of structural lesions on brain MRI, and (3) absence of any other neurological diseases, including stroke, demyelinating disease, neurodegenerative diseases, or epilepsy, and other medical conditions such as diabetes mellitus, hypertension, or dyslipidemia. Exclusion criteria included patients with secondary RLS due to iron deficiency (serum Ferritin <75 μg/L), pregnancy, chronic kidney disease, or peripheral neuropathy. As a control group, we recruited healthy individuals of comparable age and sex who did not meet the RLS diagnostic criteria according to the diagnostic questionnaire [23], and had normal brain MRI and no medical conditions.
The International RLS scale was used to assess the severity of RLS symptoms [24, 25] along with (1) the RLS Quality of Life Questionnaire [26], (2) the Insomnia Severity Index (ISI) [27], (3) the Pittsburgh Sleep Quality Index (PSQI) [28], and (4) the Hospital Anxiety and Depression Scale [29].
DTI acquisition
RLS patients and healthy controls underwent DTI using the same scanner. All MRI scans were conducted during the daytime, between 09:00 am and 12:00 am, to minimize the influence of RLS symptoms. The DTI was carried out with spin-echo single-shot echo-planar pulse sequences in 32 distinct diffusion directions (repetition time [TR]/ echo time [TE] = 8620/85 ms, fractional anisotropy = 90°, slice thickness = 2.25 mm, acquisition matrix = 120 × 120, field of view = 240 × 240 mm2, and b-value = 1,000 s/mm2). A 3.0T scanner with a 32-channel head coil was utilized for all MRI scans. The DTI data utilized in this study were recruited from the imaging data of patients with RLS and healthy controls that were previously included in our prior study [30].
Functional assessment of glymphatic system using DTI-ALPS method
We determined the DTI-ALPS index to examine the function of the glymphatic system in patients with RLS and healthy controls using the DSI studio program (Figure 1). Initially, we accessed the source DTI images and established a mask to filter the background region, enhance the reconstruction efficacy, and facilitate further visualization, with thresholding, smoothing, and defragmentation performed as subsequent steps. In addition, we preprocessed using FSL’s top-up and eddy to account for susceptibility artifacts and eddy current distortion. Next, using a generalized q-sampling imaging method, we generated one fiber orientation per voxel and associated anisotropy and diffusivity measures. We drew an ROI and extracted the fiber orientation and diffusivities along the x, y, and z axes as voxel values within the ROI. Finally, the DTI-ALPS index was computed using the following formula [16]:
![The process of developing diffusion tensor image analysis along the perivascular space (DTI-ALPS) method to assess glymphatic system function. This figure has been modified from our previous publication in our group [18].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/sleep/46/11/10.1093_sleep_zsad239/1/m_zsad239_fig1.jpeg?Expires=1747928064&Signature=GC9~AKjd~FGiW6mVD-iuH-yfhb77pne95lN5Lnbx697uoTbJPtmCep5kRwC1ws-lozQjoS96K8DLfSoXY0m5EixRNCJp1v9xwPko5VzwgHFRyVI4t1f5TPIU8QE4v6bK11mueFzSzia0cbwPq-4cAoo04zWQ3HzByngKmUWZH0mEzrJis5pI6xOmMZxWoAXB0-4WFFop1TualhEjYUufkAtoDZP6rRbuag4hAOS86YSPPwBEWMhcJUm8jhULsEOMJix8UIHFJdymFvhZ5dUHZor~pSsGO62LDH~wsPuTu0rhG0flkj-fIT6JMwdhpia8X2uGpzpIWuenVGic9trwqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The process of developing diffusion tensor image analysis along the perivascular space (DTI-ALPS) method to assess glymphatic system function. This figure has been modified from our previous publication in our group [18].
where Dxxproj is the diffusivity along the x-axis in the projection fiber, Dxxassoci is the diffusivity along the x-axis in the association fiber, Dyyproj is the diffusivity along the y-axis in the projection fiber, and Dzzassoci is the diffusivity along the z-axis in the association fiber.
We compared the DTI-ALPS index between patients with RLS and healthy controls. We also investigated the differences in the DTI-ALPS index in patients with RLS according to the medications used to relieve symptoms. To evaluate the possible influence of α2δ ligands, which could enhance slow-wave sleep and subsequently affect the index, we carried out further analysis to investigate any differences in the DTI-ALPS index between the group receiving α2δ ligands medications and dopamine agonists. Additional analyses were conducted in the healthy control group by categorizing individuals based on sleep quality using the PSQI questionnaire. The healthy control group was divided into two subgroups: good sleep group (N. 29, PSQI score ≤ 4) and poor sleep group (N. 21, PSQI score ≥ 5).
Correlation analysis
In patients with RLS, we performed a correlation analysis between the DTI-ALPS index and clinical characteristics including age, age of RLS onset, symptom duration, International RLS Severity Scale (IRLS), RLS Quality of Life Questionnaire, PSQI, ISI, Hospital Anxiety Scale, and Hospital Depression Scale.
Statistical analysis
Chi-square test was utilized to test the difference between categorical variables, whereas the independent samples t-test or Mann–Whitney test was utilized for continuous variables. Pearson’s test was utilized for correlation analysis. The frequency was expressed as a percentage for categorical variables, while mean values with standard deviation (SD) or median values with interquartile range were provided for continuous variables. Results with p < 0.05 were considered statistically significant. While analyzing diffusivities along the axis of the fibers, multiple corrections were applied (Bonferroni correction, p = 0.0055 [0.05/9]). For all statistical analysis, version 20.014 of the MedCalc Statistical Software (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021) was used.
Results
Clinical characteristics of patients with RLS
We recruited 69 patients with primary RLS and 51 healthy controls. The clinical characteristics of both groups are listed in Table 1. There were no significant differences in age and sex (57.0 vs. 55.9 years, p = 0.443; 20/69 vs. 13/51, p = 0.672) between the two groups. Of the 69 patients with RLS, 44 patients (63.8%) were drug-naïve state for RLS, whereas 25 patients (36.2%) were already taking medication to relieve RLS symptoms before MRI scanning (patients living with RLS with a drug-treated state). Of the 25 patients treated with medications, dopamine agonists were used as monotherapy in 17 individuals. Additionally, five patients used a combination of dopamine agonists and α2δ ligands, while two patients utilized dopamine agonists, α2δ ligands, and clonazepam together. One patient relied on α2δ ligands and clonazepam.
| . | Patients with RLS (N = 69) . | Drug-naïve RLS Patients (N = 44) . | Treated RLS Patients (N = 25) . | Healthy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 57.0 ± 6.7 | 56.3 ± 6.3 | 58.3 ± 7.2 | 55.9 ± 8.1 | 0.418 | 0.791 | 0.213 |
| Male, n (%) | 20 (28.9) | 13 (29.5) | 7 (28.0) | 13 (25.4) | 0.673 | 0.660 | 0.817 |
| Age of onset, years | 47 (41.7–54.0) | 48 (40.5–54.0) | 58 (53.5–65.0) | ||||
| Symptom duration, months | 120 (39–162) | 84 (36–144) | 120 (48–249) | ||||
| IRLS | 27.1 ± 6.4 | 27.2 ± 6.8 | 26.9 ± 5.9 | ||||
| Disease-specific quality of life | 8.7 ± 3.3 | 8.8 ± 3.5 | 8.6 ± 3.1 | ||||
| PSQI | 12 (9.0–14.2) | 12 (9.0–15.5) | 12 (9.5–13.3) | 4 (3–5) | <0.001 | <0.001 | <0.001 |
| ISI | 16 (11–23) | 18 (11–23) | 13 (11.0–21.5) | 3 (1–4) | <0.001 | <0.001 | <0.001 |
| HAS | 7 (4–9) | 7 (4–10) | 6 (3–8) | 4 (2.2–5) | <0.001 | <0.001 | 0.011 |
| HDS | 8 (5–11) | 9 (5–11) | 6(4–10) | 5 (4–7) | 0.007 | <0.001 | 0.433 |
| . | Patients with RLS (N = 69) . | Drug-naïve RLS Patients (N = 44) . | Treated RLS Patients (N = 25) . | Healthy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 57.0 ± 6.7 | 56.3 ± 6.3 | 58.3 ± 7.2 | 55.9 ± 8.1 | 0.418 | 0.791 | 0.213 |
| Male, n (%) | 20 (28.9) | 13 (29.5) | 7 (28.0) | 13 (25.4) | 0.673 | 0.660 | 0.817 |
| Age of onset, years | 47 (41.7–54.0) | 48 (40.5–54.0) | 58 (53.5–65.0) | ||||
| Symptom duration, months | 120 (39–162) | 84 (36–144) | 120 (48–249) | ||||
| IRLS | 27.1 ± 6.4 | 27.2 ± 6.8 | 26.9 ± 5.9 | ||||
| Disease-specific quality of life | 8.7 ± 3.3 | 8.8 ± 3.5 | 8.6 ± 3.1 | ||||
| PSQI | 12 (9.0–14.2) | 12 (9.0–15.5) | 12 (9.5–13.3) | 4 (3–5) | <0.001 | <0.001 | <0.001 |
| ISI | 16 (11–23) | 18 (11–23) | 13 (11.0–21.5) | 3 (1–4) | <0.001 | <0.001 | <0.001 |
| HAS | 7 (4–9) | 7 (4–10) | 6 (3–8) | 4 (2.2–5) | <0.001 | <0.001 | 0.011 |
| HDS | 8 (5–11) | 9 (5–11) | 6(4–10) | 5 (4–7) | 0.007 | <0.001 | 0.433 |
Values with mean ± standard deviation or median (interquartile range).
RLS, Restless Legs Syndrome; IRLS, International Restless Legs Syndrome Severity Scale; PSQI, Pittsburgh sleep quality index; ISI, Insomnia severity index; HAS, Hospital anxiety scale; HDS, Hospital depression scale.
*P-value between patients with RLS and healthy controls.
†P-value between patients with drug-naïve RLS and healthy controls.
‡P-value between patients living with drug-treated RLS and healthy controls.
| . | Patients with RLS (N = 69) . | Drug-naïve RLS Patients (N = 44) . | Treated RLS Patients (N = 25) . | Healthy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 57.0 ± 6.7 | 56.3 ± 6.3 | 58.3 ± 7.2 | 55.9 ± 8.1 | 0.418 | 0.791 | 0.213 |
| Male, n (%) | 20 (28.9) | 13 (29.5) | 7 (28.0) | 13 (25.4) | 0.673 | 0.660 | 0.817 |
| Age of onset, years | 47 (41.7–54.0) | 48 (40.5–54.0) | 58 (53.5–65.0) | ||||
| Symptom duration, months | 120 (39–162) | 84 (36–144) | 120 (48–249) | ||||
| IRLS | 27.1 ± 6.4 | 27.2 ± 6.8 | 26.9 ± 5.9 | ||||
| Disease-specific quality of life | 8.7 ± 3.3 | 8.8 ± 3.5 | 8.6 ± 3.1 | ||||
| PSQI | 12 (9.0–14.2) | 12 (9.0–15.5) | 12 (9.5–13.3) | 4 (3–5) | <0.001 | <0.001 | <0.001 |
| ISI | 16 (11–23) | 18 (11–23) | 13 (11.0–21.5) | 3 (1–4) | <0.001 | <0.001 | <0.001 |
| HAS | 7 (4–9) | 7 (4–10) | 6 (3–8) | 4 (2.2–5) | <0.001 | <0.001 | 0.011 |
| HDS | 8 (5–11) | 9 (5–11) | 6(4–10) | 5 (4–7) | 0.007 | <0.001 | 0.433 |
| . | Patients with RLS (N = 69) . | Drug-naïve RLS Patients (N = 44) . | Treated RLS Patients (N = 25) . | Healthy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 57.0 ± 6.7 | 56.3 ± 6.3 | 58.3 ± 7.2 | 55.9 ± 8.1 | 0.418 | 0.791 | 0.213 |
| Male, n (%) | 20 (28.9) | 13 (29.5) | 7 (28.0) | 13 (25.4) | 0.673 | 0.660 | 0.817 |
| Age of onset, years | 47 (41.7–54.0) | 48 (40.5–54.0) | 58 (53.5–65.0) | ||||
| Symptom duration, months | 120 (39–162) | 84 (36–144) | 120 (48–249) | ||||
| IRLS | 27.1 ± 6.4 | 27.2 ± 6.8 | 26.9 ± 5.9 | ||||
| Disease-specific quality of life | 8.7 ± 3.3 | 8.8 ± 3.5 | 8.6 ± 3.1 | ||||
| PSQI | 12 (9.0–14.2) | 12 (9.0–15.5) | 12 (9.5–13.3) | 4 (3–5) | <0.001 | <0.001 | <0.001 |
| ISI | 16 (11–23) | 18 (11–23) | 13 (11.0–21.5) | 3 (1–4) | <0.001 | <0.001 | <0.001 |
| HAS | 7 (4–9) | 7 (4–10) | 6 (3–8) | 4 (2.2–5) | <0.001 | <0.001 | 0.011 |
| HDS | 8 (5–11) | 9 (5–11) | 6(4–10) | 5 (4–7) | 0.007 | <0.001 | 0.433 |
Values with mean ± standard deviation or median (interquartile range).
RLS, Restless Legs Syndrome; IRLS, International Restless Legs Syndrome Severity Scale; PSQI, Pittsburgh sleep quality index; ISI, Insomnia severity index; HAS, Hospital anxiety scale; HDS, Hospital depression scale.
*P-value between patients with RLS and healthy controls.
†P-value between patients with drug-naïve RLS and healthy controls.
‡P-value between patients living with drug-treated RLS and healthy controls.
Glymphatic system function analysis
Differences in diffusivities and DTI-ALPS index between patients with RLS and healthy controls are listed in Table 2. The y-axis diffusivities in the projection fiber were greater in patients with RLS than in healthy controls (0.46 × 10−3 vs. 0.42 × 10−3, p = 0.005). However, the other diffusivities along the x-axis and z-axis in the projection fiber, as well as the x-axis, y-axis, and z-axis in the association and subcortical fibers, were comparable between the groups. The DTI-ALPS index differed significantly between patients with RLS and healthy controls (Figure 2); patients with RLS had a lower DTI-ALPS index than healthy controls (1.48 vs. 1.60, p = 0.008). In the subgroup analysis, the DTI-ALPS index in drug-naïve RLS patients was also lower than healthy controls (1.47 vs. 1.60, p = 0.014). However, there was no significant difference between the drug-treated RLS patients and controls (1.49 vs. 1.60, p = 0.076). No statistically significant difference was observed between groups who administered dopamine agonists and those receiving α2δ ligands (1.552 ± 0.231 vs. 1.383 ± 0.110, p = 0.064). In addition, there was no significant difference in the DTI-ALPS index between drug-naïve and drug-treated RLS patients (1.47 vs. 1.49, p = 0.667). There was also no significant difference in the DTI-ALPS index between the good sleep group and poor sleep group (1.60 vs. 1.62, p = 0.530) in healthy controls.
Differences in Diffusivity and Diffusion Tensor Image Analysis Along the Perivascular Space Index Between Patients With Restless Legs Syndrome and Healthy Controls
| . | Patients with restless legs syndrome (N = 69) . | Patients with drug-naïve RLS (N = 44) . | Treated patients with RLS (N = 25) . | Heathy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . | §P-value . |
|---|---|---|---|---|---|---|---|---|
| Projection fiber | ||||||||
| Dxx | 0.610 ± 0.081 | 0.611 ± 0.085 | 0.611 ± 0.077 | 0.629 ± 0.066 | 0.194 | 0.246 | 0.288 | 0.125 |
| Dyy | 0.467 ± 0.094 | 0.471 ± 0.095 | 0.460 ± 0.094 | 0.422 ± 0.074 | 0.005 | 0.006 | 0.062 | 0.064 |
| Dzz | 1.112 ± 0.096 | 1.115 ± 0.105 | 1.153 ± 0.074 | 1.112 ± 0.092 | 0.612 | 0.813 | 0.124 | 0.399 |
| Association fiber | ||||||||
| Dxx | 0.627 ± 0.095 | 0.614 ± 0.100 | 0.651 ± 0.084 | 0.636 ± 0.097 | 0.626 | 0.281 | 0.519 | 0.995 |
| Dyy | 1.159 ± 0.130 | 1.181 ± 0.134 | 1.121 ± 0.115 | 1.114 ± 0.134 | 0.067 | 0.018 | 0.836 | 0.638 |
| Dzz | 0.384 ± 0.083 | 0.378 ± 0.084 | 0.396 ± 0.085 | 0.381 ± 0.089 | 0.879 | 0.82 | 0.522 | 0.118 |
| Subcortical fiber | ||||||||
| Dxx | 0.997 ± 0.144 | 1.015 ± 0.184 | 0.967 ± 0.137 | 1.013 ± 0.134 | 0.558 | 0.931 | 0.168 | 0.183 |
| Dyy | 0.648 ± 0.144 | 0.649 ± 0.150 | 0.647 ± 0.136 | 0.654 ± 0.122 | 0.787 | 0.827 | 0.802 | 0.964 |
| Dzz | 0.627 ± 0.133 | 0.629 ± 0.120 | 0.624 ± 0.156 | 0.598 ± 0.131 | 0.232 | 0.233 | 0.445 | 0.883 |
| DTI-ALPS index | 1.481 ± 0.241 | 1.4715 ± 0.259 | 1.498 ± 0.213 | 1.601 ± 0.245 | 0.008 | 0.014 | 0.076 | 0.667 |
| . | Patients with restless legs syndrome (N = 69) . | Patients with drug-naïve RLS (N = 44) . | Treated patients with RLS (N = 25) . | Heathy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . | §P-value . |
|---|---|---|---|---|---|---|---|---|
| Projection fiber | ||||||||
| Dxx | 0.610 ± 0.081 | 0.611 ± 0.085 | 0.611 ± 0.077 | 0.629 ± 0.066 | 0.194 | 0.246 | 0.288 | 0.125 |
| Dyy | 0.467 ± 0.094 | 0.471 ± 0.095 | 0.460 ± 0.094 | 0.422 ± 0.074 | 0.005 | 0.006 | 0.062 | 0.064 |
| Dzz | 1.112 ± 0.096 | 1.115 ± 0.105 | 1.153 ± 0.074 | 1.112 ± 0.092 | 0.612 | 0.813 | 0.124 | 0.399 |
| Association fiber | ||||||||
| Dxx | 0.627 ± 0.095 | 0.614 ± 0.100 | 0.651 ± 0.084 | 0.636 ± 0.097 | 0.626 | 0.281 | 0.519 | 0.995 |
| Dyy | 1.159 ± 0.130 | 1.181 ± 0.134 | 1.121 ± 0.115 | 1.114 ± 0.134 | 0.067 | 0.018 | 0.836 | 0.638 |
| Dzz | 0.384 ± 0.083 | 0.378 ± 0.084 | 0.396 ± 0.085 | 0.381 ± 0.089 | 0.879 | 0.82 | 0.522 | 0.118 |
| Subcortical fiber | ||||||||
| Dxx | 0.997 ± 0.144 | 1.015 ± 0.184 | 0.967 ± 0.137 | 1.013 ± 0.134 | 0.558 | 0.931 | 0.168 | 0.183 |
| Dyy | 0.648 ± 0.144 | 0.649 ± 0.150 | 0.647 ± 0.136 | 0.654 ± 0.122 | 0.787 | 0.827 | 0.802 | 0.964 |
| Dzz | 0.627 ± 0.133 | 0.629 ± 0.120 | 0.624 ± 0.156 | 0.598 ± 0.131 | 0.232 | 0.233 | 0.445 | 0.883 |
| DTI-ALPS index | 1.481 ± 0.241 | 1.4715 ± 0.259 | 1.498 ± 0.213 | 1.601 ± 0.245 | 0.008 | 0.014 | 0.076 | 0.667 |
(×10−3 mm2/ s).
Data are mean ± standard deviation values.
Dxx, diffusivity along the x-axis; Dyy, diffusivity along the y-axis; Dzz, diffusivity along the z-axis.
*P-value between patients with RLS and healthy controls.
†P-value between patients with drug-naïve RLS and healthy controls.
‡P-value between patients with drug-treated RLS and healthy controls.
§P-value for drug-naïve- versus drug-treated patients with RLS.
Differences in Diffusivity and Diffusion Tensor Image Analysis Along the Perivascular Space Index Between Patients With Restless Legs Syndrome and Healthy Controls
| . | Patients with restless legs syndrome (N = 69) . | Patients with drug-naïve RLS (N = 44) . | Treated patients with RLS (N = 25) . | Heathy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . | §P-value . |
|---|---|---|---|---|---|---|---|---|
| Projection fiber | ||||||||
| Dxx | 0.610 ± 0.081 | 0.611 ± 0.085 | 0.611 ± 0.077 | 0.629 ± 0.066 | 0.194 | 0.246 | 0.288 | 0.125 |
| Dyy | 0.467 ± 0.094 | 0.471 ± 0.095 | 0.460 ± 0.094 | 0.422 ± 0.074 | 0.005 | 0.006 | 0.062 | 0.064 |
| Dzz | 1.112 ± 0.096 | 1.115 ± 0.105 | 1.153 ± 0.074 | 1.112 ± 0.092 | 0.612 | 0.813 | 0.124 | 0.399 |
| Association fiber | ||||||||
| Dxx | 0.627 ± 0.095 | 0.614 ± 0.100 | 0.651 ± 0.084 | 0.636 ± 0.097 | 0.626 | 0.281 | 0.519 | 0.995 |
| Dyy | 1.159 ± 0.130 | 1.181 ± 0.134 | 1.121 ± 0.115 | 1.114 ± 0.134 | 0.067 | 0.018 | 0.836 | 0.638 |
| Dzz | 0.384 ± 0.083 | 0.378 ± 0.084 | 0.396 ± 0.085 | 0.381 ± 0.089 | 0.879 | 0.82 | 0.522 | 0.118 |
| Subcortical fiber | ||||||||
| Dxx | 0.997 ± 0.144 | 1.015 ± 0.184 | 0.967 ± 0.137 | 1.013 ± 0.134 | 0.558 | 0.931 | 0.168 | 0.183 |
| Dyy | 0.648 ± 0.144 | 0.649 ± 0.150 | 0.647 ± 0.136 | 0.654 ± 0.122 | 0.787 | 0.827 | 0.802 | 0.964 |
| Dzz | 0.627 ± 0.133 | 0.629 ± 0.120 | 0.624 ± 0.156 | 0.598 ± 0.131 | 0.232 | 0.233 | 0.445 | 0.883 |
| DTI-ALPS index | 1.481 ± 0.241 | 1.4715 ± 0.259 | 1.498 ± 0.213 | 1.601 ± 0.245 | 0.008 | 0.014 | 0.076 | 0.667 |
| . | Patients with restless legs syndrome (N = 69) . | Patients with drug-naïve RLS (N = 44) . | Treated patients with RLS (N = 25) . | Heathy controls (N = 51) . | *P-value . | †P-value . | ‡P-value . | §P-value . |
|---|---|---|---|---|---|---|---|---|
| Projection fiber | ||||||||
| Dxx | 0.610 ± 0.081 | 0.611 ± 0.085 | 0.611 ± 0.077 | 0.629 ± 0.066 | 0.194 | 0.246 | 0.288 | 0.125 |
| Dyy | 0.467 ± 0.094 | 0.471 ± 0.095 | 0.460 ± 0.094 | 0.422 ± 0.074 | 0.005 | 0.006 | 0.062 | 0.064 |
| Dzz | 1.112 ± 0.096 | 1.115 ± 0.105 | 1.153 ± 0.074 | 1.112 ± 0.092 | 0.612 | 0.813 | 0.124 | 0.399 |
| Association fiber | ||||||||
| Dxx | 0.627 ± 0.095 | 0.614 ± 0.100 | 0.651 ± 0.084 | 0.636 ± 0.097 | 0.626 | 0.281 | 0.519 | 0.995 |
| Dyy | 1.159 ± 0.130 | 1.181 ± 0.134 | 1.121 ± 0.115 | 1.114 ± 0.134 | 0.067 | 0.018 | 0.836 | 0.638 |
| Dzz | 0.384 ± 0.083 | 0.378 ± 0.084 | 0.396 ± 0.085 | 0.381 ± 0.089 | 0.879 | 0.82 | 0.522 | 0.118 |
| Subcortical fiber | ||||||||
| Dxx | 0.997 ± 0.144 | 1.015 ± 0.184 | 0.967 ± 0.137 | 1.013 ± 0.134 | 0.558 | 0.931 | 0.168 | 0.183 |
| Dyy | 0.648 ± 0.144 | 0.649 ± 0.150 | 0.647 ± 0.136 | 0.654 ± 0.122 | 0.787 | 0.827 | 0.802 | 0.964 |
| Dzz | 0.627 ± 0.133 | 0.629 ± 0.120 | 0.624 ± 0.156 | 0.598 ± 0.131 | 0.232 | 0.233 | 0.445 | 0.883 |
| DTI-ALPS index | 1.481 ± 0.241 | 1.4715 ± 0.259 | 1.498 ± 0.213 | 1.601 ± 0.245 | 0.008 | 0.014 | 0.076 | 0.667 |
(×10−3 mm2/ s).
Data are mean ± standard deviation values.
Dxx, diffusivity along the x-axis; Dyy, diffusivity along the y-axis; Dzz, diffusivity along the z-axis.
*P-value between patients with RLS and healthy controls.
†P-value between patients with drug-naïve RLS and healthy controls.
‡P-value between patients with drug-treated RLS and healthy controls.
§P-value for drug-naïve- versus drug-treated patients with RLS.
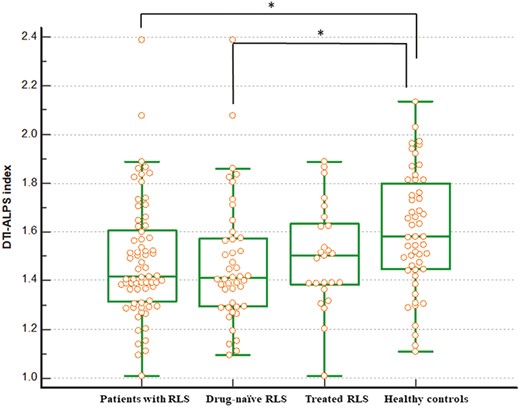
The variations in the diffusion tensor image analysis along the perivascular space (DTI-ALPS) index were observed between individuals diagnosed with RLS and the healthy controls. The figure shows a decreased DTI-ALPS index in patients compared to healthy controls, suggesting glymphatic system dysfunction in patients with RLS. The DTI-ALPS index in patients living with drug-naïve RLS is also lower than that in healthy controls.
Correlation between DTI-ALPS index and the clinical characteristics
There were no significant correlations between DTI-ALPS index and clinical characteristics, including age (r = −0.128, p = 0.295), age of RLS onset (r = −0.034, p = 0.784), symptom duration (r = −0.058, p = 0.636), IRLS (r = 0.108, p = 0.376), RLS Quality of Life Questionnaire (r = 0.071, p = 0.563), PSQI (r = −0.046, p = 0.708), ISI (r = 0.016, p = 0.897), Hospital Anxiety Scale (r = 0.095, p = 0.439), and Hospital Depression Scale (r = −0.003, p = 0.978) in the patients with RLS.
Discussion
The glymphatic system includes both CSF inflow through the periarterial spaces and ISF outflow through perivenous spaces. It facilitates the transportation of immune cells and nutrients as well as disposal of the waste metabolites. Decreased activity of this system has been shown in a variety of sleep-related and neurodegenerative disorders [6]. Currently, there are no direct methods for assessment of inflow and outflow of CSF and ISF as a measure of drainage function of the glymphatic system. The DTI-ALPS method assumes that perivascular ISF movement is prominent along the parallel medullary veins near the lateral ventricles [16]. A low DTI-ALPS index means decreased water diffusivity in perivascular space, indicative of glymphatic system dysfunction [16]. We demonstrated a significantly lower DTI-ALPS index in patients with RLS compared to healthy controls. To the best of our knowledge, this is the first study to evaluate the glymphatic system function in patients with RLS.
The glymphatic system, a mass transport system between the CSF and ISF, is important for channeling metabolic waste and avoiding the accumulation of neurotoxic materials [31].
To assess cortical influx of CSF, in vivo two-photon imaging in awake and sleeping mice demonstrated a 95% reduction in periarterial and parenchymal tracer influx in the awake state compared to sleep.
Xie et al. [32]. It can be hypothesized that diminished interstitial space volume during wakefulness heightens resistance to the flow of convective fluid, consequently inhibiting the influx of CSF. Recent studies reported that the glymphatic system function is associated with sleep disorders or sleep parameters of physiologic conditions [33, 34]. We found that the DTI-ALPS index was lower in the RLS group than in healthy controls which suggests dysfunction in glymphatic system (Figure 3).
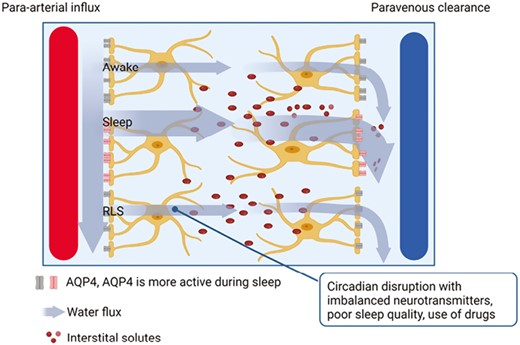
Model of glymphatic system function in awake and sleep state, and in individuals with RLS. Cerebrospinal fluid enters brain tissue via periarterial routes, aided by the presence of aquaporin-4 (AQP4) water channel in astrocytic end-feet. It eliminates solutes from the interstitial space and drains along the veins. AQP4 water channel activity is higher during sleep compared to wakefulness. Based on this, we hypothesize that neurophysiological evidence such as circadian disruption, poor sleep quality, and drug use may be attributed to the glymphatic system dysfunction in patients with RLS.
It is not clear whether the glymphatic system dysfunction is caused by a circadian disruption or, conversely, it is the glymphatic system dysfunction that impairs the balance in levels of neurotransmitters such as dopamine, glutamate, and adenosine in RLS. Particularly, altered dopaminergic neurotransmission is the key mechanism underlying both akathisia and periodic leg movement in RLS, and pre-synaptic hyper-dopaminergic signaling was reported in patients with RLS [1]. Increased dopaminergic stimulation induces postsynaptic down-regulation of the dopamine 2 receptor (D2R, downregulated in the morning, and mildly upregulated in the afternoon). A strong circadian dopaminergic activity aggravates RLS symptoms after sunset [3]. It modulates melanopsin mRNA through D2R in the retina and influences suprachiasmatic nuclei (SCN) development and clock regulation via D1R signaling [3, 35]. Aberrant dopamine signaling disrupts sleep and circadian cycles. Glutamate is a key neurotransmitter involved in brain metabolism and the regulation of the sleep–wake cycle. Glutamate transporters, such as vesicular glutamate transporters and excitatory amino acid transporters, control extracellular glutamate levels in the brain. Their activity is likely modulated in a day–night pattern by the circadian rhythm system [36]. In addition to glutamate, adenosine emerges as a promising candidate for regulating SCN activity. Adenosine receptors play a role in controlling circadian rhythms, sleep homeostasis, and certain neuro-immunological processes. Adenosinergic signaling is involved in regulating various aspects of the circadian clock [37].
Poor sleep quality in patients with RLS could affect the glymphatic system function [38, 39]. The presence of several sleep abnormalities in individuals with RLS, such as delayed sleep onset, reduced total sleep time, decreased sleep efficiency, elevated arousal index, frequent stage shifts, and prolonged REM sleep latency, is well-established [40]. Moreover, patients with RLS exhibit increased wakefulness and N1 sleep alongside decreased sleep stage 2 and REM sleep. Additionally, the PLMS indices and sleep fragmentation index are significantly elevated in RLS individuals [40]. Recent animal studies have revealed that sleep fragmentation, particularly in dementia models, exacerbates the alteration in AQP4 water channel expression and renders susceptibility to glymphatic system dysfunction [41]. Research exploring the link between inadequate sleep quality and glymphatic system performance has shown evidence of the buildup of amyloid-beta and tau, along with the expansion of perivascular spaces in the basal ganglia [42]. Therefore, we categorized the healthy control group based on sleep quality and conducted a subgroup analysis. In this study, we did not observe a significant difference in the DTI-ALPS index based on sleep quality. Nevertheless, it should be noted that the healthy control group had a limited sample size, and within the poor sleep group, most individuals had PSQI scores concentrated around 5 or 6.
Interestingly, drug-treated RLS patients tended to have lower glymphatic system function index compared to the normal group, but there was no statistical difference. Also, no significant difference was seen between patients living with drug-naïve RLS and patients with drug-treated RLS. Due to the limited sample size, the potential impact of medications cannot be ruled out. While it is well-established that α2δ ligands demonstrate greater improvement in sleep architecture, primarily by increasing the percentage of slow-wave sleep, compared to dopamine agonists [43], it was challenging to make a direct comparison in the patient population of this study due to the very low proportion of patients solely using α2δ ligands. Therefore, future studies should include larger cohorts, and it would be beneficial to verify our findings through longitudinal studies that compare the drug-naïve and drug-treated states within the same patients before and after treatment.
The function of glymphatic system may also be impaired in the aging brain which is more affected by RLS [44]. The main reasons attributed to age-related glymphatic system dysfunction are noted as the loss of perivascular AQP4 polarization and a decrease in cerebral arterial pulsatility [44]. Impaired distribution of neurotransmitters, carrier proteins, and other solutes due to aging is exacerbated by glymphatic system dysfunctions. However, contrary to our initial hypothesis, we found no correlation between the DTI-ALPS index and age in patients with RLS. These factors could be attributed to medication usage, the size of the sample, and the limited age range within the RLS patient group.
This was the first study to evaluate the glymphatic system function in patients with RLS. However, this study has several limitations. First, the DTI-ALPS method itself has limitations. This method is only capable of calculating diffusivity along the x, y, and z axes, which are components of the diffusion tensors. As a result, it is only possible to independently evaluate diffusivity along the perivascular space in the outer region of the lateral ventricle, particularly within the plane of the lateral ventricle body. In areas where the perivascular space does not align with the x, y, or z axes, or in regions where the perivascular space and the predominant fiber tract have parallel orientations, it is not possible to isolate and evaluate the diffusivity along the perivascular space. Secondly, MRI scans were conducted during daytime hours for both individuals with RLS and the healthy control group. If the MRI had been performed during the nighttime when RLS symptoms were present, the results may have been different. However, the analysis of the DTI-ALPS index based on DTI acquisition time demonstrated an intraclass correlation coefficient of 0.828 in four test–retest iterations, indicating that it is not influenced by data acquisition time, as previously confirmed in prior studies [45]. Third, we could not evaluate the direction of causality between glymphatic system function and RLS in this cross-sectional study. Lastly, a selection bias cannot be excluded due to the single-center study design and ethnic background. To validate our findings, a longitudinal multicenter study is necessary.
Conclusion
The DTI-ALPS method may function as a biomarker in evaluating the glymphatic system function in patients with RLS. Although the direction of causality is not clear, this study revealed a decrease in DTI-ALPS index in individuals with RLS, suggesting glymphatic system dysfunction. However, no correlation between the index and clinical manifestation was observed.
Acknowledgment
None.
Disclosure statements
Financial disclosure: None. Nonfinancial disclosure: None.
Author Contributions
K.M.P. and K.T.K. equally contributed to paper writing and data analysis as first authors. Y.W.C. and K.T.K. contributed to patient recruitment, data control, and organization of the collected data. D.A.L and G.K.M contributed to the first manuscript draft and review. Y.W.C. supervised the paper writing. All the authors participated in the analysis and interpretation of data. All the authors revised the manuscript critically and approved the manuscript in its final form.
Data Availability
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data are not available.
References
Author notes
Kang Min Park and Keun Tae Kim contributed equally to this study.






Comments