-
PDF
- Split View
-
Views
-
Cite
Cite
Donald L Bliwise, Ting-Chuan Wang, Vladimir Svetnik, Gary Zammit, Peining Tao, Christopher Lines, W Joseph Herring, Phase advance of bedtimes in Alzheimer’s disease, Sleep, Volume 46, Issue 11, November 2023, zsad191, https://doi.org/10.1093/sleep/zsad191
Close - Share Icon Share
Dear Editor,
Dysfunction of the circadian timing system is a distinguishing feature of many neurodegenerative diseases [1]. We report here on an apparent phase advance in the self-selected bedtimes of patients with Alzheimer’s disease (AD) observed during baseline of a clinical trial.
This analysis used baseline data from two randomized controlled trials evaluating suvorexant versus placebo in participants with insomnia (MSD Protocols 028 and 029; clinicaltrials.gov NCT01097616 and NCT01097629) [2] and a randomized controlled trial in participants with AD and insomnia (MSD Protocol 061; clinicaltrials.gov NCT02750306) [3]. All three trials were conducted in accordance with principles of Good Clinical Practice and were approved by Institutional Review Boards. Written Informed Consent was provided by the participant or their legal representative. Eligible participants for the two non-AD trials were ≥ 18 years of age, met Diagnostic and Statistical Manual of Mental Disorders (DSM)—4th Edition Text Revision criteria for primary insomnia, and had to have a regular bedtime between 9 PM and 1 AM. Eligible participants for the AD trial were 50–90 years of age, met National Institute on Aging-Alzheimer’s Association or DSM—5th Edition (DSM-5) clinical criteria for probable Alzheimer’s disease dementia, as well as DSM-5 criteria for insomnia, and had to have a regular bedtime between 8 PM and 1 AM. In all three trials, overnight polysomnography in a sleep laboratory, with lights-off at the participant’s habitual bedtime, was performed at a screening visit 14 days before randomization (which also served as a laboratory adaptation night) and at a baseline visit 7 days before randomization. Use of sedating medications was prohibited before and during the trials, and there were restrictions on the use of alcohol, caffeine and tobacco. Participants with AD could be taking an acetylcholinesterase inhibitor and/or memantine provided they were on stable doses prior to screening.
For the purposes of this post hoc analysis comparing “elderly insomnia” (EI) participants versus “AD+insomnia” (ADI) participants, we limited the analysis to the subset of participants who were 55–80 years of age in the trials. The analysis was based on data from the baseline polysomnography visit. Data from the two non-AD trials were combined. Bedtimes were determined on the initial sleep lab night based on the participant’s median habitual bedtime as reported using sleep diaries. To account for the 1-hour earlier habitual bedtime inclusion criteria in the ADI study, we calculated frequency distributions by quarter hour for only those participants with lights-off times (i.e., habitual bedtimes) between 9 PM and 1 AM in both the EI and ADI populations.
The number of participants age 55–80 years was 869 in the EI studies and 235 in the ADI study. The EI and ADI samples did not differ in the proportions of participants who were men (37.9% for EI; 36.2% for ADI) but differed in the proportions of participants who self-identified as non-White (6.4% for EI; 40.4% for ADI). All participants in both studies were negative for major depression, psychosis, drug abuse, and other comorbid neurologic conditions.
When comparing the samples, 845 (97%) in the EI studies and 186 (79%) in the ADI study had lights-off times between 9 PM and 1 AM, with the lower percentage in the ADI population reflecting the 1-hour earlier habitual bedtime inclusion criteria in that study (but not included in our analysis). The frequency distributions of lights-off times between 9 PM and 1 AM in the two populations are shown in Figure 1 and indicate a clear leftwards shift toward earlier bedtimes in the ADI population. The median bedtime in the ADI population was 21:52 in the ADI population versus 22:41 in the EI population, a difference of 49 minutes. The difference in bedtime distributions met statistical significance in a post hoc chi-square analysis using 1-hour bins to generate a 2 × 4 chi-square (χ2 = 101, df = 3, P < 0.001).
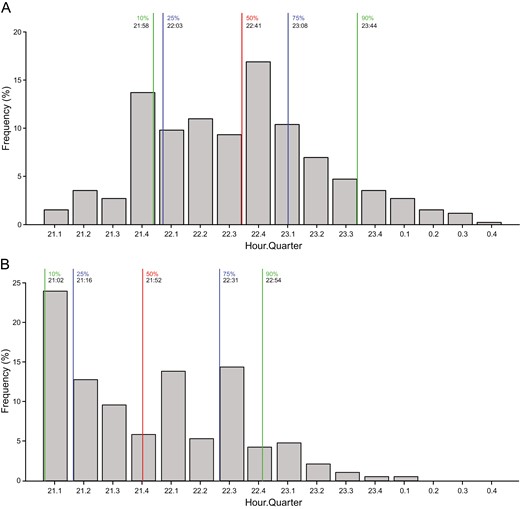
Frequency distributions of lights-off times by hour quarter for EI (A) and ADI (B).
The altered chronobiology of people with AD has been investigated previously. The most commonly cited finding has been a reduction in the “amplitude” of the sleep–wake rhythm, typically inferred from changes in actigraphically measured rest–activity rhythms [4], which portend dementia. These findings have been corroborated by neuropathologic studies [5] linking such rhythm changes to deterioration of the primary circadian pacemaker in the mammalian nervous system, the suprachiasmatic nucleus (SCN).
Associated changes in circadian phase in AD have been studied less often and frequently conflict [6]. Summaries of this literature typically have described phase delay in people with AD [7, 8]. This predominant perspective has been grounded in seminal studies on small series (n’s ≤ 30) of select cases encompassing ante-mortem actigraphic observations when compared to post-mortem observations of neuronal loss, though not necessarily amyloidosis, in the human SCN [5, 9–11]. Although these elegant neuropathological studies remain unique contributions to the literature, they do not jibe with other early work suggesting that AD patients have earlier bedtimes and/or earlier peaks in body temperature [12–14] relative to elderly controls [6]. More recent population-based data involving large cohorts of hundreds or thousands of participants using either actigraphy [15–17] or self-reports [18–20] have also suggested that cognitive decline is associated with earlier peaks in activity rhythms or earlier bedtimes, though a few cohort studies have not reported this [21, 22] or the finding appears dependent upon the methods of analyses of actigraphy [17, 23].
Phase advances in rest–activity rhythms have long been recognized as a key chronobiologic feature of aging in other mammalian species [24]. If cell loss in the SCN represents the substrate for changes in circadian rhythms in human AD [10], then one might also expect that experimental models involving lesions in this region would produce similar phase advances. Davis and Gorski [25] reported that the magnitude of experimental lesions in the rodent SCN (expressed as a percentage of volume loss) was strongly correlated (r = .73) with a phase advance of free-running rest–activity rhythms recorded under constant conditions. To the extent that neuronal loss in the human SCN in living persons with AD is likely to represent a graded effect, these basic science findings and the studies of human populations suggesting early bedtimes in dementing illness are consistent with each other.
Our post hoc analyses of baseline data from randomized clinical trials suggest that people with AD and their caregivers “elect” earlier bedtimes when undergoing polysomnography in a laboratory environment. This difference approached 50 minutes and was observed despite truncating bedtimes occurring within the 1-hour earlier allowable bedtime window in the AD protocol. Whether this observation represents a true change in the endogenous circadian timing system independent of entrainment in individuals with AD or instead represents an issue related to convenience for caregivers (or sleep laboratory staff), or differing conventions used by investigative sites in the EI studies versus the ADI study for scheduling participants, cannot be determined, but the former remains a possibility. Alternatively, earlier bedtimes might also suggest a weakening of the homeostatic regulation of wakefulness and sleep with advanced age and in AD [26]. Finally, the higher proportion of non-White individuals among the ADI study participants might have played a role as well.
In summary, these data suggest that people with AD self-select earlier bedtimes, which could be compatible with the phase advance of endogenous circadian rhythms, possibly resulting from graded and progressive SCN deterioration.
Funding
The study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD).
Conflict of Interest Statement
DLB has acted as a consultant for MSD, Jazz, Eisai, Idorsia, and Ferring. TCW, VS, PT, CL and WJH are employees of, and own stock options in, MSD. GZ is an employee of Clinilabs Drug Development Corporation and a stockholder of Clinilabs Drug Development Corporation, Home Sleep and Respiratory Care, and Sleep Disorders Institute. He has acted as consultant or contractor for MSD, Idorsia Pharmaceuticals Eisai, Janssen, Jazz Pharmaceuticals, Purdue, and Takeda.
Data Availability
MSD’s data sharing policy, including restrictions, is available at https://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical trial data can be submitted through the EngageZone site or via email to [email protected].





Comments