-
PDF
- Split View
-
Views
-
Cite
Cite
Anne-Sophie Winter, Christian Haverkamp, Christian Gratzke, Roman Huber, Ann-Kathrin Lederer, Valerian and postoperative sleep: a retrospective cohort analysis of gynecological, urologic, and general surgical patients, Sleep, Volume 45, Issue 10, October 2022, zsac122, https://doi.org/10.1093/sleep/zsac122
Close - Share Icon Share
Abstract
Postoperative sleep disturbances appear to be a common complication after surgery being treated with sleep-promoting medication such as valerian, but robust data and evidence of medicinal approaches are lacking.
We performed a retrospective cohort analysis of all 21 168 urological, gynecological, and general surgical patients of the University Medical Center Freiburg, Germany, who underwent surgery between 2015 and 2020. Target parameters were the usage of sleep-promoting medication to estimate the occurrence of postoperative sleep disturbances as well as the kind of sleep medication with a special focus on herbal medication such as valerian.
Drug-treated sleep disturbances occurred in 15% (n = 3083) of the patients. Valerian was the second most applied drug (n = 814, 26.4%) after classic benzodiazepines (n = 1 138, 36.9%). The majority of patients got valerian as monotherapy. Age, length of stay, and comorbidities were associated with demand for sleep medication in general (p < .001). Valerian monotherapy was more common in women (OR 1.53, 95% CI: 1.33–1.77, p < .001), elderly patients (OR 1.50, 95% CI: 1.29–1.75, p < .001), and patients with prolonged hospital stay (OR 2.23, 95% CI: 1.91–2.61, p < .001).
Valerian plays an important role in the treatment of postoperative sleep disturbances clinically, and it appears to be a promising therapeutic approach especially in women, older and sicker patients, and those with prolonged hospital stay. Further research has to clarify the efficacy of valerian postoperatively.
DRKS00027903, https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00027903
Sleep disturbances after surgery are suggested to negatively affect patients’ recovery process. Therefore, it is important to learn more about the frequency of postoperative sleep disturbances and the appropriate treatment approaches to improve postoperative sleep, which might also improve surgical outcome. As patients’ interest in complementary and alternative medicine increases, questions about its plausibility, applicability, and efficacy arise. This is the first trial to investigate the occurrence of postoperative sleep disturbances as well as the kind of sleep medication with a special focus on valerian in gynecological, urologic, and general surgical patients.
Introduction
Sleep disturbances are very common in Western countries as approximately 6% of the adult population suffer regularly and chronically from symptoms of insomnia [1]. Patients with insomnia report about reduced sleep quality, problems falling asleep, and staying asleep through the night as well as early awakening without subjectively feeling rested [2, 3]. Sleep disturbances can lead to daytime fatigue, flaws in attention, as well as in job related and social impairments [3]. They often occur in relation to stress or in combination with psychiatric diseases such as depression [4]. One example of exposing not only the body but also the mind and psyche to massive stress are surgical interventions [5, 6]. Hence, postoperative sleep disturbances are one of the most common complications after surgery being associated with acute changes of sleep structure, mostly reduced time of deep sleep, or REM sleep [7–10]. Multiple factors including unfamiliar surroundings, pain, or physical discomfort promote reduced sleep quality postoperatively [11]. The problem is often neglected but can be classified as “acute insomnia” which starts at a definable time and in most cases, even if some patients report persistent difficulty with sleeping after being discharged, usually lasts less than three months [12]. It is known that reduction of stress and pain after surgery is necessary to support the postoperative healing process [13]. As recent research shows that sleep disturbances aggravate stress and decrease resistance of patients, it can be assumed that sleep disturbances have negative effects on the recovery of (surgical) patients [7, 9, 14]. Recent research shows that sleep disturbances increase the physical stress reaction of the human body and disturb the circadian rhythm [15]. Sleep deprivation leads to higher inflammatory markers and cortisol levels [16]. In patients with sleep disturbances, postoperative delirium is also more likely to occur [17]. Scientific evidence for medical treatment of acute postoperative sleep disturbances is widely lacking. Commonly, acute and chronic sleep disturbances are treated by hypnotics (benzodiazepine receptor agonists, Z-substances), antihistamines, and melatonin receptor agonists as well as herbal medicine such as valerian root extracts. Valerian has been used for nervous restlessness and sleep problems in non-professional medicine for more than 300 years [18]. The drug is extracted from the root of Valeriana officinalis L., a plant species native to Europe and Asia.
The main constituents include valpotriates, volatile sesquiterpenes, and valerenic acid and lignans [19]. It is suggested that a lignan derivate named olivil, which binds to adenosine A1- and GABA-A receptors, may be responsible for valerian’s sedative effects [20]. Clinical studies suggest positive effects of valerian on chronic insomnia, but the significance of these studies is limited due to methodical flaws [21]. Trials with surgical patients are lacking and international guidelines do not recommend valerian and other herbal sleep medication in the treatment of insomnia disorders [1]. At the University Medical Center Freiburg, valerian extract LI 156 (Sedonium Klosterfrau Berlin GmbH) has been used. The aim of this study was evaluating the frequency of medical treatment of postoperative sleep disturbances as well as evaluating the kind and frequency of postoperative use of valerian in gynecological, urologic, and visceral surgical patients of Medical Center, University of Freiburg, a tertiary care center in South-West Germany.
Methods
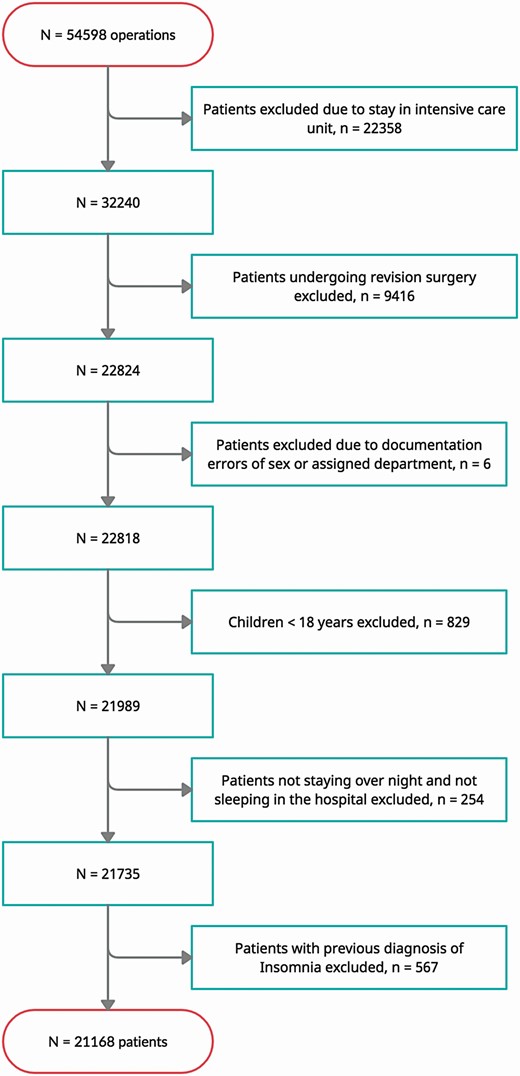
We performed a monocentric, retrospective cohort study at the University Medical Center Freiburg, one of the largest university centers in Germany with more than 2000 hospital beds and 90 000 patients per year. The study was approved by the ethics committee of the University of Freiburg (EK FR 158/20) before onset. The study was registered at the German Clinical Trials Register (DRKS00027903). Pre-defined inclusion criteria were elective surgery at the Department of Gynecology, the Department of Urology or the Department of General- and Visceral Surgery between January 2015 and December 2019 and age >18 years. Excluded were unknown sex, patients who could not clearly be assigned to a treating clinical department, children (< 18 years of age), previous diagnosis of insomnia, patients staying on intensive care unit, and patients who underwent revision surgery due to postoperative complications.
None of the departments had a postoperative standard operating procedure for treatment of postoperative sleep disturbances.
Data acquisition
For retrospective data collection, general patient information (clinical department, date of admission, discharge and surgery, and patient ID), demographic details (date of birth, age, and sex) as well as information on medication (Anatomical Therapeutic Chemical Classification System (ATC)), previous illnesses (International Statistical Classification of Diseases and Related Health Problems [ICD]), main diagnosis (ICD), and German procedure classification (OPS) classifying type of surgery were obtained from the data integration center established within the medical informatics initiative [22]. Source data for the medication administration were the electronic patient chart “Meona” (Meona GmbH, Freiburg, Germany). A postoperative period of 5 days was considered for analysis with one day starting at 06:00 am and lasting 24 hr by definition. If patients stayed in hospital overnight but less than 24 hr, they were also included. Comorbidities were evaluated using the Elixhauser Comorbidity Index and the corresponding van Walraven Score, which condenses Elixhauser’s Index into a single numeric score based on its 31 ICD-10 diagnoses. The scoring system was initially created “to be used with administrative databases for predicting in-hospital mortality“ [23] with a range from −19 (less likely for in-hospital death) to 89 (more likely for in-hospital death) [24].
Patients were only included once. If patients were attributed to multiple cases, the first surgical treatment was chosen. Patients were separated in a cohort with and a cohort without postoperative sleep disturbances. As seen in a precursor trial for feasibility, we found no standardized electronic documentation on acute postoperative sleep disturbances in all patients. We decided to estimate the occurrence of postoperative sleep disturbances in patients, who received sleep medication postoperatively given out by medical staff. This is also justified by the fact that subjective expression of symptoms is sufficient for the diagnosis of non-organic sleep disorders (ICD F51.0) [25]. Patients who needed sleep medication were descriptively separated in patients receiving monotherapy, patients receiving combined therapy and patients in need of rescue therapy after the use of valerian and other treatment regimes. Therapy groups and types of sleep medication are shown in Table 1. For comparison of a potential efficacy of valerian, the cohort was separated into three groups: First “no sleep medication received,” second “valerian monotherapy,” and third “valerian combination therapy,” the latter including also valerian rescue therapy as a subgroup.
| . | Definition . | Included medication . |
|---|---|---|
| No therapy received | Patients without sleep medication | |
| Monotherapy | Single drug use for treating sleep disorder during the five day observation period. For clarity, only drugs applicated in more than 50 patients were considered | -Valerian -Classic benzodiazepines (BZ; i.e. midazolam, flurazepam, lormetazepam) -Benzodiazepine receptor agonists (BZRA; i.e. zopiclone, zolpidem) -Melperone -Quetiapine -Risperidone and haloperidol |
| Combination therapy | Treatment of sleep disorder using more than one drug during the 5 day observation period | -Valerian and BZ -Valerian and BZRA -Valerian, BZ and other drugs -Valerian and other drugs |
| Subgroup of combination therapy: Rescue therapy | Application of another sleep medication within 12 hr after usage of valerian | - BZ, BZRA |
| Other* | Other drugs of the ATC N05 group “Psycholeptics” (N05 A- Antipsychotics, N05B- Anxiolytics, N05C-Hypnotics and Sedatives) possibly used to treat sleep disorders | -Dexmedetomidin -Thioridazin -Tiaprid, sulpirid -Distraneurin -Zuclopentixol -Aripiprazol -Levopromazin -Chlorprothixene -Chloral hydrate -Lithium -Clozapine, olanzapine -Melantonin receptor agonists -Prothipendyl -Pipamperone |
| . | Definition . | Included medication . |
|---|---|---|
| No therapy received | Patients without sleep medication | |
| Monotherapy | Single drug use for treating sleep disorder during the five day observation period. For clarity, only drugs applicated in more than 50 patients were considered | -Valerian -Classic benzodiazepines (BZ; i.e. midazolam, flurazepam, lormetazepam) -Benzodiazepine receptor agonists (BZRA; i.e. zopiclone, zolpidem) -Melperone -Quetiapine -Risperidone and haloperidol |
| Combination therapy | Treatment of sleep disorder using more than one drug during the 5 day observation period | -Valerian and BZ -Valerian and BZRA -Valerian, BZ and other drugs -Valerian and other drugs |
| Subgroup of combination therapy: Rescue therapy | Application of another sleep medication within 12 hr after usage of valerian | - BZ, BZRA |
| Other* | Other drugs of the ATC N05 group “Psycholeptics” (N05 A- Antipsychotics, N05B- Anxiolytics, N05C-Hypnotics and Sedatives) possibly used to treat sleep disorders | -Dexmedetomidin -Thioridazin -Tiaprid, sulpirid -Distraneurin -Zuclopentixol -Aripiprazol -Levopromazin -Chlorprothixene -Chloral hydrate -Lithium -Clozapine, olanzapine -Melantonin receptor agonists -Prothipendyl -Pipamperone |
*Other drugs were not considered for group comparison.
| . | Definition . | Included medication . |
|---|---|---|
| No therapy received | Patients without sleep medication | |
| Monotherapy | Single drug use for treating sleep disorder during the five day observation period. For clarity, only drugs applicated in more than 50 patients were considered | -Valerian -Classic benzodiazepines (BZ; i.e. midazolam, flurazepam, lormetazepam) -Benzodiazepine receptor agonists (BZRA; i.e. zopiclone, zolpidem) -Melperone -Quetiapine -Risperidone and haloperidol |
| Combination therapy | Treatment of sleep disorder using more than one drug during the 5 day observation period | -Valerian and BZ -Valerian and BZRA -Valerian, BZ and other drugs -Valerian and other drugs |
| Subgroup of combination therapy: Rescue therapy | Application of another sleep medication within 12 hr after usage of valerian | - BZ, BZRA |
| Other* | Other drugs of the ATC N05 group “Psycholeptics” (N05 A- Antipsychotics, N05B- Anxiolytics, N05C-Hypnotics and Sedatives) possibly used to treat sleep disorders | -Dexmedetomidin -Thioridazin -Tiaprid, sulpirid -Distraneurin -Zuclopentixol -Aripiprazol -Levopromazin -Chlorprothixene -Chloral hydrate -Lithium -Clozapine, olanzapine -Melantonin receptor agonists -Prothipendyl -Pipamperone |
| . | Definition . | Included medication . |
|---|---|---|
| No therapy received | Patients without sleep medication | |
| Monotherapy | Single drug use for treating sleep disorder during the five day observation period. For clarity, only drugs applicated in more than 50 patients were considered | -Valerian -Classic benzodiazepines (BZ; i.e. midazolam, flurazepam, lormetazepam) -Benzodiazepine receptor agonists (BZRA; i.e. zopiclone, zolpidem) -Melperone -Quetiapine -Risperidone and haloperidol |
| Combination therapy | Treatment of sleep disorder using more than one drug during the 5 day observation period | -Valerian and BZ -Valerian and BZRA -Valerian, BZ and other drugs -Valerian and other drugs |
| Subgroup of combination therapy: Rescue therapy | Application of another sleep medication within 12 hr after usage of valerian | - BZ, BZRA |
| Other* | Other drugs of the ATC N05 group “Psycholeptics” (N05 A- Antipsychotics, N05B- Anxiolytics, N05C-Hypnotics and Sedatives) possibly used to treat sleep disorders | -Dexmedetomidin -Thioridazin -Tiaprid, sulpirid -Distraneurin -Zuclopentixol -Aripiprazol -Levopromazin -Chlorprothixene -Chloral hydrate -Lithium -Clozapine, olanzapine -Melantonin receptor agonists -Prothipendyl -Pipamperone |
*Other drugs were not considered for group comparison.
Estimation of a potential therapeutic effect of valerian
The evaluation of a potential therapeutic effect of valerian was exploratory. Due to the retrospective study character, it is not possible to differentiate between a placebo- or drug-intended efficacy of valerian. In patients with valerian monotherapy, we assumed that application of valerian was sufficient in the treatment of postoperative sleep disturbances, as patients did not ask for further sleep medication. In patients with valerian combination therapy (one day valerian, another day alternative sleep medication), we assumed that valerian was rather not sufficient in the treatment of postoperative sleep disturbances. Finally, we assumed that valerian was clearly not sufficient in patients who required immediate (within 12 hr) rescue therapy after valerian was applied.
Primary and secondary targets
The primary target of the study was to identify the total number of patients being treated with sleep medication during the first and fifth postoperative day after gynecological, urologic, or general surgery. On the basis of these data, we aimed to estimate the frequency of postoperative sleep disturbances. The secondary aim was to capture the kind of sleep-promoting medication to compare herbal and classic sleep medication including also potentially influencing sociodemographic data (age, sex, surgical therapy, length of hospital stay, and comorbidities). Furthermore, as a secondary target, we also tried to estimate efficacy of valerian comparing patients with valerian monotherapy and those with valerian combination therapy (including the rescue therapy mentioned above).
Statistical analysis
Statistical analysis was performed using R (R PBC, Boston, USA). The majority of the analysis was carried out descriptively including information either in number or percentage, mean and standard deviation as well as median and range. A p-value < .05 was considered statistically significant. Chi-squared test was performed to examine the relation between sociodemographic parameters (age, gender, length of hospital stay, and weighted Elixhauser comorbidity score by van Walraven [23]) and primary targets. Logistic regression was applied to assess the association between sociodemographic parameters and the primary and secondary targets. Results are shown as odds ratio (OR) with 95% confidence interval (CI).
Results
From January 2015 to January 2020, a total of 22824 patients underwent surgery at the departments of Urology, Gynecology, and General and Visceral Surgery at Medical Center, University of Freiburg, without staying at an intensive care unit and without need of revision surgery. In total, 21 168 patients were eligible for study inclusion (Figure 1). Most patients were treated in General and Visceral Surgery (n = 9516, 45%). The mean age of included patients was 56.99 years (range 18–106 years, SD ± 17.73 years) with 10 302 patients older than 60 years. More than half of the patients were female (n = 1610, 52.2%). The mean length of stay was 6.51 days (range 0.21–99.25 days, SD ± 6.24 days; patients, staying <1 day, had a hospital stay of less than 24 hr, but with at least one night in hospital). Almost 55% of all patients (n = 11633) stayed less than 5 days. Elixhauser Comorbidity Score was 4.21 points on average (range (−14)−58 points, SD ± 6.59 points). Overall, 18 085 patients did not receive sleep medication during the 5-day observation period, whereas 3083 patients did receive sleep medication. Less than 10% of patients (n = 1532, 8.4%) stayed for one night, and only one of these patients received sleep medication. Different medical sleep therapies, localization of surgery, as well as demographic data are summarized in Table 2.
| . | Sleep medication (n = 3083) . | No sleep medication (n = 18 085) . | Total (n = 21 168) . |
|---|---|---|---|
| Sex | |||
| Male | 1473 (47.8%) | 8718 (48.2%) | 10191 (48.1%) |
| Female | 1610 (52.2%) | 9367 (51.8%) | 10977 (51.9%) |
| Age | |||
| Mean (SD) | 62.40 (16.44) | 56.06 (17.78) | 56.99 (17.73) |
| Range | 18.00 - 98.00 | 18.00 - 106.00 | 18.00 - 106.00 |
| Age categories | |||
| <60 years | 1160 (37.6%) | 9706 (53.7%) | 10866 (51.3%) |
| 60+ years | 1923 (62.4%) | 8379 (46.3%) | 10302 (48.7%) |
| Department of hospitalization | |||
| General and visceral Surgery | 1482 (48.1%) | 8034 (44.4%) | 9516 (45.0%) |
| Urology | 881 (28.6%) | 5430 (30.0%) | 6311 (29.8%) |
| Gynecology | 720 (23.4%) | 4621 (25.6%) | 5341 (25.2%) |
| Hospital stay | |||
| Mean (SD, days) | 9.33 (7.49) | 6.03 (5.87) | 6.51 (6.24) |
| Range | 1.42 - 77.92 | 0.21 - 99.25 | 0.21 - 99.25 |
| ≤5 days | 920 (29.8%) | 10713 (59.2%) | 11633 (55.0%) |
| >5 days | 2163 (70.2%) | 7372 (40.8%) | 9535 (45.0%) |
| Sleep medication | |||
| Mono: Valerian | 814 (26.4%) | 0 (0.0%) | 814 (3.8%) |
| Mono: Classic benzodiazepines | 1138 (36.9%) | 0 (0.0%) | 1138 (5.4%) |
| Mono: Benzodiazepine receptor agonists | 538 (17.5%) | 0 (0.0%) | 538 (2.5%) |
| Comb: Valerian/benzodiazepine | 191 (6.2%) | 0 (0.0%) | 191 (0.9%) |
| Comb: Valerian/benzodiazepine/ other | 5 (0.2%) | 0 (0.0%) | 5 (0.0%) |
| Comb: Valerian/other | 22 (0.7%) | 0 (0.0%) | 22 (0.1%) |
| Mono: Risperidone or haloperidol | 62 (2.0%) | 0 (0.0%) | 62 (0.3%) |
| Mono: Melperone | 84 (2.7%) | 0 (0.0%) | 84 (0.4%) |
| Mono: Quetiapine | 54 (1.8%) | 0 (0.0%) | 54 (0.3%) |
| Other | 175 (5.7%) | 0 (0.0%) | 175 (0.8%) |
| Rescue therapy, valerian to benzodiazepines within twelve hours | 121 (55.5%) | 0 | 121 (55.5%) |
| No rescue | 97 (44.5%) | 0 | 97 (44.5%) |
| Localization of surgery | |||
| Hernia repair | 85 (2.8%) | 654 (3.6%) | 739 (3.5%) |
| Digestive tract | 837 (27.1%) | 4848 (26.8%) | 5685 (26.9%) |
| Urinary tract | 513 (16.6%) | 3015 (16.7%) | 3528 (16.7%) |
| Male reproductive organs | 377 (12.2%) | 2166 (12.0%) | 2543 (12.0%) |
| Female reproductive organs | 222 (7.2%) | 2282 (12.6%) | 2504 (11.8%) |
| Obstetrics | 6 (0.2%) | 120 (0.7%) | 126 (0.6%) |
| Breast surgery | 331 (10.7%) | 1409 (7.8%) | 1740 (8.2%) |
| Other | 712 (23.1%) | 3591 (19.9%) | 4303 (20.3%) |
| Elixhauser, van Walraven Score | |||
| Mean (SD, points) | 6.05 (7.53) | 3.89 (6.37) | 4.21 (6.59) |
| Range | −14.00–53.00 | −11.00–58.00 | −14.00–58.00 |
| . | Sleep medication (n = 3083) . | No sleep medication (n = 18 085) . | Total (n = 21 168) . |
|---|---|---|---|
| Sex | |||
| Male | 1473 (47.8%) | 8718 (48.2%) | 10191 (48.1%) |
| Female | 1610 (52.2%) | 9367 (51.8%) | 10977 (51.9%) |
| Age | |||
| Mean (SD) | 62.40 (16.44) | 56.06 (17.78) | 56.99 (17.73) |
| Range | 18.00 - 98.00 | 18.00 - 106.00 | 18.00 - 106.00 |
| Age categories | |||
| <60 years | 1160 (37.6%) | 9706 (53.7%) | 10866 (51.3%) |
| 60+ years | 1923 (62.4%) | 8379 (46.3%) | 10302 (48.7%) |
| Department of hospitalization | |||
| General and visceral Surgery | 1482 (48.1%) | 8034 (44.4%) | 9516 (45.0%) |
| Urology | 881 (28.6%) | 5430 (30.0%) | 6311 (29.8%) |
| Gynecology | 720 (23.4%) | 4621 (25.6%) | 5341 (25.2%) |
| Hospital stay | |||
| Mean (SD, days) | 9.33 (7.49) | 6.03 (5.87) | 6.51 (6.24) |
| Range | 1.42 - 77.92 | 0.21 - 99.25 | 0.21 - 99.25 |
| ≤5 days | 920 (29.8%) | 10713 (59.2%) | 11633 (55.0%) |
| >5 days | 2163 (70.2%) | 7372 (40.8%) | 9535 (45.0%) |
| Sleep medication | |||
| Mono: Valerian | 814 (26.4%) | 0 (0.0%) | 814 (3.8%) |
| Mono: Classic benzodiazepines | 1138 (36.9%) | 0 (0.0%) | 1138 (5.4%) |
| Mono: Benzodiazepine receptor agonists | 538 (17.5%) | 0 (0.0%) | 538 (2.5%) |
| Comb: Valerian/benzodiazepine | 191 (6.2%) | 0 (0.0%) | 191 (0.9%) |
| Comb: Valerian/benzodiazepine/ other | 5 (0.2%) | 0 (0.0%) | 5 (0.0%) |
| Comb: Valerian/other | 22 (0.7%) | 0 (0.0%) | 22 (0.1%) |
| Mono: Risperidone or haloperidol | 62 (2.0%) | 0 (0.0%) | 62 (0.3%) |
| Mono: Melperone | 84 (2.7%) | 0 (0.0%) | 84 (0.4%) |
| Mono: Quetiapine | 54 (1.8%) | 0 (0.0%) | 54 (0.3%) |
| Other | 175 (5.7%) | 0 (0.0%) | 175 (0.8%) |
| Rescue therapy, valerian to benzodiazepines within twelve hours | 121 (55.5%) | 0 | 121 (55.5%) |
| No rescue | 97 (44.5%) | 0 | 97 (44.5%) |
| Localization of surgery | |||
| Hernia repair | 85 (2.8%) | 654 (3.6%) | 739 (3.5%) |
| Digestive tract | 837 (27.1%) | 4848 (26.8%) | 5685 (26.9%) |
| Urinary tract | 513 (16.6%) | 3015 (16.7%) | 3528 (16.7%) |
| Male reproductive organs | 377 (12.2%) | 2166 (12.0%) | 2543 (12.0%) |
| Female reproductive organs | 222 (7.2%) | 2282 (12.6%) | 2504 (11.8%) |
| Obstetrics | 6 (0.2%) | 120 (0.7%) | 126 (0.6%) |
| Breast surgery | 331 (10.7%) | 1409 (7.8%) | 1740 (8.2%) |
| Other | 712 (23.1%) | 3591 (19.9%) | 4303 (20.3%) |
| Elixhauser, van Walraven Score | |||
| Mean (SD, points) | 6.05 (7.53) | 3.89 (6.37) | 4.21 (6.59) |
| Range | −14.00–53.00 | −11.00–58.00 | −14.00–58.00 |
| . | Sleep medication (n = 3083) . | No sleep medication (n = 18 085) . | Total (n = 21 168) . |
|---|---|---|---|
| Sex | |||
| Male | 1473 (47.8%) | 8718 (48.2%) | 10191 (48.1%) |
| Female | 1610 (52.2%) | 9367 (51.8%) | 10977 (51.9%) |
| Age | |||
| Mean (SD) | 62.40 (16.44) | 56.06 (17.78) | 56.99 (17.73) |
| Range | 18.00 - 98.00 | 18.00 - 106.00 | 18.00 - 106.00 |
| Age categories | |||
| <60 years | 1160 (37.6%) | 9706 (53.7%) | 10866 (51.3%) |
| 60+ years | 1923 (62.4%) | 8379 (46.3%) | 10302 (48.7%) |
| Department of hospitalization | |||
| General and visceral Surgery | 1482 (48.1%) | 8034 (44.4%) | 9516 (45.0%) |
| Urology | 881 (28.6%) | 5430 (30.0%) | 6311 (29.8%) |
| Gynecology | 720 (23.4%) | 4621 (25.6%) | 5341 (25.2%) |
| Hospital stay | |||
| Mean (SD, days) | 9.33 (7.49) | 6.03 (5.87) | 6.51 (6.24) |
| Range | 1.42 - 77.92 | 0.21 - 99.25 | 0.21 - 99.25 |
| ≤5 days | 920 (29.8%) | 10713 (59.2%) | 11633 (55.0%) |
| >5 days | 2163 (70.2%) | 7372 (40.8%) | 9535 (45.0%) |
| Sleep medication | |||
| Mono: Valerian | 814 (26.4%) | 0 (0.0%) | 814 (3.8%) |
| Mono: Classic benzodiazepines | 1138 (36.9%) | 0 (0.0%) | 1138 (5.4%) |
| Mono: Benzodiazepine receptor agonists | 538 (17.5%) | 0 (0.0%) | 538 (2.5%) |
| Comb: Valerian/benzodiazepine | 191 (6.2%) | 0 (0.0%) | 191 (0.9%) |
| Comb: Valerian/benzodiazepine/ other | 5 (0.2%) | 0 (0.0%) | 5 (0.0%) |
| Comb: Valerian/other | 22 (0.7%) | 0 (0.0%) | 22 (0.1%) |
| Mono: Risperidone or haloperidol | 62 (2.0%) | 0 (0.0%) | 62 (0.3%) |
| Mono: Melperone | 84 (2.7%) | 0 (0.0%) | 84 (0.4%) |
| Mono: Quetiapine | 54 (1.8%) | 0 (0.0%) | 54 (0.3%) |
| Other | 175 (5.7%) | 0 (0.0%) | 175 (0.8%) |
| Rescue therapy, valerian to benzodiazepines within twelve hours | 121 (55.5%) | 0 | 121 (55.5%) |
| No rescue | 97 (44.5%) | 0 | 97 (44.5%) |
| Localization of surgery | |||
| Hernia repair | 85 (2.8%) | 654 (3.6%) | 739 (3.5%) |
| Digestive tract | 837 (27.1%) | 4848 (26.8%) | 5685 (26.9%) |
| Urinary tract | 513 (16.6%) | 3015 (16.7%) | 3528 (16.7%) |
| Male reproductive organs | 377 (12.2%) | 2166 (12.0%) | 2543 (12.0%) |
| Female reproductive organs | 222 (7.2%) | 2282 (12.6%) | 2504 (11.8%) |
| Obstetrics | 6 (0.2%) | 120 (0.7%) | 126 (0.6%) |
| Breast surgery | 331 (10.7%) | 1409 (7.8%) | 1740 (8.2%) |
| Other | 712 (23.1%) | 3591 (19.9%) | 4303 (20.3%) |
| Elixhauser, van Walraven Score | |||
| Mean (SD, points) | 6.05 (7.53) | 3.89 (6.37) | 4.21 (6.59) |
| Range | −14.00–53.00 | −11.00–58.00 | −14.00–58.00 |
| . | Sleep medication (n = 3083) . | No sleep medication (n = 18 085) . | Total (n = 21 168) . |
|---|---|---|---|
| Sex | |||
| Male | 1473 (47.8%) | 8718 (48.2%) | 10191 (48.1%) |
| Female | 1610 (52.2%) | 9367 (51.8%) | 10977 (51.9%) |
| Age | |||
| Mean (SD) | 62.40 (16.44) | 56.06 (17.78) | 56.99 (17.73) |
| Range | 18.00 - 98.00 | 18.00 - 106.00 | 18.00 - 106.00 |
| Age categories | |||
| <60 years | 1160 (37.6%) | 9706 (53.7%) | 10866 (51.3%) |
| 60+ years | 1923 (62.4%) | 8379 (46.3%) | 10302 (48.7%) |
| Department of hospitalization | |||
| General and visceral Surgery | 1482 (48.1%) | 8034 (44.4%) | 9516 (45.0%) |
| Urology | 881 (28.6%) | 5430 (30.0%) | 6311 (29.8%) |
| Gynecology | 720 (23.4%) | 4621 (25.6%) | 5341 (25.2%) |
| Hospital stay | |||
| Mean (SD, days) | 9.33 (7.49) | 6.03 (5.87) | 6.51 (6.24) |
| Range | 1.42 - 77.92 | 0.21 - 99.25 | 0.21 - 99.25 |
| ≤5 days | 920 (29.8%) | 10713 (59.2%) | 11633 (55.0%) |
| >5 days | 2163 (70.2%) | 7372 (40.8%) | 9535 (45.0%) |
| Sleep medication | |||
| Mono: Valerian | 814 (26.4%) | 0 (0.0%) | 814 (3.8%) |
| Mono: Classic benzodiazepines | 1138 (36.9%) | 0 (0.0%) | 1138 (5.4%) |
| Mono: Benzodiazepine receptor agonists | 538 (17.5%) | 0 (0.0%) | 538 (2.5%) |
| Comb: Valerian/benzodiazepine | 191 (6.2%) | 0 (0.0%) | 191 (0.9%) |
| Comb: Valerian/benzodiazepine/ other | 5 (0.2%) | 0 (0.0%) | 5 (0.0%) |
| Comb: Valerian/other | 22 (0.7%) | 0 (0.0%) | 22 (0.1%) |
| Mono: Risperidone or haloperidol | 62 (2.0%) | 0 (0.0%) | 62 (0.3%) |
| Mono: Melperone | 84 (2.7%) | 0 (0.0%) | 84 (0.4%) |
| Mono: Quetiapine | 54 (1.8%) | 0 (0.0%) | 54 (0.3%) |
| Other | 175 (5.7%) | 0 (0.0%) | 175 (0.8%) |
| Rescue therapy, valerian to benzodiazepines within twelve hours | 121 (55.5%) | 0 | 121 (55.5%) |
| No rescue | 97 (44.5%) | 0 | 97 (44.5%) |
| Localization of surgery | |||
| Hernia repair | 85 (2.8%) | 654 (3.6%) | 739 (3.5%) |
| Digestive tract | 837 (27.1%) | 4848 (26.8%) | 5685 (26.9%) |
| Urinary tract | 513 (16.6%) | 3015 (16.7%) | 3528 (16.7%) |
| Male reproductive organs | 377 (12.2%) | 2166 (12.0%) | 2543 (12.0%) |
| Female reproductive organs | 222 (7.2%) | 2282 (12.6%) | 2504 (11.8%) |
| Obstetrics | 6 (0.2%) | 120 (0.7%) | 126 (0.6%) |
| Breast surgery | 331 (10.7%) | 1409 (7.8%) | 1740 (8.2%) |
| Other | 712 (23.1%) | 3591 (19.9%) | 4303 (20.3%) |
| Elixhauser, van Walraven Score | |||
| Mean (SD, points) | 6.05 (7.53) | 3.89 (6.37) | 4.21 (6.59) |
| Range | −14.00–53.00 | −11.00–58.00 | −14.00–58.00 |
Sleep therapy in detail
During the observation period, almost 15% of the study population needed sleep medication. Most patients received classic benzodiazepines (n = 1138, 36.9%). The second most applied medication was valerian (n = 814, 26.4%) followed by benzodiazepine receptor agonists (n = 538, 17.5%). Less than 10% of the patients (n = 218) received a combination therapy of valerian and another sleep medication during the five postoperative days. More than half of these patients (55.5%, n = 121) received another sleep medication within 12 hr after valerian. Hence, they were in need of immediate rescue therapy after application of valerian. More than 50 patients took risperidone or haloperidol (n = 62, 2.0%), melperone (n = 84, 2.7%), or quetiapine (n = 54, 1.8%). All other sleep-promoting drugs were given to less than 50 patients (n = 175, 5.7%) and were not considered for further analysis (see Table 1 for detailed information).
Association between sociodemographic factors and treatment groups
We found a significant association between patients’ age, length of hospital stay, patients’ Elixhauser comorbidity score, and the analyzed treatment groups (p < .001, Table 3). Patients older than 60 years and patients staying in hospital for more than 5 days presented with higher odds to take sleep medication in general and to receive valerian monotherapy in both uni- and multivariable logistic regression (p < .001). A longer stay in hospital was associated with a higher OR of taking valerian combination therapy by 8.09 (5.56–12.22, p < .001) in univariable and 7.67 (5.20–11.70, p < .001) in multivariable analysis. Here, higher age was only statistically significant in the univariable analysis. A higher rate of comorbidities expressed in the Elixhauser comorbidity score was associated with a higher OR of applying sleep medication, valerian mono- or combination therapy only in univariable analysis.
Chi-squared test on relation between age, sex, length of hospital stay, and comorbidity score on treatment groups “no sleep medication received,” “valerian monotherapy,” “valerian combination therapy”
| . | . | TRUE . | FALSE . | Df* . | Chi^2** . | P . |
|---|---|---|---|---|---|---|
| No sleep medication received | ||||||
| Age | <60 years | 9706 | 1160 | 1 | 270.73 | <0.001 |
| 60+ years | 8379 | 1923 | ||||
| Sex | Male | 8718 | 1473 | 1 | 0.18 | 0.6747 |
| Female | 9367 | 1610 | ||||
| Hospital stay | <5 days | 10713 | 920 | 1 | 918.27 | <0.001 |
| >5 days | 7372 | 2163 | ||||
| Elixhauser Score | Above median | 8790 | 1969 | 1 | 244.89 | <0.001 |
| Below median | 9295 | 1114 | ||||
| Valerian monotherapy | ||||||
| Age | <60 years | 327 | 10539 | 1 | 45.64 | <0.001 |
| 60+ years | 496 | 9806 | ||||
| Sex | Male | 329 | 9862 | 1 | 22.54 | <0.001 |
| Female | 494 | 10483 | ||||
| Hospital stay | <5 days | 284 | 11349 | 1 | 143.77 | <0.001 |
| >5 days | 539 | 8996 | ||||
| Elixhauser Score | Above median | 487 | 10272 | 1 | 23.52 | <0.001 |
| Below median | 336 | 10073 | ||||
| Valerian combination therapy or rescue therapy | ||||||
| Age | <60 years | 80 | 10786 | 1 | 18.30 | <0.001 |
| 60+ years | 138 | 10164 | ||||
| Sex | Male | 96 | 10095 | 1 | 1.33 | 0.2494 |
| Female | 122 | 10855 | ||||
| Hospital stay | <5 days | 29 | 11604 | 1 | 152.68 | <0.001 |
| >5 days | 189 | 9346 | ||||
| Elixhauser Score | Above median | 157 | 10602 | 1 | 38.73 | <0.001 |
| Below median | 61 | 10348 |
| . | . | TRUE . | FALSE . | Df* . | Chi^2** . | P . |
|---|---|---|---|---|---|---|
| No sleep medication received | ||||||
| Age | <60 years | 9706 | 1160 | 1 | 270.73 | <0.001 |
| 60+ years | 8379 | 1923 | ||||
| Sex | Male | 8718 | 1473 | 1 | 0.18 | 0.6747 |
| Female | 9367 | 1610 | ||||
| Hospital stay | <5 days | 10713 | 920 | 1 | 918.27 | <0.001 |
| >5 days | 7372 | 2163 | ||||
| Elixhauser Score | Above median | 8790 | 1969 | 1 | 244.89 | <0.001 |
| Below median | 9295 | 1114 | ||||
| Valerian monotherapy | ||||||
| Age | <60 years | 327 | 10539 | 1 | 45.64 | <0.001 |
| 60+ years | 496 | 9806 | ||||
| Sex | Male | 329 | 9862 | 1 | 22.54 | <0.001 |
| Female | 494 | 10483 | ||||
| Hospital stay | <5 days | 284 | 11349 | 1 | 143.77 | <0.001 |
| >5 days | 539 | 8996 | ||||
| Elixhauser Score | Above median | 487 | 10272 | 1 | 23.52 | <0.001 |
| Below median | 336 | 10073 | ||||
| Valerian combination therapy or rescue therapy | ||||||
| Age | <60 years | 80 | 10786 | 1 | 18.30 | <0.001 |
| 60+ years | 138 | 10164 | ||||
| Sex | Male | 96 | 10095 | 1 | 1.33 | 0.2494 |
| Female | 122 | 10855 | ||||
| Hospital stay | <5 days | 29 | 11604 | 1 | 152.68 | <0.001 |
| >5 days | 189 | 9346 | ||||
| Elixhauser Score | Above median | 157 | 10602 | 1 | 38.73 | <0.001 |
| Below median | 61 | 10348 |
*Df, Degrees of freedom;
**Chi^2: Chi-square statistic value.
Chi-squared test on relation between age, sex, length of hospital stay, and comorbidity score on treatment groups “no sleep medication received,” “valerian monotherapy,” “valerian combination therapy”
| . | . | TRUE . | FALSE . | Df* . | Chi^2** . | P . |
|---|---|---|---|---|---|---|
| No sleep medication received | ||||||
| Age | <60 years | 9706 | 1160 | 1 | 270.73 | <0.001 |
| 60+ years | 8379 | 1923 | ||||
| Sex | Male | 8718 | 1473 | 1 | 0.18 | 0.6747 |
| Female | 9367 | 1610 | ||||
| Hospital stay | <5 days | 10713 | 920 | 1 | 918.27 | <0.001 |
| >5 days | 7372 | 2163 | ||||
| Elixhauser Score | Above median | 8790 | 1969 | 1 | 244.89 | <0.001 |
| Below median | 9295 | 1114 | ||||
| Valerian monotherapy | ||||||
| Age | <60 years | 327 | 10539 | 1 | 45.64 | <0.001 |
| 60+ years | 496 | 9806 | ||||
| Sex | Male | 329 | 9862 | 1 | 22.54 | <0.001 |
| Female | 494 | 10483 | ||||
| Hospital stay | <5 days | 284 | 11349 | 1 | 143.77 | <0.001 |
| >5 days | 539 | 8996 | ||||
| Elixhauser Score | Above median | 487 | 10272 | 1 | 23.52 | <0.001 |
| Below median | 336 | 10073 | ||||
| Valerian combination therapy or rescue therapy | ||||||
| Age | <60 years | 80 | 10786 | 1 | 18.30 | <0.001 |
| 60+ years | 138 | 10164 | ||||
| Sex | Male | 96 | 10095 | 1 | 1.33 | 0.2494 |
| Female | 122 | 10855 | ||||
| Hospital stay | <5 days | 29 | 11604 | 1 | 152.68 | <0.001 |
| >5 days | 189 | 9346 | ||||
| Elixhauser Score | Above median | 157 | 10602 | 1 | 38.73 | <0.001 |
| Below median | 61 | 10348 |
| . | . | TRUE . | FALSE . | Df* . | Chi^2** . | P . |
|---|---|---|---|---|---|---|
| No sleep medication received | ||||||
| Age | <60 years | 9706 | 1160 | 1 | 270.73 | <0.001 |
| 60+ years | 8379 | 1923 | ||||
| Sex | Male | 8718 | 1473 | 1 | 0.18 | 0.6747 |
| Female | 9367 | 1610 | ||||
| Hospital stay | <5 days | 10713 | 920 | 1 | 918.27 | <0.001 |
| >5 days | 7372 | 2163 | ||||
| Elixhauser Score | Above median | 8790 | 1969 | 1 | 244.89 | <0.001 |
| Below median | 9295 | 1114 | ||||
| Valerian monotherapy | ||||||
| Age | <60 years | 327 | 10539 | 1 | 45.64 | <0.001 |
| 60+ years | 496 | 9806 | ||||
| Sex | Male | 329 | 9862 | 1 | 22.54 | <0.001 |
| Female | 494 | 10483 | ||||
| Hospital stay | <5 days | 284 | 11349 | 1 | 143.77 | <0.001 |
| >5 days | 539 | 8996 | ||||
| Elixhauser Score | Above median | 487 | 10272 | 1 | 23.52 | <0.001 |
| Below median | 336 | 10073 | ||||
| Valerian combination therapy or rescue therapy | ||||||
| Age | <60 years | 80 | 10786 | 1 | 18.30 | <0.001 |
| 60+ years | 138 | 10164 | ||||
| Sex | Male | 96 | 10095 | 1 | 1.33 | 0.2494 |
| Female | 122 | 10855 | ||||
| Hospital stay | <5 days | 29 | 11604 | 1 | 152.68 | <0.001 |
| >5 days | 189 | 9346 | ||||
| Elixhauser Score | Above median | 157 | 10602 | 1 | 38.73 | <0.001 |
| Below median | 61 | 10348 |
*Df, Degrees of freedom;
**Chi^2: Chi-square statistic value.
Sex was not significantly associated with valerian combination therapy (p = .249) or receiving sleep medication in general (p = .675). The relation between sex and valerian monotherapy was significant (p < .001). Women were more likely to receive valerian monotherapy with an OR of 1.41 (1.23–1.63, p < .001) in the univariable and 1.53 (1.33–1.77, p < .001) in the multivariable logistic regression.
All results of Chi-squared test and of univariable and multivariable logistic regression are shown in Tables 3 and 4.
Association between age, sex, length of hospital stay, and Elixhauser comorbidity score and probability for assigned treatment group
| . | . | Regression coefficient . | OR—univariable (95% CI, p-value) . | OR—multivariable (95% CI, p-value)* . |
|---|---|---|---|---|
| No sleep medication received | ||||
| Age | <60 years | Reference | ||
| 60+ years | −0.423 | 0.52 (0.48–0.56, p < .001) | 0.67 (0.62–0.73, p < .001) | |
| Sex | Male | Reference | ||
| Female | −0.115 | 0.98 (0.91–1.06, p = .661) | 0.89 (0.82–0.97, p = .005) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | −1.100 | 0.29 (0.27–0.32, p < .001) | 0.33 (0.30–0.36, p < .001) | |
| Elixhauser Score | Mean | −0.009 | 0.96 (0.95–0.96, p < .001) | 0.99 (0.99–1.00, p = .004) |
| Valerian monotherapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.408 | 1.63 (1.41−1.88, p < .001) | 1.50 (1.29–1.75, p < .001) | |
| Sex | Male | Reference | ||
| Female | 0.427 | 1.41 (1.23–1.63, p < .001) | 1.53 (1.33–1.77, p < .001) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 0.801 | 2.39 (2.07–2.77, p < .001) | 2.23 (1.91–2.61, p < .001) | |
| Elixhauser Score | Mean | −0.003 | 1.02 (1.01–1.03, p < .001) | 1.00 (0.99–1.01, p = .621) |
| Valerian combination therapy or rescue therapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.278 | 1.83 (1.39–2.42, p < .001) | 1.28 (0.96–1.71, p = .094) | |
| Sex | Male | Reference | ||
| Female | 0.230 | 1.18 (0.90–1.55, p = .223) | 1.26 (0.96–1.66, p = .099) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 2.028 | 8.09 (5.56–12.22, p < .001) | 7.67 (5.20–11.70, p < .001) | |
| Elixhauser Score | Mean | 0.001 | 1.04 (1.03–1.06, p < .001) | 1.00 (0.98–1.02, p = .976) |
| . | . | Regression coefficient . | OR—univariable (95% CI, p-value) . | OR—multivariable (95% CI, p-value)* . |
|---|---|---|---|---|
| No sleep medication received | ||||
| Age | <60 years | Reference | ||
| 60+ years | −0.423 | 0.52 (0.48–0.56, p < .001) | 0.67 (0.62–0.73, p < .001) | |
| Sex | Male | Reference | ||
| Female | −0.115 | 0.98 (0.91–1.06, p = .661) | 0.89 (0.82–0.97, p = .005) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | −1.100 | 0.29 (0.27–0.32, p < .001) | 0.33 (0.30–0.36, p < .001) | |
| Elixhauser Score | Mean | −0.009 | 0.96 (0.95–0.96, p < .001) | 0.99 (0.99–1.00, p = .004) |
| Valerian monotherapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.408 | 1.63 (1.41−1.88, p < .001) | 1.50 (1.29–1.75, p < .001) | |
| Sex | Male | Reference | ||
| Female | 0.427 | 1.41 (1.23–1.63, p < .001) | 1.53 (1.33–1.77, p < .001) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 0.801 | 2.39 (2.07–2.77, p < .001) | 2.23 (1.91–2.61, p < .001) | |
| Elixhauser Score | Mean | −0.003 | 1.02 (1.01–1.03, p < .001) | 1.00 (0.99–1.01, p = .621) |
| Valerian combination therapy or rescue therapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.278 | 1.83 (1.39–2.42, p < .001) | 1.28 (0.96–1.71, p = .094) | |
| Sex | Male | Reference | ||
| Female | 0.230 | 1.18 (0.90–1.55, p = .223) | 1.26 (0.96–1.66, p = .099) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 2.028 | 8.09 (5.56–12.22, p < .001) | 7.67 (5.20–11.70, p < .001) | |
| Elixhauser Score | Mean | 0.001 | 1.04 (1.03–1.06, p < .001) | 1.00 (0.98–1.02, p = .976) |
Univariable and multivariable logistic regression with odds ratio (OR), 95% CI, and p-value.
*The multivariable logistic regression models were performed using Age, Sex, Hospital Stay, and Elixhauser Score as explanatory variables for each multivariable model.
Association between age, sex, length of hospital stay, and Elixhauser comorbidity score and probability for assigned treatment group
| . | . | Regression coefficient . | OR—univariable (95% CI, p-value) . | OR—multivariable (95% CI, p-value)* . |
|---|---|---|---|---|
| No sleep medication received | ||||
| Age | <60 years | Reference | ||
| 60+ years | −0.423 | 0.52 (0.48–0.56, p < .001) | 0.67 (0.62–0.73, p < .001) | |
| Sex | Male | Reference | ||
| Female | −0.115 | 0.98 (0.91–1.06, p = .661) | 0.89 (0.82–0.97, p = .005) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | −1.100 | 0.29 (0.27–0.32, p < .001) | 0.33 (0.30–0.36, p < .001) | |
| Elixhauser Score | Mean | −0.009 | 0.96 (0.95–0.96, p < .001) | 0.99 (0.99–1.00, p = .004) |
| Valerian monotherapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.408 | 1.63 (1.41−1.88, p < .001) | 1.50 (1.29–1.75, p < .001) | |
| Sex | Male | Reference | ||
| Female | 0.427 | 1.41 (1.23–1.63, p < .001) | 1.53 (1.33–1.77, p < .001) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 0.801 | 2.39 (2.07–2.77, p < .001) | 2.23 (1.91–2.61, p < .001) | |
| Elixhauser Score | Mean | −0.003 | 1.02 (1.01–1.03, p < .001) | 1.00 (0.99–1.01, p = .621) |
| Valerian combination therapy or rescue therapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.278 | 1.83 (1.39–2.42, p < .001) | 1.28 (0.96–1.71, p = .094) | |
| Sex | Male | Reference | ||
| Female | 0.230 | 1.18 (0.90–1.55, p = .223) | 1.26 (0.96–1.66, p = .099) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 2.028 | 8.09 (5.56–12.22, p < .001) | 7.67 (5.20–11.70, p < .001) | |
| Elixhauser Score | Mean | 0.001 | 1.04 (1.03–1.06, p < .001) | 1.00 (0.98–1.02, p = .976) |
| . | . | Regression coefficient . | OR—univariable (95% CI, p-value) . | OR—multivariable (95% CI, p-value)* . |
|---|---|---|---|---|
| No sleep medication received | ||||
| Age | <60 years | Reference | ||
| 60+ years | −0.423 | 0.52 (0.48–0.56, p < .001) | 0.67 (0.62–0.73, p < .001) | |
| Sex | Male | Reference | ||
| Female | −0.115 | 0.98 (0.91–1.06, p = .661) | 0.89 (0.82–0.97, p = .005) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | −1.100 | 0.29 (0.27–0.32, p < .001) | 0.33 (0.30–0.36, p < .001) | |
| Elixhauser Score | Mean | −0.009 | 0.96 (0.95–0.96, p < .001) | 0.99 (0.99–1.00, p = .004) |
| Valerian monotherapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.408 | 1.63 (1.41−1.88, p < .001) | 1.50 (1.29–1.75, p < .001) | |
| Sex | Male | Reference | ||
| Female | 0.427 | 1.41 (1.23–1.63, p < .001) | 1.53 (1.33–1.77, p < .001) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 0.801 | 2.39 (2.07–2.77, p < .001) | 2.23 (1.91–2.61, p < .001) | |
| Elixhauser Score | Mean | −0.003 | 1.02 (1.01–1.03, p < .001) | 1.00 (0.99–1.01, p = .621) |
| Valerian combination therapy or rescue therapy | ||||
| Age | <60 years | Reference | ||
| 60+ years | 0.278 | 1.83 (1.39–2.42, p < .001) | 1.28 (0.96–1.71, p = .094) | |
| Sex | Male | Reference | ||
| Female | 0.230 | 1.18 (0.90–1.55, p = .223) | 1.26 (0.96–1.66, p = .099) | |
| Hospital stay | <5 days | Reference | ||
| >5 days | 2.028 | 8.09 (5.56–12.22, p < .001) | 7.67 (5.20–11.70, p < .001) | |
| Elixhauser Score | Mean | 0.001 | 1.04 (1.03–1.06, p < .001) | 1.00 (0.98–1.02, p = .976) |
Univariable and multivariable logistic regression with odds ratio (OR), 95% CI, and p-value.
*The multivariable logistic regression models were performed using Age, Sex, Hospital Stay, and Elixhauser Score as explanatory variables for each multivariable model.
Discussion
In this retrospective cohort study, we aimed to evaluate the frequency of postoperative sleep disturbances in urological, gynecological and general surgical patients as well as the kind of medicinal therapy with a special focus on herbal medication. With 15% occurrence of drug-treated sleep disturbances in our study population, we found sleep disturbances to occur commonly in postoperative surgical patients. To the best of our knowledge, this study is the first to investigate the role of valerian in the treatment of postoperative sleep disturbances. We found valerian to be the second most applied medication in all departments.
We were able to evaluate data of more than 20 000 surgical patients. However, the results of retrospective cohort analyses are always limited due to documentation errors and selection bias. The issue of missing documentations became evident in our study as there was no accurate and standardized documentation about postoperative sleep disturbances making it impossible to capture the precise frequency of postoperative sleep disturbances. Due to the retrospective design of this study, we could not apply validated instruments (e.g., Insomnia Severity Index [ISI], Pittsburgh Sleep Quality Index [PSQI], SF-A) to prove existence of sleep disturbances in the included surgical patients. We estimated the occurrence of postoperative sleep disturbances in patients, who received sleep medication postoperatively which is justified by the definition of acute sleep disorders according to the ICD [1]. The risk of incorrect or missing documentation of the distribution of sleep medication is low as all drugs given out to patients must be documented in the electronic patient record. The number of patients suffering from postoperative sleep disturbances might be higher than captured as it is assumable that not all of the patients with sleep disturbances demanded for medication. It is, however, also possible that sleep medication was given out in advance and without the patients suffering from sleep disturbances. Notably, these considerations emphasize the missing recognition of postoperative sleep disturbances and the necessity of capturing symptoms of insomnia by a valid tool to avoid over-treatment as well as not-treatment of postoperative sleep disturbances. To the best of our knowledge, just one partially comparable study regarding frequency of sleep disturbances in surgical patients exists. Suzuki et al. showed a rate of 10% of postoperative sleeping disturbances of patients admitted to an otolaryngology ward [10], which is similar to our results.
There are multiple factors influencing postoperative sleep quality, Rosenberg-Adamsen et al. name “environmental factors, surgical stress response, pain, starvation, psychological factors” among others to “contribute to postoperative sleep disturbance” [8]. Knowing this, we excluded patients staying in the intensive care unit to minimize environmental and stress related factors. We also excluded children and patients with previous diagnosis of insomnia, as their sleeping pattern and, for the latter, the history of sleep medication could have had an influence on the usage of sleep medication.
The high rate of medicinally treated postoperative sleep disturbances in our study emphasizes that postoperative sleep disturbances are a relevant issue and need to be considered more in surgical patients. Su and Wang, for example, found that sleep disturbances can lead to “higher risk of delirium, increased sensitivity to pain, more cardiovascular events, and poorer recovery” [7]. Furthermore, poor sleep quality has been found to increase systemic inflammation [16, 26], and higher levels of systemic inflammatory markers are assumed to contribute to postoperative complications after surgery [27]. Improvement of postoperative sleep might, therefore, also be able to prevent postoperative complications.
Interestingly, although valerian and other herbal medication are explicitly not recommended in the European guideline for the diagnosis and treatment of insomnia, valerian was the second most applied sleep medication in our study [1]. One reason for this could be the fact that valerian, as a herbal sleep aid, is commonly considered to be very well tolerated with little to no side effects [28]. Recent surveys indicate that approximately 70% of the German population are experienced with complementary medicine, especially with herbal medication and dietary supplements [29–31]. Kilper et al. surveyed orthopedic and trauma surgical patients, who “perceived complementary and alternative medicine as a gentle therapeutic approach” [31]. The majority found complementary and alternative medicine to be “harmless” was “satisfied with it” and would hence “recommend its usage” [31]. Noteworthy, it is remarkable that the participating departments in this study did not provide any internal guidelines for the treatment of acute postoperative sleep disturbances. As a consequence, it can be assumed that the applied drugs were a free choice made by the attending physicians and nurses.
In general, recommendations for reasonable clinical dosing of valerian in acute sleep disturbances are not available, and recent research does not provide any information about the usage of valerian in surgical patients. Systematic reviews dealing with valerian are mostly focused on valerian in chronic sleep disorders, which are treated with a continuous application of valerian and not with a single dose as performed in our study. The included trials are often inhomogeneous and the systematic reviews are, therefore, not able to draw firm conclusion due to methodologic flaws [1, 21, 25, 32]. Meta-analyses show large differences in dosage of valerian and different types of valerian extracts are used. To evaluate the efficacy of valerian, it is still unclear how to choose the right dose. This emphasizes the necessity for further research [21, 33]. A systematic review by Taibi et al. using a 70% ethanolic extract of valerian, which is similar to the drug used in our study, in chronic sleep disorders reported no superiority versus placebo, but equivalence to oxazepam and other benzodiazepins [33]. Mineo et al. showed an effect of a single dose of 900 mg of valerian on the intracortical facilitation circuits, which are reported to play a role in regulation of awakening [34, 35]. Besides the scientific gap regarding application of valerian in acute sleep disturbances, the efficacy of valerian in surgical patients is largely unclear. In our study, more than three times as many patients received valerian monotherapy than valerian combination therapy. By our definition of valerian monotherapy, including only patients not asking for other sleep medication after receiving valerian, we can carefully assume that the application of valerian was sufficient to treat most patients’ sleeping problems. If the observed efficacy is caused by the drug or by placebo, it is not differentiable. The efficacy of sleep-promoting drugs is matter of scientific discussions as research shows that placebo treatment is also able to improve sleep [36, 37].
In our study, especially women seem to receive and maybe profit from valerian monotherapy, next to older people, those with longer hospital stay and more comorbidities. In previous research, female sex and higher age have been found to be risk factors for the development of sleep disturbances [3]. Especially a patient’s age is suggested to be an important factor to contribute to developing postoperative sleep disturbances [5]. As surgical patients become older, treating postoperative sleep disturbances will be a growing problem [7]. Older patients are also more likely to develop side effects (dizziness, risk of falling, etc.) or paradoxical reactions using benzodiazepines for sedation purposes or promotion of sleep [38]. Valerian, here, can be a good alternative to avoid these reactions as there are little to no side effects reported in previous trials [33]. In our study, we were not able to report side effects caused by valerian, as there was no documentation in the electronic patient record. The interest in complementary and alternative medicine is known to be higher in women than in men, which is shown by data from Germany as well as from the United States [29, 39, 40]. In our study, sex was not associated with other treatment groups whereas age, duration of stay and amount of comorbidities generally seem to increase the probability of needing sleep medication. The duration of hospital stay can be linked to morbidity, surgical therapy, and (postoperative) complications and is related to the occurrence of sleep disturbances. Our data do not indicate clearly in which type of patient valerian was combined or why rescue therapy was needed. Future prospective trials need to focus on the evaluation of the right dose and preparation of valerian, its efficacy in the treatment of postoperative sleep disturbances as well as confounding factors and side effects.
In conclusion, our results emphasize the necessity of capturing and evaluating postoperative sleep disturbances to improve postoperative sleep. Postoperative sleep disturbances appear to be a common postoperative complication leading to a request for sleep medication in approximately 15% of patients after surgery. Besides conventional sleep medication such as benzodiazepines, valerian is of clinical relevance in the treatment of postoperative sleep disturbances, at least in our tertiary care hospital. Valerian appears to be a promising therapeutic approach especially in women, older and sicker patients and those with longer duration of hospital stay. Further research should discriminate between drug- and placebo-caused effects of sleep promoting medication in surgical patients.
Disclosure Statement
(a) Financial disclosure: The authors declare that there are no financial ties.
(b) Non-financial disclosure: The authors declare that there is no conflict of interest.
References
Author notes
These authors contributed equally to this work.





Comments