-
PDF
- Split View
-
Views
-
Cite
Cite
Honghong Ren, Zongchang Li, Jinguang Li, Jun Zhou, Ying He, Chunwang Li, Qianjin Wang, Xiaogang Chen, Jinsong Tang, Correlation Between Cortical Thickness Abnormalities of the Olfactory Sulcus and Olfactory Identification Disorder and Persistent Auditory Verbal Hallucinations in Chinese Patients With Chronic Schizophrenia, Schizophrenia Bulletin, Volume 50, Issue 5, September 2024, Pages 1232–1242, https://doi.org/10.1093/schbul/sbae040
Close - Share Icon Share
Abstract
Persistent auditory verbal hallucinations (pAVHs) and olfactory identification impairment are common in schizophrenia (SCZ), but the neuroimaging mechanisms underlying both pAVHs and olfactory identification impairment are unclear. This study aimed to investigate whether pAVHs and olfactory identification impairment in SCZ patients are associated with changes in cortical thickness.
In this study, cortical thickness was investigated in 78 SCZ patients with pAVHs (pAVH group), 58 SCZ patients without AVHs (non-AVH group), and 83 healthy controls (HC group) using 3T magnetic resonance imaging. The severity of pAVHs was assessed by the Auditory Hallucination Rating Scale. Olfactory identification deficits were assessed using the Odor Stick Identification Test for Japanese (OSIT-J). In addition, the relationship between the severity of pAVHs and olfactory identification disorder and cortical thickness abnormalities was determined.
Significant reductions in cortical thickness were observed in the right medial orbital sulcus (olfactory sulcus) and right orbital sulcus (H-shaped sulcus) in the pAVH group compared to both the non-AVH and HC groups (P < .003, Bonferroni correction). Furthermore, the severity of pAVHs was found to be negatively correlated with the reduction in cortical thickness in the olfactory sulcus and H-shaped sulcus. Additionally, a decrease in cortical thickness in the olfactory sulcus showed a positive correlation with the OSIT-J scores (P < .05, false discovery rate correction).
Cortical thickness abnormalities in the olfactory sulcus may be a common neuroimaging mechanism for pAVHs and olfactory identification deficits in SCZ patients.
Introduction
Auditory verbal hallucinations (AVHs) are a defining positive symptom and a significant diagnostic criterion of schizophrenia (SCZ),1 affecting around 60%–80% of patients with the disorder.2 While antipsychotic medications can effectively reduce the frequency and severity of AVHs for most patients,3 there still remains a subgroup (approximately 25%–30% of cases) that do not respond to traditional antipsychotics and continue to experience persistent AVHs (pAVHs).4,5 Therefore, patients diagnosed with this form of SCZ are classified as having treatment-resistant SCZ.6 The pAVHs represent a major and persistent burden on patients, commonly associated with social and occupational dysfunction as well as negative outcomes.5,7,8
Research on olfactory functioning in individuals with SCZ has been extensively studied, focusing primarily on deficits in olfactory identification ability.9,10 These deficits remain significant even after controlling for factors such as gender11 and smoking.12 Olfactory impairment is evident regardless of medication status, as unmedicated patients consistently exhibit substantial deficits in olfactory identification.13 Further research has shown that olfactory deficits are also present in first-degree relatives of those with SCZ,14 as well as in young adults and adolescents with prodromal symptoms of psychosis,15 and in patients newly diagnosed with psychotic conditions.16 In neuroleptic-naive first-episode psychosis patients, the severity of olfactory dysfunction does not improve with medication stabilization16 and has shown predictive value for poor long-term outcomes.17
Growing evidence indicates that olfactory dysfunction may serve as an endophenotype of SCZ.9,15,18 Furthermore, olfactory dysfunction has also been documented in individuals with SCZ who experience pAVHs concurrently.19,20 This is not surprising, considering that olfactory processing involves several brain regions that are also implicated in the illness, such as the ventromedial temporal lobe, the amygdala, hippocampus, basal forebrain, prefrontal cortex, and diencephalon. Therefore, the olfactory system shares a common neural substrate with many of the cognitive and emotional processes that are disrupted in SCZ.16,21,22
Neuroimaging studies provide compelling evidence that AVHs in SCZ patients are often associated with structural, functional, and neurometabolic abnormalities in the frontotemporal region.23–25 The frontal lobe, which is connected to the temporal lobe via the arcuate fasciculus, plays an important role in the brain’s language network26 and is involved in auditory perception and language processing.27 Previous studies have shown that patients with AVHs have reduced frontal gray matter volume (GMV)28 and altered functional connectivity29 compared with patients without AVHs and health controls (HCs). Voxel-based morphometry (VBM) analyses showed that structural changes also occurred in sensory regions other than the auditory cortex, including reduced gray matter in the left insular cortex and adjacent temporal pole,30 thalamus, cerebellum,31 left transverse gyrus (Heschl),32 left superior limbic gyrus, and dorsolateral prefrontal cortex.33
In addition, more neuroimaging studies have also previously reported the presence of structural and functional34 brain alterations in SCZ patients with olfactory impairment. Alterations in brain structure were mainly focused on GMV indicators, and specific brain regions were mainly concentrated in the olfactory bulb, olfactory sulcus,35,36 hippocampus, rectus,37 insula, temporal pole,38 and orbitofrontal lobe.39 To the best of our knowledge, current research on olfactory deficits and cortical thickness has focused on patients with dementia, Parkinson’s disease,40 and nasal disorders.41,42 Cortical thickness is a direct reflection of the cellular composition and organization of cortical neurons, neuropil, and neuroglia. It plays a crucial role in modulating processes such as synaptogenesis, synaptic pruning, and myelination within the context of neuropathological conditions.43–47 However, studies on olfactory impairment and cortical thickness in patients with SCZ are lacking.
In order to explore the neuroimaging mechanisms in which brain regions pAVHs and olfactory dysfunction are shared or overlapped in SCZ patients, this study explored cortical thickness alterations in specific brain regions of SCZ patients and their relationship with olfactory dysfunction and pAVHs based on surface-based morphometry. We hypothesized that in SCZ patients with concomitant pAVHs, there is an overlap between brain regions with reduced cortical thickness associated with the severity of pAVHs and brain regions with reduced cortical thickness associated with olfactory dysfunction.
Materials and Methods
Participants
A total of 136 individuals diagnosed with SCZ were selected from the psychiatric outpatient clinic at the Second Xiangya Hospital of Central South University. Meanwhile, 83 HCs were recruited through local community advertisements. The diagnosis of SCZ for all patients was conducted by 2 experienced psychiatrists in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). To be included in the study, participants needed to meet several criteria: (1) Han Chinese ethnicity and aged between 16 and 45 years; (2) right-handed; (3) possessing normal hearing and intelligence; (4) having no prior history of substance abuse; and (5) having no prior history of major medical or neurological diseases or trauma. The patients were categorized into 2 subgroups based on the P3 hallucination item (P3) of the Positive and Negative Syndrome Scale (PANSS).48 The pAVH group consisted of 78 patients with P3 scores ≥4, indicating the presence of pAVHs. Conversely, the non-AVH group comprised 58 patients with P3 scores = 1, indicating the absence of AVHs.49,50 Patients in the non-AVH group of SCZ had no history of AVHs at any point from the onset to the present throughout their entire course of the disease. Additionally, the severity of pAVHs was assessed utilizing the Auditory Hallucinations Rating Scale (AHRS),51 which measures various aspects of pAVHs, including their frequency, duration, intensity, impact, and level of discomfort experienced by the patient. None of the HC participants met the diagnostic criteria for any DSM-5 mental disorder, and they had no history of early-onset mental disorder or family history of mental illnesses.
Olfactory Function Evaluation
To assess the olfactory function of the subjects, we utilized the Odor Stick Identification Test for Japanese (OSIT-J).52,53 The OSIT-J comprises 12 odors, and a score <9 indicates olfactory impairment. The specific steps for olfactory assessment were described in a previous publication.21 To mitigate confounding factors such as taste and olfactory memory, the following points were taken into consideration: (1) participants abstained from eating for at least 30 min prior to the assessment; (2) the order in which odors were presented was randomized; (3) no pre-assessment odor training was provided.
Acquisition of Magnetic Resonance Imaging Data
Magnetic resonance imaging (MRI) data were acquired within 24 h of enrollment using a 3.0T scanner (Siemens Skyra) with a 16-channel headcoil at the Magnetic Imaging Center in Changsha, China. During the scanning, patients were instructed to keep their eyes closed and remain quiet, while foam pads and earplugs were utilized to minimize motion and noise interference. Anatomical T1-weighted MRI data were obtained using a 3D magnetization preparing rapid acquisition gradient echo (3D MPRAGE) sequence with the following parameters: repetition time = 2530 ms, echo time = 2.33 ms, flip angle = 7°, field of view = 256 × 256 mm, slice thickness = 1 mm, slice gap = 0 mm, and 192 slices. Datasets were examined for distortion and motion artifacts, with no major scanner upgrades or instrument replacements occurring during the study.
Cortical Thickness Measurement
MRI images were processed using FreeSurfer software (version 7.1.0, http://surfer.nmr.mgh.harvard.edu), a validated tool described in previous studies.54,55 Preprocessing steps included motion correction, skull stripping, brain extraction, Talairach transformation, intensity correction, brain tissue segmentation, automatic topology correction, and surface deformation.1 Cortical thickness was determined by reconstructing the boundaries of gray and white matter and the cortical surface, and calculating the distance between those surfaces at each point across the cortical mantle.54 The resulting cortical surfaces were carefully reviewed and manually corrected for technical accuracy. Vertex-wise cortical thickness measurements were then mapped to a standardized spherical system and smoothed using a Gaussian kernel with a full width at half maximum of 10 mm. Quality assurance was performed by 2 seasoned assessors in accordance with the ENIGMA protocol (http://enigma.ini.usc.edu/). This study also reports the group-average Euler numbers from Freesurfer as a quantitative indicator of data quality. This information is included in supplementary material for further reference. The cerebral cortex, as delineated by the Destrieux atlas,56 is segmented into approximately 148 distinct brain regions, with an equal distribution of 74 regions per hemisphere.
Statistical Analysis
Statistical analyses were conducted using SPSS 26 (SPSS Inc.), and graphs were generated using GraphPad Prism 8.0.1. The normality of variables was assessed using the Kolmogorov-Smirnov test. Between-group comparisons of demographic and clinical data were performed using chi-squared tests, 1-way ANOVA, or Mann-Whitney U tests as appropriate. Univariate ANCOVA was used to compare OSIT-J scores and cortical thickness across brain regions among the 3 groups, with age, gender, education, chlorpromazine (CPZ) equivalent dose, and estimated total intracranial volume (eTIV) as covariates. Post hoc tests with Bonferroni correction were conducted if significant differences were observed. Notably, CPZ equivalent dose was included as a covariate only when post hoc comparisons were made between the pAVH and non-AVH groups. Partial correlation analysis was employed to examine the relationship between the severity of pAVHs and cortical thickness of specific brain regions, while controlling for age, gender, education, CPZ equivalent dose, and eTIV. The significance threshold was set at P < .05 (2-tailed).
Results
Demographic and Clinical Characteristics
The data in this study belong to the same cohort as a previous study in our group, albeit with the addition of new MRI data.21 Some MRI data were excluded due to quality control requirements for preprocessing. Ultimately, the study included 83 HCs, 78 SCZ patients with pAVHs, and 58 SCZ patients without AVHs. Table 1 summarizes the demographic and clinical characteristics. There were no significant differences in age, gender, smoking status, and drinking status across the 3 groups (P > .05). Both the pAVH and non-AVH groups had significantly lower education levels compared to the HC group, and the pAVH group had significantly lower education level than the non-AVH group (post hoc results for pAVH vs non-AVH, P = .02; pAVH vs HC, P < .001; non-AVH vs HC, P = .02). There were no significant differences between the pAVH and non-AVH groups in terms of age at onset, illness duration, CPZ equivalent dosage, PANSS-P, PANSS-N, PANSS-G, and PANSS-T scores (P > .05).
Demographic and Clinical Characteristics of Patients and Healthy Controls Groups
| Characteristics . | HC (n = 83) . | Patients (n = 136) . | Significance . | ||||
|---|---|---|---|---|---|---|---|
| pAVH (n = 78) . | Non-AVH (n = 58) . | 3 Groups . | HC vs Non-AVH . | HC vs pAVH . | pAVH vs Non-AVH . | ||
| P value . | |||||||
| Gender (M/F), n | 38/45 | 38/40 | 35/23 | χ2 = 3.08 (.21) | .09 | .71 | .18 |
| Age (y) (M ± SD)a | 26.80 ± 5.91 | 25.64 ± 5.52 | 26.95 ± 5.77 | F = 1.14 (.32) | 1.00 | .61 | .57 |
| Education (y) (M ± SD)a | 14.43 ± 2.65 | 11.69 ± 3.18 | 13.09 ± 2.82 | F = 18.04 (<.001)*** | .02* | <.001*** | .02* |
| Smoker/nonsmoker, n | 12/71 | 12/66 | 14/44 | χ2 = 2.56 (.28) | .15 | .87 | .20 |
| Drinker/nondrinker, n | 2/81 | 0/78 | 1/57 | χ2 = 1.80 (.41) | .78 | .17 | .24 |
| Age at disease onset (y) (M ± SD) | – | 19.74 ± 4.08 | 20.84 ± 4.48 | – | – | – | U = 1939 (.15) |
| Illness duration (y) (M ± SD) | – | 7.21 ± 4.61 | 5.90 ± 3.86 | – | – | – | U = 1893 (.10) |
| PANSS-P (M ± SD) | – | 13.62 ± 2.83 | 12.93 ± 3.70 | – | – | – | U = 1953 (.21) |
| PANSS-N (M ± SD) | – | 15.01 ± 5.67 | 14.09 ± 7.30 | – | – | – | U = 1857 (.09) |
| PANSS-G (M ± SD) | – | 26.79 ± 6.59 | 26.95 ± 8.78 | – | – | – | U = 2051 (.42) |
| PANSS-T (M ± SD) | – | 54.72 ± 12.52 | 53.97 ± 16.78 | – | – | – | U = 1873 (.09) |
| AHRS (M ± SD) | – | 26.31 ± 4.51 | – | – | – | – | – |
| OSIT-J (M ± SD)b | 8.49 ± 1.67 | 6.71 ± 2.08 | 6.86 ± 1.86 | F = 11.27 (<.001)*** | <.001*** | <.001*** | .54 |
| CPZ equivalent (mg/d) (M ± SD) | – | 650.41 ± 280.59 | 593.48 ± 337.16 | – | – | – | U = 1912 (.12) |
| Characteristics . | HC (n = 83) . | Patients (n = 136) . | Significance . | ||||
|---|---|---|---|---|---|---|---|
| pAVH (n = 78) . | Non-AVH (n = 58) . | 3 Groups . | HC vs Non-AVH . | HC vs pAVH . | pAVH vs Non-AVH . | ||
| P value . | |||||||
| Gender (M/F), n | 38/45 | 38/40 | 35/23 | χ2 = 3.08 (.21) | .09 | .71 | .18 |
| Age (y) (M ± SD)a | 26.80 ± 5.91 | 25.64 ± 5.52 | 26.95 ± 5.77 | F = 1.14 (.32) | 1.00 | .61 | .57 |
| Education (y) (M ± SD)a | 14.43 ± 2.65 | 11.69 ± 3.18 | 13.09 ± 2.82 | F = 18.04 (<.001)*** | .02* | <.001*** | .02* |
| Smoker/nonsmoker, n | 12/71 | 12/66 | 14/44 | χ2 = 2.56 (.28) | .15 | .87 | .20 |
| Drinker/nondrinker, n | 2/81 | 0/78 | 1/57 | χ2 = 1.80 (.41) | .78 | .17 | .24 |
| Age at disease onset (y) (M ± SD) | – | 19.74 ± 4.08 | 20.84 ± 4.48 | – | – | – | U = 1939 (.15) |
| Illness duration (y) (M ± SD) | – | 7.21 ± 4.61 | 5.90 ± 3.86 | – | – | – | U = 1893 (.10) |
| PANSS-P (M ± SD) | – | 13.62 ± 2.83 | 12.93 ± 3.70 | – | – | – | U = 1953 (.21) |
| PANSS-N (M ± SD) | – | 15.01 ± 5.67 | 14.09 ± 7.30 | – | – | – | U = 1857 (.09) |
| PANSS-G (M ± SD) | – | 26.79 ± 6.59 | 26.95 ± 8.78 | – | – | – | U = 2051 (.42) |
| PANSS-T (M ± SD) | – | 54.72 ± 12.52 | 53.97 ± 16.78 | – | – | – | U = 1873 (.09) |
| AHRS (M ± SD) | – | 26.31 ± 4.51 | – | – | – | – | – |
| OSIT-J (M ± SD)b | 8.49 ± 1.67 | 6.71 ± 2.08 | 6.86 ± 1.86 | F = 11.27 (<.001)*** | <.001*** | <.001*** | .54 |
| CPZ equivalent (mg/d) (M ± SD) | – | 650.41 ± 280.59 | 593.48 ± 337.16 | – | – | – | U = 1912 (.12) |
Note: M, mean; SD, standard deviation; n, number; M/F, male/female; pAVH, persistent auditory verbal hallucination; non-AVH, without auditory verbal hallucination; HC, health control; PANSS, Positive and Negative Symptoms Scale; PANSS-T, PANSS total score; PANSS-P, PANSS positive score; PANSS-N, PANSS negative score; PANSS-G, PANSS general psychopathology score; AHRS, Auditory Hallucinations Rating Scale; OSIT-J, Odor Stick Identification Test Japan; CPZ, chlorpromazine; -, not applicable.
aANOVA was performed.
bANCOVA was performed.
*P < .05.
***P < .001.
Demographic and Clinical Characteristics of Patients and Healthy Controls Groups
| Characteristics . | HC (n = 83) . | Patients (n = 136) . | Significance . | ||||
|---|---|---|---|---|---|---|---|
| pAVH (n = 78) . | Non-AVH (n = 58) . | 3 Groups . | HC vs Non-AVH . | HC vs pAVH . | pAVH vs Non-AVH . | ||
| P value . | |||||||
| Gender (M/F), n | 38/45 | 38/40 | 35/23 | χ2 = 3.08 (.21) | .09 | .71 | .18 |
| Age (y) (M ± SD)a | 26.80 ± 5.91 | 25.64 ± 5.52 | 26.95 ± 5.77 | F = 1.14 (.32) | 1.00 | .61 | .57 |
| Education (y) (M ± SD)a | 14.43 ± 2.65 | 11.69 ± 3.18 | 13.09 ± 2.82 | F = 18.04 (<.001)*** | .02* | <.001*** | .02* |
| Smoker/nonsmoker, n | 12/71 | 12/66 | 14/44 | χ2 = 2.56 (.28) | .15 | .87 | .20 |
| Drinker/nondrinker, n | 2/81 | 0/78 | 1/57 | χ2 = 1.80 (.41) | .78 | .17 | .24 |
| Age at disease onset (y) (M ± SD) | – | 19.74 ± 4.08 | 20.84 ± 4.48 | – | – | – | U = 1939 (.15) |
| Illness duration (y) (M ± SD) | – | 7.21 ± 4.61 | 5.90 ± 3.86 | – | – | – | U = 1893 (.10) |
| PANSS-P (M ± SD) | – | 13.62 ± 2.83 | 12.93 ± 3.70 | – | – | – | U = 1953 (.21) |
| PANSS-N (M ± SD) | – | 15.01 ± 5.67 | 14.09 ± 7.30 | – | – | – | U = 1857 (.09) |
| PANSS-G (M ± SD) | – | 26.79 ± 6.59 | 26.95 ± 8.78 | – | – | – | U = 2051 (.42) |
| PANSS-T (M ± SD) | – | 54.72 ± 12.52 | 53.97 ± 16.78 | – | – | – | U = 1873 (.09) |
| AHRS (M ± SD) | – | 26.31 ± 4.51 | – | – | – | – | – |
| OSIT-J (M ± SD)b | 8.49 ± 1.67 | 6.71 ± 2.08 | 6.86 ± 1.86 | F = 11.27 (<.001)*** | <.001*** | <.001*** | .54 |
| CPZ equivalent (mg/d) (M ± SD) | – | 650.41 ± 280.59 | 593.48 ± 337.16 | – | – | – | U = 1912 (.12) |
| Characteristics . | HC (n = 83) . | Patients (n = 136) . | Significance . | ||||
|---|---|---|---|---|---|---|---|
| pAVH (n = 78) . | Non-AVH (n = 58) . | 3 Groups . | HC vs Non-AVH . | HC vs pAVH . | pAVH vs Non-AVH . | ||
| P value . | |||||||
| Gender (M/F), n | 38/45 | 38/40 | 35/23 | χ2 = 3.08 (.21) | .09 | .71 | .18 |
| Age (y) (M ± SD)a | 26.80 ± 5.91 | 25.64 ± 5.52 | 26.95 ± 5.77 | F = 1.14 (.32) | 1.00 | .61 | .57 |
| Education (y) (M ± SD)a | 14.43 ± 2.65 | 11.69 ± 3.18 | 13.09 ± 2.82 | F = 18.04 (<.001)*** | .02* | <.001*** | .02* |
| Smoker/nonsmoker, n | 12/71 | 12/66 | 14/44 | χ2 = 2.56 (.28) | .15 | .87 | .20 |
| Drinker/nondrinker, n | 2/81 | 0/78 | 1/57 | χ2 = 1.80 (.41) | .78 | .17 | .24 |
| Age at disease onset (y) (M ± SD) | – | 19.74 ± 4.08 | 20.84 ± 4.48 | – | – | – | U = 1939 (.15) |
| Illness duration (y) (M ± SD) | – | 7.21 ± 4.61 | 5.90 ± 3.86 | – | – | – | U = 1893 (.10) |
| PANSS-P (M ± SD) | – | 13.62 ± 2.83 | 12.93 ± 3.70 | – | – | – | U = 1953 (.21) |
| PANSS-N (M ± SD) | – | 15.01 ± 5.67 | 14.09 ± 7.30 | – | – | – | U = 1857 (.09) |
| PANSS-G (M ± SD) | – | 26.79 ± 6.59 | 26.95 ± 8.78 | – | – | – | U = 2051 (.42) |
| PANSS-T (M ± SD) | – | 54.72 ± 12.52 | 53.97 ± 16.78 | – | – | – | U = 1873 (.09) |
| AHRS (M ± SD) | – | 26.31 ± 4.51 | – | – | – | – | – |
| OSIT-J (M ± SD)b | 8.49 ± 1.67 | 6.71 ± 2.08 | 6.86 ± 1.86 | F = 11.27 (<.001)*** | <.001*** | <.001*** | .54 |
| CPZ equivalent (mg/d) (M ± SD) | – | 650.41 ± 280.59 | 593.48 ± 337.16 | – | – | – | U = 1912 (.12) |
Note: M, mean; SD, standard deviation; n, number; M/F, male/female; pAVH, persistent auditory verbal hallucination; non-AVH, without auditory verbal hallucination; HC, health control; PANSS, Positive and Negative Symptoms Scale; PANSS-T, PANSS total score; PANSS-P, PANSS positive score; PANSS-N, PANSS negative score; PANSS-G, PANSS general psychopathology score; AHRS, Auditory Hallucinations Rating Scale; OSIT-J, Odor Stick Identification Test Japan; CPZ, chlorpromazine; -, not applicable.
aANOVA was performed.
bANCOVA was performed.
*P < .05.
***P < .001.
Intergroup Differences in Cortical Thickness
Statistical analysis utilizing ANCOVA with Bonferroni correction across 3 participant groups revealed significant differences in several brain regions. Notably, within the left hemisphere, regions including the opercular part of the inferior frontal gyrus, orbital part of the inferior frontal gyrus, orbital gyri, anterior segment of the circular sulcus of the insula, inferior segment of the circular sulcus of the insula, superior segment of the circular sulcus of the insula, inferior frontal sulcus, superior frontal sulcus, and orbital sulci (H-shaped sulci) demonstrated significant variance (all P < .05/148 = .00034, Bonferroni correction). Conversely, in the right hemisphere, significant differences were observed in the anterior part of the cingulate gyrus and sulcus, superior frontal gyrus, orbital gyri, straight gyrus/gyrus rectus, superior segment of the circular sulcus of the insula, superior frontal gyrus, medial orbital sulcus (olfactory sulcus), and orbital sulci (H-shaped sulci) (all P < .00034). Multiple comparative analyses revealed significant reductions in cortical thickness of the right medial orbital sulcus (olfactory sulcus) (P = .002) and the right orbital sulci (H-shaped sulci) (P = .0005) in the pAVH group compared to both the non-AVH and HC groups (P < 0.05/17 = .003, Bonferroni correction). However, there were no significant differences in cortical thickness between the non-AVH and HC groups (P > .003). See table 2 and figure 1 for details.
Brain Regions With Cortical Thickness Differences in Patients and Healthy Controls
| Variable . | Cortical Thickness (mm) M ± SD . | ANCOVA . | Pairwise Comparisons P Value . | ||||
|---|---|---|---|---|---|---|---|
| pAVH . | Non-AVH . | HC . | pAVH vs Non-AVH . | pAVH vs HC . | Non-AVH vs HC . | ||
| Left hemisphere | |||||||
| Opercular part of the inferior frontal gyrus | 2.54 ± 0.16 | 2.58 ± 0.19 | 2.64 ± 0.13 | F = 9.79 (<.00034) | .29 | <.003 | .03 |
| Orbital part of the inferior frontal gyrus | 2.64 ± 0.21 | 2.67 ± 0.23 | 2.76 ± 0.19 | F = 8.17 (<.00034) | .57 | <.003 | .04 |
| Orbital gyri | 2.62 ± 0.14 | 2.67 ± 0.15 | 2.73 ± 0.12 | F = 15.58 (<.00034) | .03 | <.003 | .02 |
| Anterior segment of the circular sulcus of the insula | 2.78 ± 0.19 | 2.86 ± 0.24 | 2.94 ± 0.17 | F = 12.81 (<.00034) | .02 | <.003 | .09 |
| Inferior segment of the circular sulcus of the insula | 2.69 ± 0.18 | 2.78 ± 0.18 | 2.81 ± 0.18 | F = 10.64 (<.00034) | .01 | <.003 | .38 |
| Superior segment of the circular sulcus of the insula | 2.41 ± 0.14 | 2.45 ± 0.14 | 2.52 ± 0.11 | F = 14.71 (<.00034) | .17 | <.003 | .004 |
| Inferior frontal sulcus | 1.97 ± 0.13 | 2.01 ± 0.13 | 2.05 ± 0.12 | F = 9.50 (<.00034) | .17 | <.003 | .07 |
| Superior frontal sulcus | 2.31 ± 0.13 | 2.35 ± 0.13 | 2.39 ± 0.12 | F = 8.82 (<.00034) | .26 | <.003 | .26 |
| Orbital sulci (H-shaped sulci) | 2.43 ± 0.18 | 2.49 ± 0.17 | 2.58 ± 0.17 | F = 15.31 (<.00034) | .07 | <.003 | .01 |
| Right hemisphere | |||||||
| Anterior part of the cingulate gyrus and sulcus (ACC) | 2.44 ± 0.17 | 2.48 ± 0.17 | 2.54 ± 0.15 | F = 9.51 (<.00034) | .07 | <.003 | .18 |
| Superior frontal gyrus | 2.68 ± 0.15 | 2.69 ± 0.17 | 2.78 ± 0.18 | F = 10.37 (<.00034) | .72 | <.003 | .01 |
| Orbital gyri | 2.69 ± 0.14 | 2.75 ± 0.15 | 2.79 ± 0.15 | F = 11.63 (<.00034) | .004 | <.003 | .66 |
| Straight gyrus/gyrus rectus | 2.54 ± 0.14 | 2.59 ± 0.19 | 2.64 ± 0.16 | F = 9.25 (<.00034) | .03 | <.003 | .44 |
| Superior segment of the circular sulcus of the insula | 2.51 ± 0.15 | 2.54 ± 0.15 | 2.61 ± 0.13 | F = 11.55, (<.00034) | .27 | <.003 | .01 |
| Superior frontal gyrus | 2.25 ± 0.11 | 2.31 ± 0.15 | 2.36 ± 0.12 | F = 16.92, (<.00034) | .01 | <.003 | .04 |
| Medial orbital sulcus (olfactory sulcus)a | 2.19 ± 0.15 | 2.28 ± 0.16 | 2.31 ± 0.16 | F = 12.94, (<.00034) | .002 | <.003 | .54 |
| Orbital sulci (H-shaped sulci)a | 2.39 ± 0.17 | 2.49 ± 0.20 | 2.56 ± 0.17 | F = 21.57, (<.00034) | .0005 | <.003 | .05 |
| Variable . | Cortical Thickness (mm) M ± SD . | ANCOVA . | Pairwise Comparisons P Value . | ||||
|---|---|---|---|---|---|---|---|
| pAVH . | Non-AVH . | HC . | pAVH vs Non-AVH . | pAVH vs HC . | Non-AVH vs HC . | ||
| Left hemisphere | |||||||
| Opercular part of the inferior frontal gyrus | 2.54 ± 0.16 | 2.58 ± 0.19 | 2.64 ± 0.13 | F = 9.79 (<.00034) | .29 | <.003 | .03 |
| Orbital part of the inferior frontal gyrus | 2.64 ± 0.21 | 2.67 ± 0.23 | 2.76 ± 0.19 | F = 8.17 (<.00034) | .57 | <.003 | .04 |
| Orbital gyri | 2.62 ± 0.14 | 2.67 ± 0.15 | 2.73 ± 0.12 | F = 15.58 (<.00034) | .03 | <.003 | .02 |
| Anterior segment of the circular sulcus of the insula | 2.78 ± 0.19 | 2.86 ± 0.24 | 2.94 ± 0.17 | F = 12.81 (<.00034) | .02 | <.003 | .09 |
| Inferior segment of the circular sulcus of the insula | 2.69 ± 0.18 | 2.78 ± 0.18 | 2.81 ± 0.18 | F = 10.64 (<.00034) | .01 | <.003 | .38 |
| Superior segment of the circular sulcus of the insula | 2.41 ± 0.14 | 2.45 ± 0.14 | 2.52 ± 0.11 | F = 14.71 (<.00034) | .17 | <.003 | .004 |
| Inferior frontal sulcus | 1.97 ± 0.13 | 2.01 ± 0.13 | 2.05 ± 0.12 | F = 9.50 (<.00034) | .17 | <.003 | .07 |
| Superior frontal sulcus | 2.31 ± 0.13 | 2.35 ± 0.13 | 2.39 ± 0.12 | F = 8.82 (<.00034) | .26 | <.003 | .26 |
| Orbital sulci (H-shaped sulci) | 2.43 ± 0.18 | 2.49 ± 0.17 | 2.58 ± 0.17 | F = 15.31 (<.00034) | .07 | <.003 | .01 |
| Right hemisphere | |||||||
| Anterior part of the cingulate gyrus and sulcus (ACC) | 2.44 ± 0.17 | 2.48 ± 0.17 | 2.54 ± 0.15 | F = 9.51 (<.00034) | .07 | <.003 | .18 |
| Superior frontal gyrus | 2.68 ± 0.15 | 2.69 ± 0.17 | 2.78 ± 0.18 | F = 10.37 (<.00034) | .72 | <.003 | .01 |
| Orbital gyri | 2.69 ± 0.14 | 2.75 ± 0.15 | 2.79 ± 0.15 | F = 11.63 (<.00034) | .004 | <.003 | .66 |
| Straight gyrus/gyrus rectus | 2.54 ± 0.14 | 2.59 ± 0.19 | 2.64 ± 0.16 | F = 9.25 (<.00034) | .03 | <.003 | .44 |
| Superior segment of the circular sulcus of the insula | 2.51 ± 0.15 | 2.54 ± 0.15 | 2.61 ± 0.13 | F = 11.55, (<.00034) | .27 | <.003 | .01 |
| Superior frontal gyrus | 2.25 ± 0.11 | 2.31 ± 0.15 | 2.36 ± 0.12 | F = 16.92, (<.00034) | .01 | <.003 | .04 |
| Medial orbital sulcus (olfactory sulcus)a | 2.19 ± 0.15 | 2.28 ± 0.16 | 2.31 ± 0.16 | F = 12.94, (<.00034) | .002 | <.003 | .54 |
| Orbital sulci (H-shaped sulci)a | 2.39 ± 0.17 | 2.49 ± 0.20 | 2.56 ± 0.17 | F = 21.57, (<.00034) | .0005 | <.003 | .05 |
Note: M, mean; SD, standard deviation; mm, millimeter; ANCOVA, univariate covariance analysis; pAVH: persistent auditory verbal hallucinations; non-AVH: without auditory verbal hallucinations; HC: health control; Bonferroni correction was used for ANCOVA (P < 0.05/148 = 0.00034) and multiple comparisons (P < 0.05/17 = 0.003). Brain region labeling was conducted utilizing the Destrieux atlas.
aThe statistical significance remained after the Bonferroni correction.
Brain Regions With Cortical Thickness Differences in Patients and Healthy Controls
| Variable . | Cortical Thickness (mm) M ± SD . | ANCOVA . | Pairwise Comparisons P Value . | ||||
|---|---|---|---|---|---|---|---|
| pAVH . | Non-AVH . | HC . | pAVH vs Non-AVH . | pAVH vs HC . | Non-AVH vs HC . | ||
| Left hemisphere | |||||||
| Opercular part of the inferior frontal gyrus | 2.54 ± 0.16 | 2.58 ± 0.19 | 2.64 ± 0.13 | F = 9.79 (<.00034) | .29 | <.003 | .03 |
| Orbital part of the inferior frontal gyrus | 2.64 ± 0.21 | 2.67 ± 0.23 | 2.76 ± 0.19 | F = 8.17 (<.00034) | .57 | <.003 | .04 |
| Orbital gyri | 2.62 ± 0.14 | 2.67 ± 0.15 | 2.73 ± 0.12 | F = 15.58 (<.00034) | .03 | <.003 | .02 |
| Anterior segment of the circular sulcus of the insula | 2.78 ± 0.19 | 2.86 ± 0.24 | 2.94 ± 0.17 | F = 12.81 (<.00034) | .02 | <.003 | .09 |
| Inferior segment of the circular sulcus of the insula | 2.69 ± 0.18 | 2.78 ± 0.18 | 2.81 ± 0.18 | F = 10.64 (<.00034) | .01 | <.003 | .38 |
| Superior segment of the circular sulcus of the insula | 2.41 ± 0.14 | 2.45 ± 0.14 | 2.52 ± 0.11 | F = 14.71 (<.00034) | .17 | <.003 | .004 |
| Inferior frontal sulcus | 1.97 ± 0.13 | 2.01 ± 0.13 | 2.05 ± 0.12 | F = 9.50 (<.00034) | .17 | <.003 | .07 |
| Superior frontal sulcus | 2.31 ± 0.13 | 2.35 ± 0.13 | 2.39 ± 0.12 | F = 8.82 (<.00034) | .26 | <.003 | .26 |
| Orbital sulci (H-shaped sulci) | 2.43 ± 0.18 | 2.49 ± 0.17 | 2.58 ± 0.17 | F = 15.31 (<.00034) | .07 | <.003 | .01 |
| Right hemisphere | |||||||
| Anterior part of the cingulate gyrus and sulcus (ACC) | 2.44 ± 0.17 | 2.48 ± 0.17 | 2.54 ± 0.15 | F = 9.51 (<.00034) | .07 | <.003 | .18 |
| Superior frontal gyrus | 2.68 ± 0.15 | 2.69 ± 0.17 | 2.78 ± 0.18 | F = 10.37 (<.00034) | .72 | <.003 | .01 |
| Orbital gyri | 2.69 ± 0.14 | 2.75 ± 0.15 | 2.79 ± 0.15 | F = 11.63 (<.00034) | .004 | <.003 | .66 |
| Straight gyrus/gyrus rectus | 2.54 ± 0.14 | 2.59 ± 0.19 | 2.64 ± 0.16 | F = 9.25 (<.00034) | .03 | <.003 | .44 |
| Superior segment of the circular sulcus of the insula | 2.51 ± 0.15 | 2.54 ± 0.15 | 2.61 ± 0.13 | F = 11.55, (<.00034) | .27 | <.003 | .01 |
| Superior frontal gyrus | 2.25 ± 0.11 | 2.31 ± 0.15 | 2.36 ± 0.12 | F = 16.92, (<.00034) | .01 | <.003 | .04 |
| Medial orbital sulcus (olfactory sulcus)a | 2.19 ± 0.15 | 2.28 ± 0.16 | 2.31 ± 0.16 | F = 12.94, (<.00034) | .002 | <.003 | .54 |
| Orbital sulci (H-shaped sulci)a | 2.39 ± 0.17 | 2.49 ± 0.20 | 2.56 ± 0.17 | F = 21.57, (<.00034) | .0005 | <.003 | .05 |
| Variable . | Cortical Thickness (mm) M ± SD . | ANCOVA . | Pairwise Comparisons P Value . | ||||
|---|---|---|---|---|---|---|---|
| pAVH . | Non-AVH . | HC . | pAVH vs Non-AVH . | pAVH vs HC . | Non-AVH vs HC . | ||
| Left hemisphere | |||||||
| Opercular part of the inferior frontal gyrus | 2.54 ± 0.16 | 2.58 ± 0.19 | 2.64 ± 0.13 | F = 9.79 (<.00034) | .29 | <.003 | .03 |
| Orbital part of the inferior frontal gyrus | 2.64 ± 0.21 | 2.67 ± 0.23 | 2.76 ± 0.19 | F = 8.17 (<.00034) | .57 | <.003 | .04 |
| Orbital gyri | 2.62 ± 0.14 | 2.67 ± 0.15 | 2.73 ± 0.12 | F = 15.58 (<.00034) | .03 | <.003 | .02 |
| Anterior segment of the circular sulcus of the insula | 2.78 ± 0.19 | 2.86 ± 0.24 | 2.94 ± 0.17 | F = 12.81 (<.00034) | .02 | <.003 | .09 |
| Inferior segment of the circular sulcus of the insula | 2.69 ± 0.18 | 2.78 ± 0.18 | 2.81 ± 0.18 | F = 10.64 (<.00034) | .01 | <.003 | .38 |
| Superior segment of the circular sulcus of the insula | 2.41 ± 0.14 | 2.45 ± 0.14 | 2.52 ± 0.11 | F = 14.71 (<.00034) | .17 | <.003 | .004 |
| Inferior frontal sulcus | 1.97 ± 0.13 | 2.01 ± 0.13 | 2.05 ± 0.12 | F = 9.50 (<.00034) | .17 | <.003 | .07 |
| Superior frontal sulcus | 2.31 ± 0.13 | 2.35 ± 0.13 | 2.39 ± 0.12 | F = 8.82 (<.00034) | .26 | <.003 | .26 |
| Orbital sulci (H-shaped sulci) | 2.43 ± 0.18 | 2.49 ± 0.17 | 2.58 ± 0.17 | F = 15.31 (<.00034) | .07 | <.003 | .01 |
| Right hemisphere | |||||||
| Anterior part of the cingulate gyrus and sulcus (ACC) | 2.44 ± 0.17 | 2.48 ± 0.17 | 2.54 ± 0.15 | F = 9.51 (<.00034) | .07 | <.003 | .18 |
| Superior frontal gyrus | 2.68 ± 0.15 | 2.69 ± 0.17 | 2.78 ± 0.18 | F = 10.37 (<.00034) | .72 | <.003 | .01 |
| Orbital gyri | 2.69 ± 0.14 | 2.75 ± 0.15 | 2.79 ± 0.15 | F = 11.63 (<.00034) | .004 | <.003 | .66 |
| Straight gyrus/gyrus rectus | 2.54 ± 0.14 | 2.59 ± 0.19 | 2.64 ± 0.16 | F = 9.25 (<.00034) | .03 | <.003 | .44 |
| Superior segment of the circular sulcus of the insula | 2.51 ± 0.15 | 2.54 ± 0.15 | 2.61 ± 0.13 | F = 11.55, (<.00034) | .27 | <.003 | .01 |
| Superior frontal gyrus | 2.25 ± 0.11 | 2.31 ± 0.15 | 2.36 ± 0.12 | F = 16.92, (<.00034) | .01 | <.003 | .04 |
| Medial orbital sulcus (olfactory sulcus)a | 2.19 ± 0.15 | 2.28 ± 0.16 | 2.31 ± 0.16 | F = 12.94, (<.00034) | .002 | <.003 | .54 |
| Orbital sulci (H-shaped sulci)a | 2.39 ± 0.17 | 2.49 ± 0.20 | 2.56 ± 0.17 | F = 21.57, (<.00034) | .0005 | <.003 | .05 |
Note: M, mean; SD, standard deviation; mm, millimeter; ANCOVA, univariate covariance analysis; pAVH: persistent auditory verbal hallucinations; non-AVH: without auditory verbal hallucinations; HC: health control; Bonferroni correction was used for ANCOVA (P < 0.05/148 = 0.00034) and multiple comparisons (P < 0.05/17 = 0.003). Brain region labeling was conducted utilizing the Destrieux atlas.
aThe statistical significance remained after the Bonferroni correction.
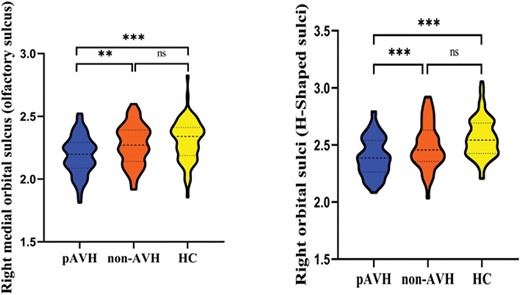
Bonferroni corrected violin graphs of cortical thickness of brain regions with significant differences among the 3 groups. Note: pAVH, persistent auditory verbal hallucination; non-AVH, without auditory verbal hallucination; HC, health control. **P < .01; ***P < .001; ns, not significant (P > .05). Bonferroni correction was used for ANCOVA (P < .05/148 = .00034) and multiple comparisons (P < .05/17 = .003).
Intergroup Differences in OSIT-J Scores
The ANCOVA analysis, after Bonferroni correction, revealed that the OSIT-J scores were significantly lower in both the pAVH (P < .001) and non-AVH (P < .001) groups compared to the HC group, while no significant differences were observed between the 2 patient groups. See table 1 and figure 2 for details.
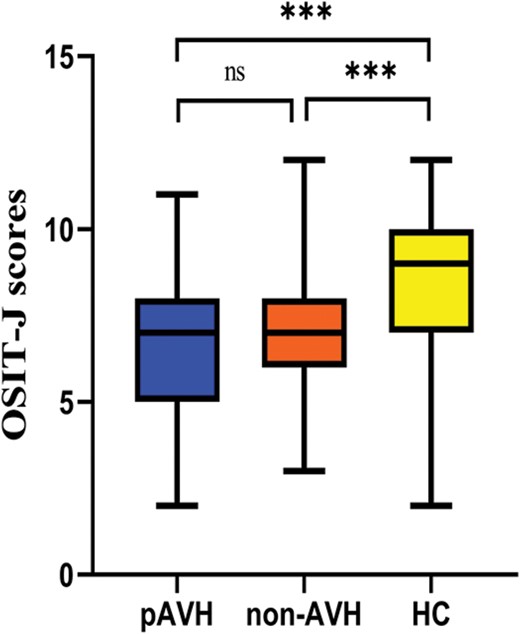
Box graph of OSIT-J scores with significant differences among the 3 groups. Note: pAVH, persistent auditory verbal hallucination; non-AVH, without auditory verbal hallucination; HC, health control. OSIT-J, Odor Stick Identification Test Japan. ***P < .001; ns, not significant (P > .05).
Correlation Analysis Results
Partial correlation analysis was conducted to investigate the relationship between differences in cortical thickness and the severity of pAVHs among SCZ patients in the pAVH group. The results showed that reductions in cortical thickness in the right medial orbital sulcus (olfactory sulcus) (r = −.31, P = .008) and the right orbital sulci (H-shaped sulci) (r = −.30, P = .009) were negatively correlated with the severity of AVHs (as measured by AHRS scores). In addition, the present study found a positive correlation between the reduction of cortical thickness in the right medial orbital sulcus (olfactory sulcus) and OSIT-J scores (r = .28, P = .018). Bonferroni correction was applied for these correlations (P < .05/4 = .0125), and table 3, figures 3 and 4 provide further details. It is important to highlight that the correlation analysis result concerning cortical thickness in the right medial orbital sulcus (olfactory sulcus) and OSIT-J scores remained significant following false discovery rate correction (P < .05) but did not withstand Bonferroni correction.
Correlation Between Cortical Thickness of Brain Regions and the Severity of pAVHs
| Variable . | AHRS . | OSIT-J . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| Right medial orbital sulcus (olfactory sulcus) | −.31 | .008a | .28 | .018b |
| Right orbital sulci (H-shaped sulci) | −.30 | .009a | .16 | .17 |
| Variable . | AHRS . | OSIT-J . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| Right medial orbital sulcus (olfactory sulcus) | −.31 | .008a | .28 | .018b |
| Right orbital sulci (H-shaped sulci) | −.30 | .009a | .16 | .17 |
Note: pAVHs, persistent auditory verbal hallucinations; AHRS, the Auditory Hallucinations Rating Scale; OSIT-J, Odor Stick Identification Test Japan; Bonferroni correction (P < .05/4 = .0125).
aSurvival with Bonferroni correction.
bSurvival with false discovery rate (FDR) correction (P < .05).
Correlation Between Cortical Thickness of Brain Regions and the Severity of pAVHs
| Variable . | AHRS . | OSIT-J . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| Right medial orbital sulcus (olfactory sulcus) | −.31 | .008a | .28 | .018b |
| Right orbital sulci (H-shaped sulci) | −.30 | .009a | .16 | .17 |
| Variable . | AHRS . | OSIT-J . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| Right medial orbital sulcus (olfactory sulcus) | −.31 | .008a | .28 | .018b |
| Right orbital sulci (H-shaped sulci) | −.30 | .009a | .16 | .17 |
Note: pAVHs, persistent auditory verbal hallucinations; AHRS, the Auditory Hallucinations Rating Scale; OSIT-J, Odor Stick Identification Test Japan; Bonferroni correction (P < .05/4 = .0125).
aSurvival with Bonferroni correction.
bSurvival with false discovery rate (FDR) correction (P < .05).
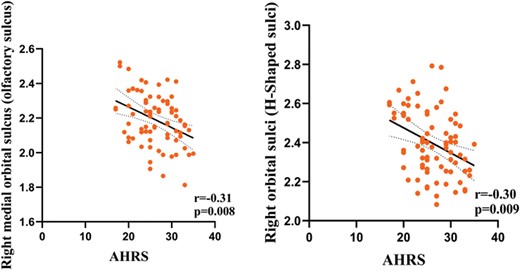
The relationship between the severity of pAVHs and cortical thickness of brain regions in the pAVH group. Note: pAVHs, persistent auditory verbal hallucinations; pAVH, persistent auditory verbal hallucination. AHRS, the Auditory Hallucinations Rating Scale. Bonferroni correction (P < .05/4 = .0125).
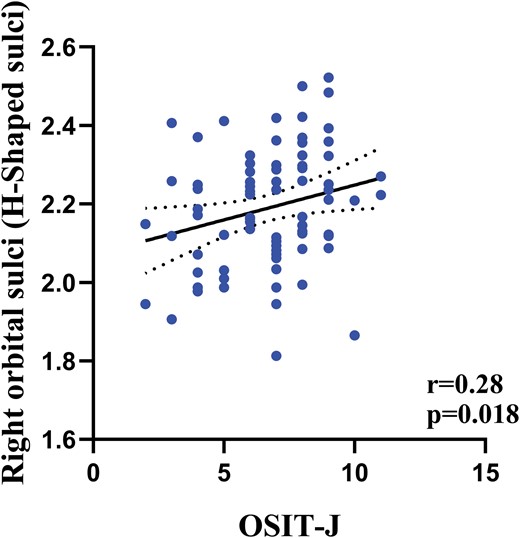
The relationship between the OSIT-J scores and cortical thickness of brain regions in the pAVH group. Note: pAVH, persistent auditory verbal hallucination. OSIT-J, Odor Stick Identification Test Japan. False discovery rate (FDR) correction (P < .05).
Additionally, partial correlation analyses were conducted to explore the association between variations in cortical thickness and the extent of olfactory dysfunction in SCZ patients within the AVH group. These analyses revealed no significant relationship between the OSIT-J scores and the cortical thickness of the right medial orbital sulcus (olfactory sulcus) (r = −.05, P = .74) and the right orbital sulci (H-shaped sulci) (r = .20, P = .16).
This study extended its exploration of the correlation between cognitive function and abnormal cortical thickness in specific brain regions among individuals diagnosed with SCZ. The results have been incorporated into supplementary tables 1 and 2. Regrettably, no association was observed between cognitive function and cortical thickness in the medial orbital sulcus (olfactory sulcus) and the orbital sulci (H-shaped sulci).
Discussion
This study comprehensively examined the cortical thickness in specific brain regions of SCZ patients with pAVHs, as well as its association with the severity of pAVHs and OSIT-J scores. The findings indicated that cortical thinning in SCZ patients with pAVHs primarily occurred in the frontal lobe, particularly in the right medial orbital sulcus (olfactory sulcus) and the right orbital sulcus (H-sulcus). Additionally, within the pAVH group, reductions in cortical thickness in the right medial orbital sulcus (olfactory sulcus) and right orbital sulcus (H-shaped sulcus) were negatively associated with the severity of pAVHs. Furthermore, a positive correlation was observed between cortical thinning in the right medial orbital sulcus (olfactory sulcus) and OSIT-J scores.
This study found that the cortical thickness of the right medial orbital sulcus (olfactory sulcus) and the right orbital sulci (H-shaped sulci) decreased in the pAVH group compared with the non-AVH and HC groups. These areas belong to the orbital frontal cortex (OFC), which is involved in language, cognition, and emotional processing.57–59 Previous studies have demonstrated that dysfunction of the OFC may lead to pAVHs like those found in SCZ. For example, structural MRI showed that the GMV and cortex thickness of the OFC in SCZ patients decreased, which was negatively correlated with the positive symptom subscale (including hallucinations).60–63 Previous studies on positron emission tomography and functional MRI have found abnormal activation of the OFC in SCZ patients with pAVHs.64,65 At present, the main theory of the neurocognitive model of pAVHs is the neurocognitive action self-monitoring system,66,67 which holds that individuals perceive events that occur within as originating from outside. Patients with SCZ, especially those with pAVHs, may have self-monitoring dysfunction,68 in which they are unable to recognize events occurring within themselves and perceive them as external. Therefore, the OFC may play a key role in the self-monitoring system that mediates the occurrence and development of pAVHs.
Previous studies have also reported structural abnormalities in other brain regions, such as the temporal1,69 and insular cortex,70 but we did not observe any differences in cortical thickness in these regions. This inconsistent finding may be attributed to methodological differences and heterogeneity in SCZ. Previous studies mostly used VBM analysis, which focuses on structural changes in GMV or density rather than cortical thickness. To the best of our knowledge, previous studies assessing cortical thickness in SCZ patients with AVH have had relatively small samples.1,71 Furthermore, SCZ patients present with a wide range of clinical symptoms and durations of illness. Evidence has demonstrated that SCZ patients show cortex thinning over time across the entire course of illness,72 suggesting that the chronicity of the illness might be an important contributor to cortical thinning. Moreover, in the present study, we did not observe significant thickness reduction in the left medial orbital sulcus (olfactory sulcus) and the left orbital sulci (H-shaped sulci). This finding concurs with previous studies demonstrating that in the frontal cortex, the morphometric changes appeared to be more consistent on the right than the left in SCZ patients.1
In addition, this study found a positive correlation between cortical thinning in the right medial orbital sulcus (olfactory sulcus) and OSIT-J scores. In other words, the thinner the cortical thickness of the right medial orbital sulcus (olfactory sulcus) in SCZ patients with pAVHs, the more severe the olfactory identification impairment. This is consistent with most previous studies that abnormalities in the structure of the olfactory sulcus are associated with olfactory impairment.35,36,39 The fetal forebrain has been seen to manifest the olfactory sulcus around 16 weeks into gestation,73 and the completeness of this structure is believed to be linked with the growth of other olfactory formations.35 It has been observed that the depth of the olfactory sulcus is correspondingly related to olfactory function in healthy individuals as well as patients with olfactory deficiencies,15,74 proposing that it could possibly represent both unimpaired and pathological embryonic development of olfactory.
Interestingly, the present study discovered that the diminished cortical thickness of the right medial orbital sulcus (olfactory sulcus) in SCZ patients who also experienced pAVHs was associated not just with the intensity of pAVHs, but also with the harshness of olfactory identification deficiencies. This indicates a potential shared neuroimaging mechanism for pAVHs and olfactory impairment in chronic SCZ patients. This finding further substantiates previous research conclusions that the olfactory pathway intersects with numerous brain regions engaged in socio-emotional information processing and cognitive activity.75,76 Consequently, in SCZ patients, structural or functional disruptions in these areas can trigger not only mental disorders but also olfactory malfunction.
Moreover, the findings of this investigation are not entirely consistent with previous studies. For instance, the research by Nguyen et al indicated that there were no differences in the bilateral olfactory sulci between SCZ patients and HC, yet they identified a significant correlation between the length of the olfactory sulcus and olfactory functional impairments. They also observed a significant reduction in the volume of the olfactory bulb in SCZ patients, which correlated with their performance on the University of Pennsylvania Smell Identification Test (UPSIT). This supports the hypothesis that the olfactory system in SCZ patients is subject to both morphological and functional alterations, emphasizing the potential of these olfactory features as biomarkers for the condition.35 Conversely, the study by Turetsky et al found that the right olfactory sulcus in SCZ patients was smaller than that in the HC group, with no differences observed in the H-shaped sulcus. Their research further highlighted that SCZ patients exhibited significant changes in olfactory responses, which appeared to be unrelated to clinical symptoms, medication, or smoking status. This suggests that there may be a fundamental issue with the olfactory receptor neurons themselves, potentially related to the growth or maturation processes of these cells in SCZ patients.22 The discrepancies between our results and those reported by Turetsky et al and Nguyen et al could be attributed to several factors. These include the heterogeneity of the samples studied (such as clinical high-risk populations, individuals with first-episode psychosis, or chronic patients), differences in sample sizes, and the methodological approaches used (such as assessments of GMV, cortical thickness, and tools for evaluating olfactory function). These differences highlight the complexity of neuroanatomical and functional changes in SCZ and emphasize the importance of considering these variables when interpreting research findings. The current study utilized cortical thickness and OSIT-J scores as measures, which are different from those used in the aforementioned studies. This methodological choice allowed us to explore the relationship between cortical thickness and olfactory function from a novel perspective.
Notably, this study showed that all SCZ patients exhibited impaired olfactory function, yet changes in cortical thickness were only observed in those with pAVHs. Several potential explanations emerge from this observation: The alterations of cortical thickness might be specifically associated with pAVHs rather than solely with olfactory impairments, suggesting a unique aspect of the brains of SCZ patients experiencing pAVHs. Additional contributing factors or comorbidities in SCZ patients with pAVHs could be driving the observed changes in cortical thickness, extending beyond olfactory dysfunction alone. It is also plausible that the study lacked sufficient power to detect subtle changes in cortical thickness in SCZ patients without AVHs, if they exist. Moreover, the relationship between olfaction and cortical thickness could be indirect or involve intricate interactions rather than a straightforward causal link.
This study has certain limitations that warrant further consideration. First, the study is cross-sectional, necessitating follow-up assessments to delve deeper into symptom progression and prognosis, and to investigate the link between symptom evolution and cerebral cortex thickness alterations. Second, all patients were on antipsychotic medication throughout the course of the study. Despite our findings showing no dosage variation between the 2 groups over the last year, the potential impact on cortical thickness cannot be disregarded. In subsequent investigations, the association between cortical thickness irregularities and clinical symptoms in first-episode, untreated SCZ patients should be more thoroughly examined. Third, while existing studies suggest notable cultural differences in cortical thickness among SCZ patients,77,78 our study’s exclusive focus on a Chinese patient cohort precludes a cross-cultural analysis. Recognizing this limitation, we emphasize the need for future research to explore these differences more comprehensively, potentially shedding light on culturally specific neurobiological characteristics of SCZ.
Conclusion
In conclusion, the present study demonstrates that SCZ patients with pAVHs have reduced cortical thickness in the right medial orbitofrontal sulcus (olfactory sulcus) and the right orbitofrontal sulcus (H sulcus), which is negatively correlated with the severity of pAVHs. Also, reduced cortical thickness in the right medial orbital sulcus (olfactory sulcus) was positively correlated with olfactory identification impairment. These findings suggest that anatomical defects in the right medial orbital sulcus (olfactory sulcus) may modulate the common neuroimaging mechanisms underlying pAVHs and olfactory deficits in SCZ patients.
Supplementary Material
Supplementary material is available at https://dbpia.nl.go.kr/schizophreniabulletin/.
Acknowledgments
We would like to express our sincere thanks to all participants in this work. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Author Contributions
The study was designed and supervised by Jinsong Tang and Xiaogang Chen. Data were collected by Honghong Ren, Jinguang Li, and Jun Zhou. The scanning was processed by Chunwang Li. The analyses and interpretation of data were performed by Honghong Ren and Qianjin Wang. The manuscript was first drafted by Honghong Ren and Qianjin Wang, and revised critically by Jinsong Tang, Xiaogang Chen, Zongchang Li, and Ying He for important intellectual content. All co-authors revised and approved the final version to be published.
Funding
This research was supported by the National Natural Science Foundation of China (82171495 to J.T.), National key R & D program of China (2022YFE0103700 to J.T.), and Natural Science Youth Foundation of Shandong Province, China (ZR2023QH565 to H.R.).
Ethical Approval
This research adhered to the principles of the Declaration of Helsinki and received the green light from the Ethics Committee of the Second Xiangya Hospital, Central South University (Approval No. S006, 2018). Comprehensive details about the study, including its potential benefits and risks, were shared with the participants, and their written informed consent was duly procured.




