-
PDF
- Split View
-
Views
-
Cite
Cite
Ting Yat Wong, Sherry Kit Wa Chan, Charlton Cheung, Christy Lai Ming Hui, Yi Nam Suen, Wing Chung Chang, Edwin Ho Ming Lee, Eric Yu Hai Chen, Dynamic Patterns of Symptoms and Functioning in Predicting Deliberate Self-harm in Patients with First-Episode Schizophrenia-Spectrum Disorders Over 3 Years, Schizophrenia Bulletin, Volume 48, Issue 5, September 2022, Pages 1043–1052, https://doi.org/10.1093/schbul/sbac057
Close - Share Icon Share
Abstract
Patients with schizophrenia have a significant risk of self-harm. We aimed to explore the dynamic relationship between symptomatology, functioning and deliberate self-harm (DSH) and evaluate the feasibility of developing a self-harm risk prediction tool for patients with first-episode schizophrenia (FES).
Patients with FES (n = 1234) were followed up for 36 months. Symptomatology, functioning, treatment adherence and self-harm information were obtained monthly over the follow-up period. A time-varying vector autoregressive (VAR) model was used to study the contribution of clinical variables to self-harm over the 36th month. Random forest models for self-harm were established to classify the individuals with self-harm and predict future self-harm events.
Over a 36-month period, 187 patients with FES had one or more self-harm events. The depressive symptoms contributed the most to self-harm prediction during the first year, while the importance of positive psychotic symptoms increased from the second year onwards. The random forest model with all static information and symptom instability achieved a good area under the receiver operating characteristic curve (AUROC = 0.77 ± 0.023) for identifying patients with DSH. With a sliding window analysis, the averaged AUROC of predicting a self-event was 0.65 ± 0.102 (ranging from 0.54 to 0.78) with the best model being 6-month predicted future 6-month self-harm for month 11–23 (AUROC = 0.7).
Results highlight the importance of the dynamic relationship of depressive and positive psychotic symptoms with self-harm and the possibility of self-harm prediction in FES with longitudinal clinical data.
Introduction
Patients with schizophrenia have 12.6 times higher risk of suicide compared to a general population.1 Self-harm, with or without suicidal intent, is a strong predictor of death by suicide2 and is associated with poor outcomes in patients with schizophrenia.2 Suicide and self-harm are more common during the early phase of the illness.3 The suicide rate is 2.7 times higher in patients with first-episode schizophrenia (FES) than in those with chronic conditions.4 In a 12-year follow-up study of patients with FES, half of the deaths by suicide were found to have occurred in the first 3 years of the illness.5 Approximately 1 in 10 patients have suicide attempts or self-harm events during the early phase of the illness.6–8 Therefore, self-harm and suicide risk detection and prevention are crucial components of early intervention programs for patients with FES.
Establishing risk factors for self-harm is the first step in developing suicide risk detection and suicide prevention strategies. Early studies in this field have mostly used a categorical classification of patients based on the presence of a suicide attempt or self-harm event and considered these variables to be static.9 More recent longitudinal studies have included a limited number of assessment time points, with intervals of months or years between the assessments.8,10,11 With these approaches, several risk factors have consistently been reported, including previous suicide attempts and the presence of depressive and positive psychotic symptoms.9,12 However, a higher variability in the occurrence of suicidal ideation is found in the first year of the illness than in later stages,13,14 and accordingly, clinical psychopathology, including positive and negative psychotic symptoms, also fluctuates over the early stage of the illness.15,16 The dynamic nature of suicidality and psychopathology and their variable interactions over time in patients with FES have not been comprehensively examined. Only limited aspects of the dynamic interaction between suicidality and clinical psychopathology in patients with schizophrenia have been explored. Mood variability has been shown to predict the course of suicidal ideation17 and a dynamic interaction between insight and suicidal ideation has been found.18
In clinical practice, the identification of self-harm risk within an actionable time frame is vital and relies mostly on clinical judgment and assessment tools, which tend to be questionnaire based. Although implementing systematic clinical suicide assessment for patients with schizophrenia is advocated in many clinical guidelines, its predictive value is modest and the adherence rate by clinicians has been low.19 More recently, machine learning approaches have been used with clinical data obtained from electronic clinical records to predict suicide in the general adult and adolescent populations. The results of these studies suggest that an algorithm-based tool is useful to facilitate and support suicide risk assessments in clinical settings.20–22 However, these studies have all been conducted in the general population, with no study focusing on populations with specific conditions such as schizophrenia.
The current study involved a re-analysis of a subset of data collected from a 3-year historical control study comparing the general outcomes of patients with FES who received an early intervention and those who received standard care.5,23 Detailed monthly clinical, functioning and self-harm measurements of the FES patients were collected over a period of 36 months. Relationships of these monthly variables have not been reported. We aimed to examine the dynamic interactions between clinical symptoms, social functioning, treatment adherence and self-harm each month for the first 36 months of the illness in patients with FES. Moreover, the feasibility of developing an algorithm-based self-harm risk prediction tool for patients in the early stage of FES was evaluated.
Methods
Sample Identification
The sample used in the current study was part of a historical control study conducted to compare the 3-year outcomes of an early intervention service (EIS) with those of standard care service (SCS).23 Consecutive patients with a diagnosis of psychotic disorders who enrolled in the early intervention service from July 1, 2001, to June 30, 2003, were identified from the Clinical Management System (CMS), a centralized hospital database maintained by the Hospital Authority (HA) of Hong Kong. Patients with first-episode psychosis who were matched for sex, age and diagnosis and who received standard care provided by the HA for the first time from July 1, 1998, to June 30, 2001, were identified. The current study focused only on patients with first episode schizophrenia-spectrum (FES) diagnoses. In total, 617 patients were identified for each group. Patients with learning difficulties or comorbid neurological conditions or those who had more than 1 month of psychiatric treatment prior to entering the respective services were excluded from the study. To achieve the aim of the present study, all patients (n = 1234) were analyzed as a whole and the respective services were considered as covariates in the analysis.
Data Collection
All demographic and clinical data of the patients during the initial 36 months of treatment were obtained from the CMS and written clinical records. The CMS is a comprehensive electronic clinical record system used by the HA in all clinical settings, and it includes the clinical consultation notes, diagnoses, admission details and medications prescribed. The clinical consultation notes are structured clinician documentation including history of illness and mental state examination. Using a standardized data entry form and operational definitions of clinical variables, data were systematically retrieved each month over the 36-month period. The demographic variables included sex, age and years of education. Premorbid information including age at FES onset, the duration of untreated psychosis (DUP), history of deliberate self-harm (DSH) during the DUP and illicit substance use prior to entering the service were also extracted. DUP was operationally defined as the period (in days) between the documented first occurrence of positive psychotic symptoms and the prescription of antipsychotic medication by a psychiatrist.24 The baseline diagnosis was determined by clinicians based on the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision criteria, using all available clinical data. Other baseline information included are smoking status, presence of substance abuse as documented by the clinicians, hospitalization at onset and days of hospitalization at first onset. The monthly data collected including positive and negative psychotic symptoms, depressive symptoms, medication adherence, social functioning and deliberate self-harm events (DSH) which includes both suicide attempts (SA) and nonsuicidal self-injury (NSSI). Positive and negative psychotic symptoms were measured using the Clinical Global Impression-Schizophrenia (CGI-SCH) scale.25 Depressive symptoms were measured using the Clinical Global Impression scale (CGIS).26 Medication adherence was measured for each patient using a score of 1–3, with 1 indicating good adherence and 3 indicating poor adherence. The Social and Occupational Functioning Assessment Scale (SOFAS) was used to assess the social functioning of patients. Deliberate self-harm events were extracted monthly based on the clinical notes of clinicians at the same time as all other clinical variables using the same standardized data entry form. Consensus meetings were conducted with the clinicians and researchers every 2 weeks during the data collection period for quality assurance. Validity and interrater reliability for the major variables were evaluated using the records of 12 patients. The intraclass correlation coefficient (ICC) was determined by comparing the assessments made by clinicians and research staff. The test results (DUP: ICC = 0.78, CGI-SCH positive: ICC = 0.89, and CGI-SCH negative: ICC = 0.77) revealed a satisfactory level of concordance.
Data Preprocessing
Missing longitudinal data were managed using carry-forward and multivariate imputation by chained equations (MICE) 27 approaches. In the carry-forward approach, a missing data point was replaced with the value of the previous time point for the individual. MICE was performed using the “mice” package in R. The main results of the current study were based on the carry-forward approach, and the results for the dataset prepared with MICE are presented in the Supplementary Materials (Supplementary Table S3–S6, Figure S3–S5).
The Role of Instability of Clinical Information in Predicting Self-harm
Patients who had a DSH event during the 36-month follow-up period were considered as the DSH group, while others were the non-DSH group. Between group differences were analyzed with the Wilcoxon rank-sum test and Pearson’s chi-square test depending on the variable characteristics. The mean and mean square of successive differences (MSSD) was computed to represent the instability in the extracted clinical data of each participant during the 36-month follow-up period. Hierarchical logistic regressions were performed to examine the ability of demographics, premorbid information, service modalities and the mean and MSSD of clinical data during the follow-up period to predict the self-harm group in this FES population. The first model (M0) included the demographic, premorbid information and service modalities (distal features) of the patients. The second model (M1) included the distal features and means of clinical parameters over the 36-month study period, and the third model (M2) included the distal features and the MSSD of clinical parameters. A chi-square test was performed to determine the additional contribution of the models that included the means or instability of clinical parameters (ie, M1 or M2) in predicting the self-harm group other than the baseline model with only the distal features (ie, M0). Akaike information criterion (AIC) Bayesian information criteria (BIC) was used to compare M1 and M2 as they are not nested models. Since mean and MSSD of clinical parameters were highly correlated in the current dataset (positive symptom: r = 0.28, P < .001; negative symptom: r = 0.56, P < .001; depressive symptom: r = 0.84, P < .001; SOFAS: r = 0.21, P < .001; medication adherence: r = 0.88, P < 0.001), we did not include both the mean and MSSD of independent variables in one single regression model to avoid potential multicollinearity issues.
Dynamic Contributions of Clinical Information to Self-harm Over Time
A time-varying vector autoregressive (VAR) model was constructed at the group level to determine the dynamic patterns of clinical information in predicting self-harm over 36 months using the “mgm” package in R.28 Time-varying models assume that the observations at each time point are not generated from stationary parameters, but from the changes in parameters over time.29 In the first-order VAR model, each variable at time point t is predicted by all variables (including itself) at time point t – 1. The VAR model calculated the prediction effect of itself at the previous time point (autoregressive effect) and the prediction effect of all other variables at the previous time point (cross-lagged effect).
Classification of Individuals with Self-harm over a 36-month Period
A random forest model 30 was implemented to classify individuals with DSH using a 3-fold cross-validation procedure. Two-thirds of the data were used for hyperparameter tuning (ntry and min_n; trees = 1000 and others were default) and model training and the remaining one-third of the data were used for model validation. As each split generated random combinations of train and test sets of participants which may affect the classification performance, we repeated the workflow 100 times to obtain a stable prediction metric (ie, an area under the receiver operating characteristic curve [AUROC]). Three models were examined: (1) only baseline information, (2) baseline information plus mean symptom severity, and (3) baseline information plus mean and MSSD of the symptom. Models were compared to examine if adding mean or MSSD of the symptom can increase the classification performance in identifying the individuals with self-harm.
Predicting Future Self-harm Events
The same random forest model workflow was applied to predict DSH events in the future window using prior clinical information. For each time point, the outcome was defined as any self-harm event in the following 6-month or 12-month windows. Distal features included the demographic, premorbid information and service modalities, whereas proximal features were defined as clinical data from the previous month, including the presence of self-harm and instability in clinical features over the previous 6-month or 12-month (Supplementary Figure S1). Therefore, four combinations of windows were examined. A SMOTE oversampling procedure was used to increase the minority class of self-harm events.
Software and Code Availability
Data preprocessing, statistical analyses and prediction model development were performed using R (version 4.1.5). All related scripts used in this study have been made available on the GitHub repository (https://github.com/kamione/selfharm_scz_36m).
Results
Of the 1234 FES patients included in the study, 27 patients passed away due to suicide death during the follow-up period and 12 patients had missing baseline information in one or more of the key variables and were therefore excluded from the current analysis (Supplementary Figure S2). Of the remaining 1195 patients, 187 patients had at least one DSH event during the follow-up period. Compared with patients without DSH, those with DSH were younger, had fewer years of education, had a younger age of onset, more of them had history of DSH (NSSI and SA) during the DUP, lifetime illicit substance use, more hospitalization at illness onset and had more clinical and functioning instability over the 36-month study period (Table 1). We further compared patients with different types of DSH (NSSI only vs SA only vs both) and no difference was found between patients with NSSI or SA only (Supplementary Table S1). Supplementary Figure S3 shows the dynamic changes in symptom severity during the 36-month follow-up period for FES patients with and without self-harm events. Most changes in symptoms occurred during the first year. Only small variations in positive psychotic symptoms were observed after 12 months.
Differences on Demographics, Premorbid Information and Clinical Variables between Patients with Deliberate Self-harm and Those Without
| Characteristic1 . | Without DSH, N = 10082 . | With DSH, N = 1872 . | P-value3 . | q-value4 . |
|---|---|---|---|---|
| Age (Baseline) | 21.4 (3.4) | 20.5 (3.2) | .001 | 0.002 |
| Sex | .9 | >0.9 | ||
| Male | 524 (52%) | 96 (51%) | ||
| Female | 484 (48%) | 91 (49%) | ||
| Treatment | .11 | 0.12 | ||
| Standard care | 485 (48%) | 102 (55%) | ||
| Early intervention | 523 (52%) | 85 (45%) | ||
| Education years | 10.84 (2.44) | 9.96 (2.15) | <.001 | <0.001 |
| Age of onset | 20.7 (3.5) | 19.9 (3.3) | .006 | 0.008 |
| DUP (Days) | 259 (425) | 219 (329) | >.9 | >0.9 |
| NSSI in DUP | 35 (3.5%) | 18 (9.6%) | <.001 | <0.001 |
| SA in DUP | 62 (6.2%) | 30 (16%) | <.001 | <0.001 |
| Illicit substance use (Lifetime) | 71 (7.0%) | 28 (15%) | <.001 | <0.001 |
| Substance abuse (Baseline) | 13 (1.3%) | 4 (2.1%) | .3 | 0.4 |
| Current smoker | 249 (25%) | 62 (34%) | .016 | 0.022 |
| Diagnosis | .002 | 0.003 | ||
| Schizophrenia | 775 (77%) | 163 (87%) | ||
| Other Schizophrenia spectrum | 233 (23%) | 24 (13%) | ||
| Hospitalization at onset | 692 (69%) | 157 (84%) | <.001 | <0.001 |
| Days of 1st hospitalization admission | 59 (77) | 72 (91) | .032 | 0.041 |
| Pos. Symptom (36 m Mean) | 1.68 (0.80) | 1.80 (0.68) | <.001 | <0.001 |
| Neg. Symptom (36 m Mean) | 1.52 (0.64) | 1.58 (0.49) | <.001 | <0.001 |
| Dep. Symptom (36 m Mean) | 1.24 (0.39) | 1.36 (0.35) | <.001 | <0.001 |
| SOFAS (36 m Mean) | 59 (11) | 54 (8) | <0.001 | <0.001 |
| Medication adherence (36 m Mean) | 1.19 (0.33) | 1.17 (0.23) | 0.037 | 0.044 |
| Pos. Symptom (36 m MSSD) | 0.56 (0.52) | 0.86 (0.61) | <0.001 | <0.001 |
| Neg. Symptom (36 m MSSD) | 0.35 (0.36) | 0.52 (0.39) | <0.001 | <0.001 |
| Dep. Symptom (36 m MSSD) | 0.34 (0.53) | 0.62 (0.57) | <0.001 | <0.001 |
| SOFAS (36 m MSSD) | 39 (39) | 54 (44) | <0.001 | <0.001 |
| Medication adherence (36 m MSSD) | 0.22 (0.38) | 0.27 (0.39) | <0.001 | 0.001 |
| Characteristic1 . | Without DSH, N = 10082 . | With DSH, N = 1872 . | P-value3 . | q-value4 . |
|---|---|---|---|---|
| Age (Baseline) | 21.4 (3.4) | 20.5 (3.2) | .001 | 0.002 |
| Sex | .9 | >0.9 | ||
| Male | 524 (52%) | 96 (51%) | ||
| Female | 484 (48%) | 91 (49%) | ||
| Treatment | .11 | 0.12 | ||
| Standard care | 485 (48%) | 102 (55%) | ||
| Early intervention | 523 (52%) | 85 (45%) | ||
| Education years | 10.84 (2.44) | 9.96 (2.15) | <.001 | <0.001 |
| Age of onset | 20.7 (3.5) | 19.9 (3.3) | .006 | 0.008 |
| DUP (Days) | 259 (425) | 219 (329) | >.9 | >0.9 |
| NSSI in DUP | 35 (3.5%) | 18 (9.6%) | <.001 | <0.001 |
| SA in DUP | 62 (6.2%) | 30 (16%) | <.001 | <0.001 |
| Illicit substance use (Lifetime) | 71 (7.0%) | 28 (15%) | <.001 | <0.001 |
| Substance abuse (Baseline) | 13 (1.3%) | 4 (2.1%) | .3 | 0.4 |
| Current smoker | 249 (25%) | 62 (34%) | .016 | 0.022 |
| Diagnosis | .002 | 0.003 | ||
| Schizophrenia | 775 (77%) | 163 (87%) | ||
| Other Schizophrenia spectrum | 233 (23%) | 24 (13%) | ||
| Hospitalization at onset | 692 (69%) | 157 (84%) | <.001 | <0.001 |
| Days of 1st hospitalization admission | 59 (77) | 72 (91) | .032 | 0.041 |
| Pos. Symptom (36 m Mean) | 1.68 (0.80) | 1.80 (0.68) | <.001 | <0.001 |
| Neg. Symptom (36 m Mean) | 1.52 (0.64) | 1.58 (0.49) | <.001 | <0.001 |
| Dep. Symptom (36 m Mean) | 1.24 (0.39) | 1.36 (0.35) | <.001 | <0.001 |
| SOFAS (36 m Mean) | 59 (11) | 54 (8) | <0.001 | <0.001 |
| Medication adherence (36 m Mean) | 1.19 (0.33) | 1.17 (0.23) | 0.037 | 0.044 |
| Pos. Symptom (36 m MSSD) | 0.56 (0.52) | 0.86 (0.61) | <0.001 | <0.001 |
| Neg. Symptom (36 m MSSD) | 0.35 (0.36) | 0.52 (0.39) | <0.001 | <0.001 |
| Dep. Symptom (36 m MSSD) | 0.34 (0.53) | 0.62 (0.57) | <0.001 | <0.001 |
| SOFAS (36 m MSSD) | 39 (39) | 54 (44) | <0.001 | <0.001 |
| Medication adherence (36 m MSSD) | 0.22 (0.38) | 0.27 (0.39) | <0.001 | 0.001 |
Note:
1DUP, Duration of Untreated Psychosis; NSSI, Non-Suicidal Self-Injury; SA, Suicidal Attempts; Pos, Positive; Neg, Negative; Dep, Depressive; SOFAS, Social and Occupational Functioning Assessment Scale; MSSD, Mean of the Squared Successive Differences.
2Mean (SD); n (%).
3Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test.
4False discovery rate correction for multiple testing.
Differences on Demographics, Premorbid Information and Clinical Variables between Patients with Deliberate Self-harm and Those Without
| Characteristic1 . | Without DSH, N = 10082 . | With DSH, N = 1872 . | P-value3 . | q-value4 . |
|---|---|---|---|---|
| Age (Baseline) | 21.4 (3.4) | 20.5 (3.2) | .001 | 0.002 |
| Sex | .9 | >0.9 | ||
| Male | 524 (52%) | 96 (51%) | ||
| Female | 484 (48%) | 91 (49%) | ||
| Treatment | .11 | 0.12 | ||
| Standard care | 485 (48%) | 102 (55%) | ||
| Early intervention | 523 (52%) | 85 (45%) | ||
| Education years | 10.84 (2.44) | 9.96 (2.15) | <.001 | <0.001 |
| Age of onset | 20.7 (3.5) | 19.9 (3.3) | .006 | 0.008 |
| DUP (Days) | 259 (425) | 219 (329) | >.9 | >0.9 |
| NSSI in DUP | 35 (3.5%) | 18 (9.6%) | <.001 | <0.001 |
| SA in DUP | 62 (6.2%) | 30 (16%) | <.001 | <0.001 |
| Illicit substance use (Lifetime) | 71 (7.0%) | 28 (15%) | <.001 | <0.001 |
| Substance abuse (Baseline) | 13 (1.3%) | 4 (2.1%) | .3 | 0.4 |
| Current smoker | 249 (25%) | 62 (34%) | .016 | 0.022 |
| Diagnosis | .002 | 0.003 | ||
| Schizophrenia | 775 (77%) | 163 (87%) | ||
| Other Schizophrenia spectrum | 233 (23%) | 24 (13%) | ||
| Hospitalization at onset | 692 (69%) | 157 (84%) | <.001 | <0.001 |
| Days of 1st hospitalization admission | 59 (77) | 72 (91) | .032 | 0.041 |
| Pos. Symptom (36 m Mean) | 1.68 (0.80) | 1.80 (0.68) | <.001 | <0.001 |
| Neg. Symptom (36 m Mean) | 1.52 (0.64) | 1.58 (0.49) | <.001 | <0.001 |
| Dep. Symptom (36 m Mean) | 1.24 (0.39) | 1.36 (0.35) | <.001 | <0.001 |
| SOFAS (36 m Mean) | 59 (11) | 54 (8) | <0.001 | <0.001 |
| Medication adherence (36 m Mean) | 1.19 (0.33) | 1.17 (0.23) | 0.037 | 0.044 |
| Pos. Symptom (36 m MSSD) | 0.56 (0.52) | 0.86 (0.61) | <0.001 | <0.001 |
| Neg. Symptom (36 m MSSD) | 0.35 (0.36) | 0.52 (0.39) | <0.001 | <0.001 |
| Dep. Symptom (36 m MSSD) | 0.34 (0.53) | 0.62 (0.57) | <0.001 | <0.001 |
| SOFAS (36 m MSSD) | 39 (39) | 54 (44) | <0.001 | <0.001 |
| Medication adherence (36 m MSSD) | 0.22 (0.38) | 0.27 (0.39) | <0.001 | 0.001 |
| Characteristic1 . | Without DSH, N = 10082 . | With DSH, N = 1872 . | P-value3 . | q-value4 . |
|---|---|---|---|---|
| Age (Baseline) | 21.4 (3.4) | 20.5 (3.2) | .001 | 0.002 |
| Sex | .9 | >0.9 | ||
| Male | 524 (52%) | 96 (51%) | ||
| Female | 484 (48%) | 91 (49%) | ||
| Treatment | .11 | 0.12 | ||
| Standard care | 485 (48%) | 102 (55%) | ||
| Early intervention | 523 (52%) | 85 (45%) | ||
| Education years | 10.84 (2.44) | 9.96 (2.15) | <.001 | <0.001 |
| Age of onset | 20.7 (3.5) | 19.9 (3.3) | .006 | 0.008 |
| DUP (Days) | 259 (425) | 219 (329) | >.9 | >0.9 |
| NSSI in DUP | 35 (3.5%) | 18 (9.6%) | <.001 | <0.001 |
| SA in DUP | 62 (6.2%) | 30 (16%) | <.001 | <0.001 |
| Illicit substance use (Lifetime) | 71 (7.0%) | 28 (15%) | <.001 | <0.001 |
| Substance abuse (Baseline) | 13 (1.3%) | 4 (2.1%) | .3 | 0.4 |
| Current smoker | 249 (25%) | 62 (34%) | .016 | 0.022 |
| Diagnosis | .002 | 0.003 | ||
| Schizophrenia | 775 (77%) | 163 (87%) | ||
| Other Schizophrenia spectrum | 233 (23%) | 24 (13%) | ||
| Hospitalization at onset | 692 (69%) | 157 (84%) | <.001 | <0.001 |
| Days of 1st hospitalization admission | 59 (77) | 72 (91) | .032 | 0.041 |
| Pos. Symptom (36 m Mean) | 1.68 (0.80) | 1.80 (0.68) | <.001 | <0.001 |
| Neg. Symptom (36 m Mean) | 1.52 (0.64) | 1.58 (0.49) | <.001 | <0.001 |
| Dep. Symptom (36 m Mean) | 1.24 (0.39) | 1.36 (0.35) | <.001 | <0.001 |
| SOFAS (36 m Mean) | 59 (11) | 54 (8) | <0.001 | <0.001 |
| Medication adherence (36 m Mean) | 1.19 (0.33) | 1.17 (0.23) | 0.037 | 0.044 |
| Pos. Symptom (36 m MSSD) | 0.56 (0.52) | 0.86 (0.61) | <0.001 | <0.001 |
| Neg. Symptom (36 m MSSD) | 0.35 (0.36) | 0.52 (0.39) | <0.001 | <0.001 |
| Dep. Symptom (36 m MSSD) | 0.34 (0.53) | 0.62 (0.57) | <0.001 | <0.001 |
| SOFAS (36 m MSSD) | 39 (39) | 54 (44) | <0.001 | <0.001 |
| Medication adherence (36 m MSSD) | 0.22 (0.38) | 0.27 (0.39) | <0.001 | 0.001 |
Note:
1DUP, Duration of Untreated Psychosis; NSSI, Non-Suicidal Self-Injury; SA, Suicidal Attempts; Pos, Positive; Neg, Negative; Dep, Depressive; SOFAS, Social and Occupational Functioning Assessment Scale; MSSD, Mean of the Squared Successive Differences.
2Mean (SD); n (%).
3Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test.
4False discovery rate correction for multiple testing.
Hierarchical logistic regression models showed that the self-harm history during the DUP and previous illicit substance use were associated with self-harm during the follow-up period (Ps < .05, M0: χ²(14) = 57.08, P < 0.001, Cragg–Uhler Pseudo-R² = 0.1). All mean clinical information was not significantly associated with self-harm (Ps > .05; M1: χ² [19] = 75.34, P < 0.001, Cragg–Uhler Pseudo-R² = 0.13) (Table 2). Finally, the model including the MSSD of the positive and affective symptoms (M2: χ²[19] = 99.19, P < .001, Cragg–Uhler Pseudo-R² = 0.17) was significantly associated with patients’ self-harm (Ps < .05). Chi-square tests showed that both M1 and M2 explained more variance than M0 (χ²(5) = 42.5/46.4, P < .001, ΔR² = 0.03/0.07). Moreover, M2 had lower penalty terms (AIC = 789.86, BIC = 884.98) compared to M1 (AIC = 839.44, BIC = 935.46), indicating that M2 was a better and preferred model. Accordingly, M2 results showed that instability in symptoms, including positive (odds ratio [OR] = 0.55, 95% confidence interval [CI] = 0.19, 0.911, P = .027) and depressive symptoms (OR = 0.58, 95% CI = 0.25, 0.91, P = .014), were also important predictors in addition to clinical history (Table 2).
Hierarchical Logistic Regression Models of Demographics, Premorbid Information, Symptoms and Functioning during Follow up and Variability of Symptoms and Functioning in Predicting Self-harm
| . | M0 . | M1 . | M2 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic1 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . |
| Age (Baseline) | −0.16 | −0.41, 0.10 | .2 | 0.39 | −0.17 | −0.42, 0.10 | .2 | 0.39 | −0.15 | −0.40, 0.12 | .2 | 0.52 |
| Sex | ||||||||||||
| Male | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Female | 0.07 | −0.30, 0.43 | .7 | 0.83 | 0.04 | −0.34, 0.42 | .8 | 0.87 | 0.07 | −0.32, 0.45 | .7 | 0.82 |
| Treatment | ||||||||||||
| Standard Care | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Early Intervention | 0.17 | −0.20, 0.53 | .4 | 0.60 | 0.24 | −0.14, 0.61 | .2 | 0.41 | 0.09 | −0.29, 0.48 | .6 | 0.82 |
| Education years | −0.10 | −0.19, -0.02 | .021 | 0.073 | −0.08 | −0.17, 0.01 | .080 | 0.22 | −0.08 | −0.18, 0.01 | .086 | 0.20 |
| Age of onset | 0.11 | −0.15, 0.35 | .4 | 0.60 | 0.10 | −0.17, 0.35 | .4 | 0.73 | 0.12 | −0.16, 0.36 | .4 | 0.63 |
| DUP (Days) | 0.00 | 0.00, 0.00 | .9 | 0.93 | 0.00 | 0.00, 0.00 | .8 | 0.87 | 0.00 | 0.00, 0.00 | .9 | 0.90 |
| NSSI in DUP | 1.1 | 0.34, 1.8 | .003 | 0.023 | 1.1 | 0.32, 1.8 | .004 | 0.041 | 1.1 | 0.28, 1.8 | .007 | 0.031 |
| SA in DUP | 0.80 | 0.26, 1.3 | .003 | 0.023 | 0.80 | 0.25, 1.3 | .004 | 0.041 | 0.72 | 0.15, 1.3 | .012 | 0.045 |
| Illicit substance use (lifetime) | 0.84 | 0.20, 1.5 | .008 | 0.039 | 0.86 | 0.23, 1.5 | .007 | 0.044 | 0.93 | 0.28, 1.6 | .005 | 0.030 |
| Substance abuse (baseline) | −0.34 | −2.0, 1.0 | .6 | 0.83 | −0.40 | −2.0, 1.0 | .6 | 0.82 | −0.28 | −1.9, 1.1 | .7 | 0.82 |
| Current smoker | −0.01 | −0.47, 0.44 | >.9 | 0.97 | −0.05 | −0.51, 0.41 | .8 | 0.87 | −0.09 | −0.58, 0.38 | .7 | 0.82 |
| Diagnosis | ||||||||||||
| Other schizophrenia spectrum | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Schizophrenia | 0.46 | −0.04, 1.0 | .080 | 0.19 | 0.59 | 0.07, 1.2 | .033 | 0.10 | 0.25 | −0.31, 0.85 | .4 | 0.63 |
| Hospitalization at onset | −0.14 | −0.70, 0.45 | .6 | 0.83 | −0.09 | −0.65, 0.51 | .8 | 0.87 | 0.05 | −0.53, 0.66 | .9 | 0.90 |
| Days of 1st hospitalization admission | 0.00 | 0.00, 0.00 | .034 | 0.10 | 0.00 | 0.00, 0.00 | .14 | 0.33 | 0.00 | 0.00, 0.00 | .043 | 0.14 |
| Pos. Symptom (36 m Mean) | 0.02 | −0.26, 0.29 | .9 | 0.87 | ||||||||
| Neg. Symptom (36 m Mean) | −0.09 | −0.42, 0.23 | .6 | 0.82 | ||||||||
| Dep. Symptom (36 m Mean) | 0.56 | 0.12, 1.0 | .014 | 0.064 | ||||||||
| SOFAS (36 m Mean) | −0.03 | −0.05, 0.00 | .022 | 0.082 | ||||||||
| Medication adherence (36 m Mean) | 0.22 | −0.50, 0.87 | .5 | 0.82 | ||||||||
| Pos. Symptom (36 m MSSD) | 0.55 | 0.19, 0.91 | .003 | 0.027 | ||||||||
| Neg. Symptom (36 m MSSD) | 0.48 | −0.02, 1.0 | .057 | 0.15 | ||||||||
| Dep. Symptom (36 m MSSD) | 0.58 | 0.25, 0.91 | <.001 | 0.014 | ||||||||
| SOFAS (36 m MSSD) | 0.00 | 0.00, 0.01 | .3 | 0.54 | ||||||||
| Medication Adherence (36 m MSSD) | 0.18 | −0.36, 0.65 | .5 | 0.69 | ||||||||
| . | M0 . | M1 . | M2 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic1 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . |
| Age (Baseline) | −0.16 | −0.41, 0.10 | .2 | 0.39 | −0.17 | −0.42, 0.10 | .2 | 0.39 | −0.15 | −0.40, 0.12 | .2 | 0.52 |
| Sex | ||||||||||||
| Male | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Female | 0.07 | −0.30, 0.43 | .7 | 0.83 | 0.04 | −0.34, 0.42 | .8 | 0.87 | 0.07 | −0.32, 0.45 | .7 | 0.82 |
| Treatment | ||||||||||||
| Standard Care | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Early Intervention | 0.17 | −0.20, 0.53 | .4 | 0.60 | 0.24 | −0.14, 0.61 | .2 | 0.41 | 0.09 | −0.29, 0.48 | .6 | 0.82 |
| Education years | −0.10 | −0.19, -0.02 | .021 | 0.073 | −0.08 | −0.17, 0.01 | .080 | 0.22 | −0.08 | −0.18, 0.01 | .086 | 0.20 |
| Age of onset | 0.11 | −0.15, 0.35 | .4 | 0.60 | 0.10 | −0.17, 0.35 | .4 | 0.73 | 0.12 | −0.16, 0.36 | .4 | 0.63 |
| DUP (Days) | 0.00 | 0.00, 0.00 | .9 | 0.93 | 0.00 | 0.00, 0.00 | .8 | 0.87 | 0.00 | 0.00, 0.00 | .9 | 0.90 |
| NSSI in DUP | 1.1 | 0.34, 1.8 | .003 | 0.023 | 1.1 | 0.32, 1.8 | .004 | 0.041 | 1.1 | 0.28, 1.8 | .007 | 0.031 |
| SA in DUP | 0.80 | 0.26, 1.3 | .003 | 0.023 | 0.80 | 0.25, 1.3 | .004 | 0.041 | 0.72 | 0.15, 1.3 | .012 | 0.045 |
| Illicit substance use (lifetime) | 0.84 | 0.20, 1.5 | .008 | 0.039 | 0.86 | 0.23, 1.5 | .007 | 0.044 | 0.93 | 0.28, 1.6 | .005 | 0.030 |
| Substance abuse (baseline) | −0.34 | −2.0, 1.0 | .6 | 0.83 | −0.40 | −2.0, 1.0 | .6 | 0.82 | −0.28 | −1.9, 1.1 | .7 | 0.82 |
| Current smoker | −0.01 | −0.47, 0.44 | >.9 | 0.97 | −0.05 | −0.51, 0.41 | .8 | 0.87 | −0.09 | −0.58, 0.38 | .7 | 0.82 |
| Diagnosis | ||||||||||||
| Other schizophrenia spectrum | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Schizophrenia | 0.46 | −0.04, 1.0 | .080 | 0.19 | 0.59 | 0.07, 1.2 | .033 | 0.10 | 0.25 | −0.31, 0.85 | .4 | 0.63 |
| Hospitalization at onset | −0.14 | −0.70, 0.45 | .6 | 0.83 | −0.09 | −0.65, 0.51 | .8 | 0.87 | 0.05 | −0.53, 0.66 | .9 | 0.90 |
| Days of 1st hospitalization admission | 0.00 | 0.00, 0.00 | .034 | 0.10 | 0.00 | 0.00, 0.00 | .14 | 0.33 | 0.00 | 0.00, 0.00 | .043 | 0.14 |
| Pos. Symptom (36 m Mean) | 0.02 | −0.26, 0.29 | .9 | 0.87 | ||||||||
| Neg. Symptom (36 m Mean) | −0.09 | −0.42, 0.23 | .6 | 0.82 | ||||||||
| Dep. Symptom (36 m Mean) | 0.56 | 0.12, 1.0 | .014 | 0.064 | ||||||||
| SOFAS (36 m Mean) | −0.03 | −0.05, 0.00 | .022 | 0.082 | ||||||||
| Medication adherence (36 m Mean) | 0.22 | −0.50, 0.87 | .5 | 0.82 | ||||||||
| Pos. Symptom (36 m MSSD) | 0.55 | 0.19, 0.91 | .003 | 0.027 | ||||||||
| Neg. Symptom (36 m MSSD) | 0.48 | −0.02, 1.0 | .057 | 0.15 | ||||||||
| Dep. Symptom (36 m MSSD) | 0.58 | 0.25, 0.91 | <.001 | 0.014 | ||||||||
| SOFAS (36 m MSSD) | 0.00 | 0.00, 0.01 | .3 | 0.54 | ||||||||
| Medication Adherence (36 m MSSD) | 0.18 | −0.36, 0.65 | .5 | 0.69 | ||||||||
Note:
1DUP, Duration of Untreated Psychosis; NSSI, Non-Suicidal Self-Injury; SA, Suicidal Attempts; Pos, Positive; Neg, Negative; Dep, Depressive; SOFAS, Social and Occupational Functioning Assessment Scale; MSSD, Mean of the Squared Successive Differences.
2OR, Odds Ratio, CI, Confidence Interval.
3False discovery rate correction for multiple testing.
Hierarchical Logistic Regression Models of Demographics, Premorbid Information, Symptoms and Functioning during Follow up and Variability of Symptoms and Functioning in Predicting Self-harm
| . | M0 . | M1 . | M2 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic1 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . |
| Age (Baseline) | −0.16 | −0.41, 0.10 | .2 | 0.39 | −0.17 | −0.42, 0.10 | .2 | 0.39 | −0.15 | −0.40, 0.12 | .2 | 0.52 |
| Sex | ||||||||||||
| Male | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Female | 0.07 | −0.30, 0.43 | .7 | 0.83 | 0.04 | −0.34, 0.42 | .8 | 0.87 | 0.07 | −0.32, 0.45 | .7 | 0.82 |
| Treatment | ||||||||||||
| Standard Care | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Early Intervention | 0.17 | −0.20, 0.53 | .4 | 0.60 | 0.24 | −0.14, 0.61 | .2 | 0.41 | 0.09 | −0.29, 0.48 | .6 | 0.82 |
| Education years | −0.10 | −0.19, -0.02 | .021 | 0.073 | −0.08 | −0.17, 0.01 | .080 | 0.22 | −0.08 | −0.18, 0.01 | .086 | 0.20 |
| Age of onset | 0.11 | −0.15, 0.35 | .4 | 0.60 | 0.10 | −0.17, 0.35 | .4 | 0.73 | 0.12 | −0.16, 0.36 | .4 | 0.63 |
| DUP (Days) | 0.00 | 0.00, 0.00 | .9 | 0.93 | 0.00 | 0.00, 0.00 | .8 | 0.87 | 0.00 | 0.00, 0.00 | .9 | 0.90 |
| NSSI in DUP | 1.1 | 0.34, 1.8 | .003 | 0.023 | 1.1 | 0.32, 1.8 | .004 | 0.041 | 1.1 | 0.28, 1.8 | .007 | 0.031 |
| SA in DUP | 0.80 | 0.26, 1.3 | .003 | 0.023 | 0.80 | 0.25, 1.3 | .004 | 0.041 | 0.72 | 0.15, 1.3 | .012 | 0.045 |
| Illicit substance use (lifetime) | 0.84 | 0.20, 1.5 | .008 | 0.039 | 0.86 | 0.23, 1.5 | .007 | 0.044 | 0.93 | 0.28, 1.6 | .005 | 0.030 |
| Substance abuse (baseline) | −0.34 | −2.0, 1.0 | .6 | 0.83 | −0.40 | −2.0, 1.0 | .6 | 0.82 | −0.28 | −1.9, 1.1 | .7 | 0.82 |
| Current smoker | −0.01 | −0.47, 0.44 | >.9 | 0.97 | −0.05 | −0.51, 0.41 | .8 | 0.87 | −0.09 | −0.58, 0.38 | .7 | 0.82 |
| Diagnosis | ||||||||||||
| Other schizophrenia spectrum | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Schizophrenia | 0.46 | −0.04, 1.0 | .080 | 0.19 | 0.59 | 0.07, 1.2 | .033 | 0.10 | 0.25 | −0.31, 0.85 | .4 | 0.63 |
| Hospitalization at onset | −0.14 | −0.70, 0.45 | .6 | 0.83 | −0.09 | −0.65, 0.51 | .8 | 0.87 | 0.05 | −0.53, 0.66 | .9 | 0.90 |
| Days of 1st hospitalization admission | 0.00 | 0.00, 0.00 | .034 | 0.10 | 0.00 | 0.00, 0.00 | .14 | 0.33 | 0.00 | 0.00, 0.00 | .043 | 0.14 |
| Pos. Symptom (36 m Mean) | 0.02 | −0.26, 0.29 | .9 | 0.87 | ||||||||
| Neg. Symptom (36 m Mean) | −0.09 | −0.42, 0.23 | .6 | 0.82 | ||||||||
| Dep. Symptom (36 m Mean) | 0.56 | 0.12, 1.0 | .014 | 0.064 | ||||||||
| SOFAS (36 m Mean) | −0.03 | −0.05, 0.00 | .022 | 0.082 | ||||||||
| Medication adherence (36 m Mean) | 0.22 | −0.50, 0.87 | .5 | 0.82 | ||||||||
| Pos. Symptom (36 m MSSD) | 0.55 | 0.19, 0.91 | .003 | 0.027 | ||||||||
| Neg. Symptom (36 m MSSD) | 0.48 | −0.02, 1.0 | .057 | 0.15 | ||||||||
| Dep. Symptom (36 m MSSD) | 0.58 | 0.25, 0.91 | <.001 | 0.014 | ||||||||
| SOFAS (36 m MSSD) | 0.00 | 0.00, 0.01 | .3 | 0.54 | ||||||||
| Medication Adherence (36 m MSSD) | 0.18 | −0.36, 0.65 | .5 | 0.69 | ||||||||
| . | M0 . | M1 . | M2 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic1 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . | log(OR)2 . | 95% CI2 . | P-value . | q-value3 . |
| Age (Baseline) | −0.16 | −0.41, 0.10 | .2 | 0.39 | −0.17 | −0.42, 0.10 | .2 | 0.39 | −0.15 | −0.40, 0.12 | .2 | 0.52 |
| Sex | ||||||||||||
| Male | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Female | 0.07 | −0.30, 0.43 | .7 | 0.83 | 0.04 | −0.34, 0.42 | .8 | 0.87 | 0.07 | −0.32, 0.45 | .7 | 0.82 |
| Treatment | ||||||||||||
| Standard Care | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Early Intervention | 0.17 | −0.20, 0.53 | .4 | 0.60 | 0.24 | −0.14, 0.61 | .2 | 0.41 | 0.09 | −0.29, 0.48 | .6 | 0.82 |
| Education years | −0.10 | −0.19, -0.02 | .021 | 0.073 | −0.08 | −0.17, 0.01 | .080 | 0.22 | −0.08 | −0.18, 0.01 | .086 | 0.20 |
| Age of onset | 0.11 | −0.15, 0.35 | .4 | 0.60 | 0.10 | −0.17, 0.35 | .4 | 0.73 | 0.12 | −0.16, 0.36 | .4 | 0.63 |
| DUP (Days) | 0.00 | 0.00, 0.00 | .9 | 0.93 | 0.00 | 0.00, 0.00 | .8 | 0.87 | 0.00 | 0.00, 0.00 | .9 | 0.90 |
| NSSI in DUP | 1.1 | 0.34, 1.8 | .003 | 0.023 | 1.1 | 0.32, 1.8 | .004 | 0.041 | 1.1 | 0.28, 1.8 | .007 | 0.031 |
| SA in DUP | 0.80 | 0.26, 1.3 | .003 | 0.023 | 0.80 | 0.25, 1.3 | .004 | 0.041 | 0.72 | 0.15, 1.3 | .012 | 0.045 |
| Illicit substance use (lifetime) | 0.84 | 0.20, 1.5 | .008 | 0.039 | 0.86 | 0.23, 1.5 | .007 | 0.044 | 0.93 | 0.28, 1.6 | .005 | 0.030 |
| Substance abuse (baseline) | −0.34 | −2.0, 1.0 | .6 | 0.83 | −0.40 | −2.0, 1.0 | .6 | 0.82 | −0.28 | −1.9, 1.1 | .7 | 0.82 |
| Current smoker | −0.01 | −0.47, 0.44 | >.9 | 0.97 | −0.05 | −0.51, 0.41 | .8 | 0.87 | −0.09 | −0.58, 0.38 | .7 | 0.82 |
| Diagnosis | ||||||||||||
| Other schizophrenia spectrum | 0.00 | — | 0.00 | — | 0.00 | — | ||||||
| Schizophrenia | 0.46 | −0.04, 1.0 | .080 | 0.19 | 0.59 | 0.07, 1.2 | .033 | 0.10 | 0.25 | −0.31, 0.85 | .4 | 0.63 |
| Hospitalization at onset | −0.14 | −0.70, 0.45 | .6 | 0.83 | −0.09 | −0.65, 0.51 | .8 | 0.87 | 0.05 | −0.53, 0.66 | .9 | 0.90 |
| Days of 1st hospitalization admission | 0.00 | 0.00, 0.00 | .034 | 0.10 | 0.00 | 0.00, 0.00 | .14 | 0.33 | 0.00 | 0.00, 0.00 | .043 | 0.14 |
| Pos. Symptom (36 m Mean) | 0.02 | −0.26, 0.29 | .9 | 0.87 | ||||||||
| Neg. Symptom (36 m Mean) | −0.09 | −0.42, 0.23 | .6 | 0.82 | ||||||||
| Dep. Symptom (36 m Mean) | 0.56 | 0.12, 1.0 | .014 | 0.064 | ||||||||
| SOFAS (36 m Mean) | −0.03 | −0.05, 0.00 | .022 | 0.082 | ||||||||
| Medication adherence (36 m Mean) | 0.22 | −0.50, 0.87 | .5 | 0.82 | ||||||||
| Pos. Symptom (36 m MSSD) | 0.55 | 0.19, 0.91 | .003 | 0.027 | ||||||||
| Neg. Symptom (36 m MSSD) | 0.48 | −0.02, 1.0 | .057 | 0.15 | ||||||||
| Dep. Symptom (36 m MSSD) | 0.58 | 0.25, 0.91 | <.001 | 0.014 | ||||||||
| SOFAS (36 m MSSD) | 0.00 | 0.00, 0.01 | .3 | 0.54 | ||||||||
| Medication Adherence (36 m MSSD) | 0.18 | −0.36, 0.65 | .5 | 0.69 | ||||||||
Note:
1DUP, Duration of Untreated Psychosis; NSSI, Non-Suicidal Self-Injury; SA, Suicidal Attempts; Pos, Positive; Neg, Negative; Dep, Depressive; SOFAS, Social and Occupational Functioning Assessment Scale; MSSD, Mean of the Squared Successive Differences.
2OR, Odds Ratio, CI, Confidence Interval.
3False discovery rate correction for multiple testing.
The results of the VAR model (Figure 1) demonstrated that the cross-lagged effects of positive and depressive symptoms played important roles in predicting self-harm at the next time point. Moreover, different patterns of effect of positive and depressive symptoms on self-harm were seen over the 36-month follow-up period. The edge strength between positive symptoms and self-harm was low during the initial 6 months and then increased gradually over 36 months. However, the edge strength between depressive symptoms and self-harm was high initially, reached a peak at month 12–16 and then decreased.
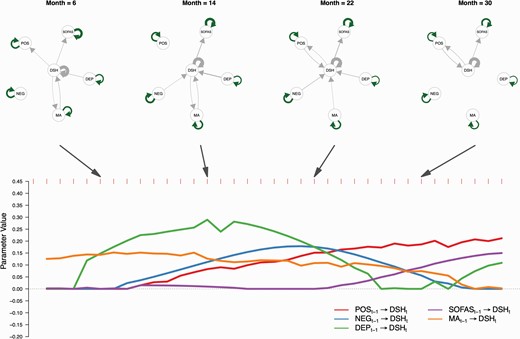
A time-varying vector autoregressive model revealed dynamic contributions of clinical information on DSH. Top panel shows lag effects among variables in selected months (6, 14, 22, and 30). Green arrows were positive parameters estimated from continuous variables while gray arrows were parameters estimated from variables containing binary values. Self-loop arrows represent autoregressive effects while direct arrows between variables represent cross-lagged effects. Bottom panel shows parameters (x-axis) of cross lagged effects from clinical information to DSH. The y-axis represents months. Note: Pos, Positive Symptoms; Neg, Negative Symptoms; Dep, Depressive Symptoms; SOFAS, Social and Occupational Functioning Assessment Scale; MA, Medication Adherence; DSH, Deliberate Self-harm.
Random forest classification models (Figure 2) showed the AUROC was 0.65 ± 0.028 with only baseline information. By adding mean of symptom severity to classify, the model performance was increased to AUROC = 0.75 ± 0.026. Further inclusion of MSSD of symptoms increased the model performance to AUROC = 0.77 ± 0.023. Pairwise Wilcoxon rank sum tests showed that model 3 (ie, baseline + mean + MSSD) had a better classification performance than model 2 (ie, baseline + mean; P < .001) and model 1 (ie, baseline; P < .001).

Adding MSSD of clinical information can better differentiate patients with DSH using a random forest classification model. Compared to random forest models with only baseline information or baseline information with 36-month mean of clinical information, the model with MSSD performed better (ie, a higher AUROC).
Figure 3 shows the performance of the prediction model for future self-harm events using distal features, clinical and functional information and the MSSD of these variables to predict self-harm. Random forest models were used with a sliding window of 6-month or 12-month prior information to predict any self-harm event in the future 6-month or 12-month windows. The results indicated that over all the possible prediction windows our models predicted self-harm above chance regardless of the combinations of prior information and prediction windows, with an average AUROC ranging from 0.648 ± 0.09 to 0.653 ± 0.073. The performance was the best (AUROC = 0.70 ± 0.071) with the model using 6-month prior information to predict self-harm over a 6-month prediction window from month 11 to 23. Details of each model’s prediction performance at each month are presented in Supplementary Table S2.
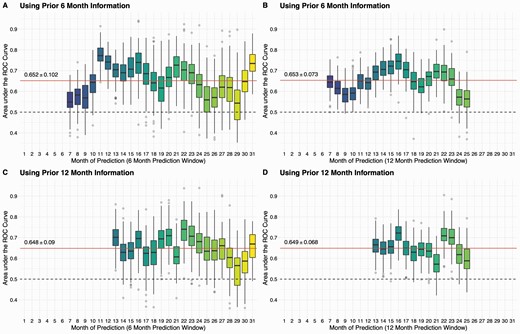
Random forest prediction models can predict future self-harm events with baseline information, clinical status of last month, and instability of clinical variables. Using sliding windows of 6 or 12 months, we examined the feasibilities of predicting future self-harm events. On average, our models can achieve a reasonable performance with AUROC of 0.65 regarding window sizes. Particularly, the performance was better when using prior 6-month information to predict future 6-month self-harm events between month 11 and 23 with a AUROC = 0.70 ± 0.071 (Panel A).
For sensitivity analysis, we reran the same analysis using an imputed dataset for missing data. The results had a similar pattern as that of the main analysis (Supplementary Materials and Table S3–S6, Figure S4–S6). However, the random model classification and prediction performance dropped in general in the imputation dataset, possibly because the imputation approach may lead to overfitting in the training data in our dataset.
Discussion
We specifically examined the dynamic interaction between clinical and functioning variables and self-harm in patients with FES during the initial 36 months of treatment and attempted to build a predictive model for self-harm during the early stage of the illness. The results suggest that in addition to distal risk factors including the different services received and the positive, negative and depressive symptoms, the instability in these symptoms also play a significant role in predicting self-harm. Furthermore, different dynamic interaction patterns of these variables with self-harm over 36 months were demonstrated. Depressive symptoms had the greatest contribution to self-harm prediction during the first and second year, while the role of positive symptoms increased gradually in the second to third years of the illness. The model with symptom instability differentiates individuals with self-harm from those without with a satisfactory performance (AUROC = 0.77 ± 0.023) and outperformed models without this information. We also attempted to predict future self-harm events with a sliding window approach using distal factors, clinical information from the previous month and the instability in clinical variables with different combinations of data and prediction timeframe. The performance was the best (AUROC = 0.70 ± 0.071) with the model using 6-month prior information to predict self-harm with a 6-month prediction window from month 11 to 23.
With the relatively fine temporal resolution of the monthly clinical and functional data extracted from the clinical notes over the initial 36 months of treatment of patients with FES, we found that the instability in psychotic and depressive symptoms, in addition to the static risk factors during the early stage of the illness, were significant in predicting self-harm. The first 2–5 years after the onset of a psychotic illness is considered to be the critical period that determines the long-term outcomes.31 This period has been characterized as showing greater instability in symptoms than the later stages.32,33 We also found that prominent symptom instability occurred in the first two years of the illness. The degree of instability in psychiatric symptoms and social functioning reflects the early characteristics of the illness. The significant relationship between these instability in clinical and functional variables and self-harm, in addition to the relationship between symptom severity and self-harm, highlighted the importance of maintaining symptom stability during the early stage of the illness. A trend significance (P < .1 after adjustment of multiple comparisons) of lowered self-harm among the patients received EIS compared with those who received SCS was found, align with the previous finding of the effectiveness of EIS in reduction of the self-harm and suicide mortality.5 EIS represents a more intense community intervention provided to patients and is likely to be an important static factor and hence is included as the distal factor in both the regression model and subsequent prediction model analysis.
Different patterns of the dynamic relationship between symptomatology and self-harm were noted for different symptoms. During the first two year of the illness, the relationship between depressive symptoms and self-harm in the following month was the most prominent. However, the strength of this relationship gradually decreased during the second half of the 36-month period. Many studies have reported a significant link between depressive symptoms and suicidal behavior in patients with first-episode psychosis.5,34 The findings of the current study further emphasized the relationship between depressive symptom instability and self-harm and indicated that such a relationship was most prominent during the first 1–2 years. One possible explanation is that there is a high prevalence of depressive symptoms among patients with FES ranging from 14.15% to 83%35–38 and is highest during the first 12 months of the illness.35 The focus of treatment after the onset of psychotic illness is often the control of psychotic symptoms. More than 70% of patients with FES respond to antipsychotic treatment with a significant reduction in their psychotic symptoms.39 The improvement in psychotic symptoms in the first year of the illness may explain the less prominent relationship between positive psychotic symptoms and self-harm during the early stage of the illness. However, the significance of positive psychotic symptoms in predicting self-harm in the following month increased gradually and continuously from 12 to 36 months. This may reflect the increase in the relapse of psychotic symptoms during the second and third years of the illness.40 These results highlight the importance of a phase-specific suicide and self-harm prevention program for patients with FES, specifically focused on identifying and managing depression during the first year of the illness and relapse prevention and psychotic symptom management during the second and third years of the illness.
The model with distal information, mean symptom severity and symptom instability had the highest AUROC in identifying patients with FES over 36-month follow up highlights the importance of symptom instability in self-harm predicting. An out-of-sample prediction model built with distal information, mean symptom severity of last month and instability of symptoms in the past 6 months, had a mean AUROC around 0.65 for predicting 6 or 12 month self-harm events. The performance was the best when using 6-month information to predict future 6 month self-harm events between month 11 and 23 (AUROC ~ 0.70). This is the first model aimed at predicting self-harm specifically in the FES population by incorporating symptom instability data. Our model using basic clinical data (25 variables) extracted from medical records had reasonable predictive performance for self-harm behavior of FES particularly in the second year of the illness. These suggest that establishing an algorithm-based self-harm risk identification system with a relatively simple set of the clinical variables incorporating symptom instability to facilitate the detection and management of self-harm risk in patients with FES during their early stage of the illness may be feasible. Further improvement and validation of the model would be required.
There are some limitations of this study that should be acknowledged. First, due to the use of the CGI-SCH scale, we were unable to explore the effect of specific symptom dimensions, such as delusion or hallucination. Second, only limited information about patients can be obtained from the CMS. Therefore, some important information, such as the presence of stressful life events, was not included in the analysis. Furthermore, the quality of the clinical data extracted from the CMS depends on the quality of clinical record entry. The sample was collected in 1998–2003 and may be limited in representing the patient with FES from recent early intervention service though the basic demographics of the current study were similar to the FES sample collected more recently from the same service.41,42 Furthermore, the exclusion criteria of the study limit the generalizability of the study results.
In summary, our study provides novel and convergent evidence for the vital role of longitudinal symptom instability, in addition to static risk factors, in predicting self-harm behaviors in patients with FES. The phasic relationships identified between different symptoms and self-harm in the early stage of the illness highlight the need for tailored phase-specific programs for self-harm and suicide prevention. The model that included static risk factors and symptom instability may have reasonable power to predict future self-harm events during the early stage of illness. These findings suggest the feasibility of developing an algorithm-based self-harm risk assessment system with ongoing basic clinical assessments including symptom instability during the early stage of schizophrenia.
Acknowledgments
We thank all clinical staff of the seven psychiatric units in Hong Kong for supporting patient recruitment and Dr Jennifer Tang in coordinating the data collection process. Dr Ting Yat Wong, Dr Charlton Cheung, Dr Christy Lai Ming Hui, Dr Yi Nam Suen declare no conflict of interest. Dr Sherry Kit Wa Chan, Dr Wing Chung Chang, Dr Edwin Ho Ming Lee, Prof Eric Yu Hai Chen are member of the working group of early intervention service for psychosis of the Hospital Authority of Hong Kong.
Funding
This study is supported by the Health and Health Service Research Grant of the Food and Health Bureau of Hong Kong (Reference number 03040141).
References
Author notes
These authors contributed equally to the article.




